Catalytic combustion of toluene over cerium modified CuMn/Al2O3/cordierite monolithic catalyst
-
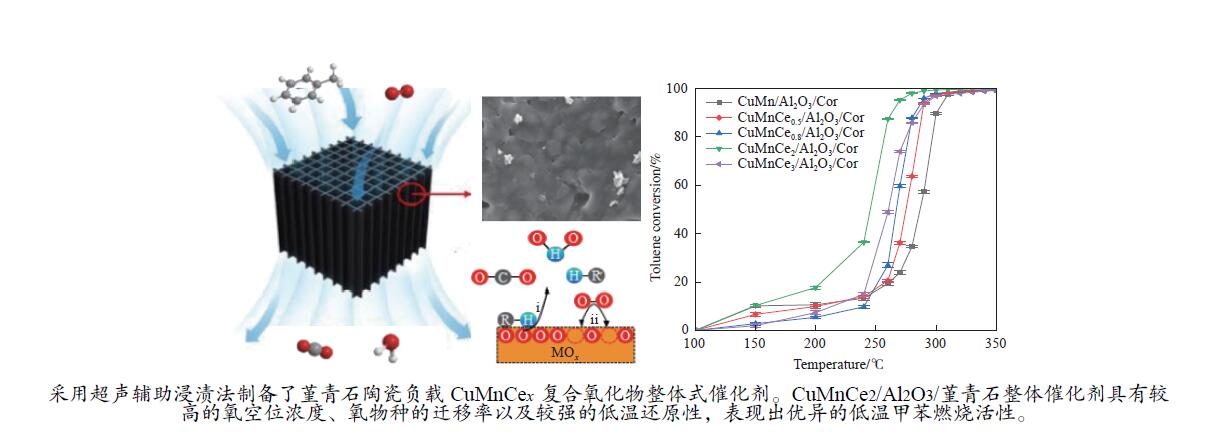
摘要: 本研究以堇青石为载体,采用超声浸渍法制备了一系列CuMnCex/Al2O3/堇青石整体式催化剂,同时,通过N2吸附-脱附、XRD、SEM、EDX、H2-TPR、O2-TPD、XPS和EPR等方法对样品的物理、化学性质进行了系统的表征分析。实验结果表明,CuMnCex/Al2O3/堇青石整体式催化剂中的Ce含量明显影响甲苯催化燃烧性能。其中,CuMnCe2/Al2O3/堇青石整体式催化剂对甲苯氧化具有最高活性,当甲苯浓度为1 g/L、空速为78000 mL/(g·h)、温度为263 °C时甲苯转化率达到90%,究其原因为CeO2在CuMnOx上分散均匀,不仅提高了氧空位的浓度和氧物种的迁移率,还增强了催化剂的低温还原性。同时,CuMnCe2/Al2O3/堇青石整体式催化剂在长期评价和循环测试中呈现良好的稳定性。
-
关键词:
- 甲苯 /
- 催化燃烧 /
- 整体式催化剂 /
- CuMnCex复合氧化物
Abstract: Catalytic combustion is an effective approach to remove volatile organic compounds, in which the development of highly active and durable catalyst is extremely crucial. Herein, a series of CuMnCex/Al2O3/cordierite monolithic catalysts were synthesized by using the ultrasonic-assisted impregnation method. The physicochemical properties were comprehensively characterized via the BET, XRD, SEM, EDX, H2-TPR, O2-TPD, XPS and EPR techniques. The results showed that the catalytic activity of CuMnCex/Al2O3/Cor for toluene combustion was strongly affected by the Ce content. The CuMnCe2/Al2O3/Cor monolithic catalyst showed the best catalytic activity with toluene conversion of 90% at 263 °C under toluene concentration of 1 g/L and space velocity of 78000 mL/(g·h). Meanwhile, the well-dispersed CeO2 in the CuMn matrix not only improved the content of oxygen vacancies and the mobility of oxygen species, but also enhanced the low-temperature reducibility of the catalyst. Moreover, the CuMnCe2/Al2O3/Cor monolithic catalyst exhibited an excellent stability in the long-term test and cycle ability test.-
Key words:
- toluene /
- catalytic combustion /
- monolithic catalyst /
- CuMnCex composite oxide
-
Table 1 Structural properties, loading percentages and weight loss of monolithic catalyst
Sample ${S_{ {\rm{BET} } }}^{\rm{a}}$
/(m2·g−1)${v_{{\rm{total}}}}^{\rm{b}} $
/(cm3·g−1)dpc
/nmLoadingd
w/%Weight losse w/% Cordierite 0.2 − − − − CuMn 11.5 0.0304 7.0 18.5 0.45 CuMnCe0.5 9.4 0.0233 6.2 19.6 0.42 CuMnCe0.8 12.4 0.0291 6.1 20.3 0.48 CuMnCe2 17.8 0.0413 6.6 26.0 0.51 CuMnCe3 10.8 0.0404 9.0 27.0 0.52 Note: a: Obtained by BET method; b: Estimated from the amount adsorbed at p/p0=0.99; c: Calculated using the BJH method; d: Active component load rate; e: Weight loss rate of active component. Table 2 Surface elemental analysis of CuMn and CuMnCex monolithic catalysts
Sample Mn4+/Mn Ce3+/Ce Cu+/Cu2+ Osur/Ototal CuMn 0.25 − 0.11 0.36 CuMnCe0.5 0.27 0.17 0.09 0.38 CuMnCe0.8 0.31 0.18 0.09 0.44 CuMnCe2 0.37 0.20 0.09 0.46 CuMnCe3 0.32 0.14 0.10 0.43 Table 3 Catalytic activity of several related catalysts for toluene combustion
Catalyst Toluene concentration
/(g·L−1)Catalyst amount
/gGHSV
/(mL·g–1·h–1)t50
/°Ct90
/°CRef. CuMn/Al2O3/Cor 1 0.1 78000 286 300 this work CuMnCe0.5/Al2O3/Cor 1 0.1 78000 274 287 this work CuMnCe0.8/Al2O3/Cor 1 0.1 78000 267 282 this work CuMnCe2/Al2O3/Cor 1 0.1 78000 244 263 this work CuMnCe3/Al2O3/Cor 1 0.1 78000 262 284 this work Cu-Co/Halloysite 0.6 0.2 60000 272 301 [58] CeO2/δ-MnO2 1 0.2 15000 237 277 [59] MnCe/ZrO2 1 0.1 36000 257 290 [60] MOx/HZSM-5 1 0.4 15000 276 285 [61] Cu-Mn-Ce/Al2O3 0.5 0.5 20000 315 340 [62] -
[1] MCDONALD B C, DE GOUW J A, GILMAN J B, et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions[J]. Science,2018,359:760−764. doi: 10.1126/science.aaq0524 [2] PENG Y, YANG Q, WANG L, et al. VOC emissions of coal-fired power plants in China based on life cycle assessment method[J]. Fuel,2021,292:120325. doi: 10.1016/j.fuel.2021.120325 [3] SEKIGUCHI K, YASUI F, FUJII E. Capturing of gaseous and particulate pollutants into liquid phase by a water/oil column using microbubbles[J]. Chemosphere,2020,256:126996. doi: 10.1016/j.chemosphere.2020.126996 [4] LI Z, JIN Y, CHEN T, et al. Trimethylchlorosilane modified activated carbon for the adsorption of VOCs at high humidity[J]. Sep Purif Technol,2021,272:118659. doi: 10.1016/j.seppur.2021.118659 [5] YI S, WAN Y. Volatile organic compounds (VOCs) recovery from aqueous solutions via pervaporation with vinyltriethoxysilane-grafted-silicalite-1/polydimethylsiloxane mixed matrix membrane[J]. Chem Eng J,2017,313:1639−1646. doi: 10.1016/j.cej.2016.11.061 [6] YAO X, ZHANG J, LIANG X, et al. Plasma-catalytic removal of toluene over the supported manganese oxides in DBD reactor: Effect of the structure of zeolites support[J]. Chemosphere,2018,208:922−930. doi: 10.1016/j.chemosphere.2018.06.064 [7] LI Y, GUO Y, XUE B. Catalytic combustion of methane over M (Ni, Co, Cu) supported on ceria-magnesia[J]. Fuel Process Technol,2009,90:652−656. doi: 10.1016/j.fuproc.2008.12.002 [8] HUANG H, XU Y, FENG Q, et al. Low temperature catalytic oxidation of volatile organic compounds: A review[J]. Catal Sci Technol,2015,5:2649−2669. doi: 10.1039/C4CY01733A [9] KAMAL M S, RAZZAK S A, HOSSAIN M M. Catalytic oxidation of volatile organic compounds (VOCs) – A review[J]. Atmos Environ,2016,140:117−134. doi: 10.1016/j.atmosenv.2016.05.031 [10] WANG Z, YANG H, LIU R, et al. Probing toluene catalytic removal mechanism over supported Pt nano- and single-atom-catalyst[J]. J Hazard Mater,2020,392:122258. doi: 10.1016/j.jhazmat.2020.122258 [11] ZHANG Z, ZHENG J, SHANGGUAN W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review[J]. Catal Today,2016,264:270−278. doi: 10.1016/j.cattod.2015.10.040 [12] KIM H S, KIM H J, KIM J H, et al. Noble-metal-based catalytic oxidation technology trends for volatile organic compound (VOC) removal[J]. Catalysts,2022,12:63. doi: 10.3390/catal12010063 [13] WANG S, GU J, SHAN R, et al. Catalytic toluene steam reforming using Ni supported catalyst from pyrolytic peat[J]. Fuel Process Technol,2021,224:107032. doi: 10.1016/j.fuproc.2021.107032 [14] LI K, LI T, DAI Y, et al. Highly active urchin-like MCo2O4 (M = Co, Cu, Ni or Zn) spinel for toluene catalytic combustion[J]. Fuel,2022,318:123648. doi: 10.1016/j.fuel.2022.123648 [15] GUO Y, WEN M, SONG S, et al. Enhanced catalytic elimination of typical VOCs over ZnCoOx catalyst derived from in situ pyrolysis of ZnCo bimetallic zeolitic imidazolate frameworks[J]. Appl Catal B: Environ,2022,308:121212. doi: 10.1016/j.apcatb.2022.121212 [16] PIUMETTI M, FINO D, RUSSO N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs[J]. Appl Catal B: Environ,2015,163:277−287. doi: 10.1016/j.apcatb.2014.08.012 [17] ZHOU L, ZHANG B, LI Z, et al. Amorphous-microcrystal combined manganese oxides for efficiently catalytic combustion of VOCs[J]. Mol Catal,2020,489:110920. doi: 10.1016/j.mcat.2020.110920 [18] CAI T, LIU Z, YUAN J, et al. The structural evolution of MnOx with calcination temperature and their catalytic performance for propane total oxidation[J]. Appl Surf Sci,2021,565:150596. doi: 10.1016/j.apsusc.2021.150596 [19] CHEN G, CAI Y, ZHANG H, et al. Pt and Mo Co-Decorated MnO2 nanorods with superior resistance to H2O, sintering, and HCl for catalytic oxidation of chlorobenzene[J]. Environ Sci Technol,2021,55:14204−14214. doi: 10.1021/acs.est.1c05086 [20] CHEN J, CHEN X, XU W, et al. Hydrolysis driving redox reaction to synthesize Mn-Fe binary oxides as highly active catalysts for the removal of toluene[J]. Chem Eng J,2017,330:281−293. doi: 10.1016/j.cej.2017.07.147 [21] HU W, HUANG J, XU J, et al. Insights into the superior performance of mesoporous MOFs-derived Cu-Mn oxides for toluene total catalytic oxidation[J]. Fuel Process Technol,2022,236:107424. doi: 10.1016/j.fuproc.2022.107424 [22] AGUILERA D A, PEREZ A, MOLINA R, et al. Cu-Mn and Co-Mn catalysts synthesized from hydrotalcites and their use in the oxidation of VOCs[J]. Appl Catal B: Environ,2011,104:144−150. doi: 10.1016/j.apcatb.2011.02.019 [23] WANG L, SUN Y, ZHU Y, et al. Revealing the mechanism of high water resistant and excellent active of CuMn oxide catalyst derived from Bimetal-Organic framework for acetone catalytic oxidation[J]. J Colloid Interface Sci,2022,622:577−590. doi: 10.1016/j.jcis.2022.04.155 [24] WANG Y, YANG D, LI S, et al. Layered copper manganese oxide for the efficient catalytic CO and VOCs oxidation[J]. Chem Eng J,2019,357:258−268. doi: 10.1016/j.cej.2018.09.156 [25] LU J, ASAHINA S, TAKAMI S, et al. Interconnected 3D framework of CeO2 with high oxygen storage capacity: High-Resolution scanning electron microscopic observation[J]. ACS Appl Nano Mater,2020,3:2346−2353. doi: 10.1021/acsanm.9b02446 [26] XIAO Y, LI H, XIE K. Activating lattice oxygen at the twisted surface in a mesoporous CeO2 single crystal for efficient and durable catalytic CO oxidation[J]. Angew Chem Int Ed,2021,60:5240−5244. doi: 10.1002/anie.202013633 [27] WANG C, ZHANG C, HUA W, et al. Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts. Chem Eng J, 2017, 315: 392-402. [28] WANG Y, XUE R, ZHAO C, et al. Effects of Ce in the catalytic combustion of toluene on CuxCe1−xFe2O4[J]. Colloid Surface A,2018,540:90−97. doi: 10.1016/j.colsurfa.2017.12.067 [29] MIAO C, LIU J, ZHAO J, et al. Catalytic combustion of toluene over CeO2-CoOx composite aerogels[J]. New J Chem,2020,44:11557−11565. doi: 10.1039/D0NJ00091D [30] GÓMEZ D M, GATICA J M, HERNÁNDEZ-GARRIDO J C, et al. A novel CoOx/La-modified-CeO2 formulation for powdered and washcoated onto cordierite honeycomb catalysts with application in VOCs oxidation[J]. Appl Catal B: Environ,2014,144:425−434. doi: 10.1016/j.apcatb.2013.07.045 [31] MA M, YANG R, JIANG Z, et al. Fabricating M/Al2O3/cordierite (M = Cr, Mn, Fe, Co, Ni and Cu) monolithic catalysts for ethyl acetate efficient oxidation: Unveiling the role of water vapor and reaction mechanism[J]. Fuel,2021,303:121244. doi: 10.1016/j.fuel.2021.121244 [32] HEIBEL A K, SORENSEN C M. Monolithic catalysts for the chemical industry[J]. Ind Eng Chem Res,2004,43:4602−4611. doi: 10.1021/ie030730q [33] ZHAO H, WANG H, QU Z. Synergistic effects in Mn-Co mixed oxide supported on cordierite honeycomb for catalytic deep oxidation of VOCs[J]. J Environ Sci,2022,112:231−243. doi: 10.1016/j.jes.2021.05.003 [34] XIONG J, LUO Z, YANG J, et al. Robust and well-controlled TiO2-Al2O3 binary nanoarray-integrated ceramic honeycomb for efficient propane combustion[J]. CrystEngComm,2019,21:2727−2735. doi: 10.1039/C8CE02012D [35] ZHOU H, GE M, WU S, et al. Iron based monolithic catalysts supported on Al2O3, SiO2, and TiO2: A comparison for NO reduction with propane[J]. Fuel,2018,220:330−338. doi: 10.1016/j.fuel.2018.01.077 [36] TIAN F, LI K, SU Y. Catalytic performance and characterization of Ce-Modified Fe catalysts supported on Al2O3 for SCR-C3H8[J]. Catal Surv Asia,2020,24:239−249. doi: 10.1007/s10563-020-09306-4 [37] LU H, ZHOU Y, HUANG H, et al. In-situ synthesis of monolithic Cu-Mn-Ce/cordierite catalysts towards VOCs combustion[J]. J Rare Earth,2011,29(9):855−860. doi: 10.1016/S1002-0721(10)60555-8 [38] BEHAR S, GONZALEZ P, AGULHON P, et al. New synthesis of nanosized Cu-Mn spinels as efficient oxidation catalysts[J]. Catal Today,2012,189:35−41. doi: 10.1016/j.cattod.2012.04.004 [39] LIU P, WEI G, LIANG X, et al. Synergetic effect of Cu and Mn oxides supported on palygorskite for the catalytic oxidation of formaldehyde: Dispersion, microstructure, and catalytic performance[J]. Appl Clay Sci,2018,161:265−273. doi: 10.1016/j.clay.2018.04.032 [40] XIAO R, QIN R, ZHANG C, et al. Catalytic decomposition of ethyl acetate over La-modified Cu-Mn oxide supported on honeycomb ceramic[J]. J Rare Earth,2021,39:817−825. doi: 10.1016/j.jre.2020.10.015 [41] SUMRUNRONNASAK S, CHANLEK N, PIMPHA N. Improved CeCuOx catalysts for toluene oxidation prepared by aqueous cationic surfactant precipitation method[J]. Mater Chem Phys,2018,216:143−152. [42] DÍAZ C C, YESTE M P, VIDAL H, et al. In situ generation of Mn1-xCex system on cordierite monolithic supports for combustion of n-hexane. Effects on activity and stability[J]. Fuel,2020,262:116564. doi: 10.1016/j.fuel.2019.116564 [43] FENG J, HOU Z Y, ZHOU X Y, et al. Low-temperature catalytic oxidation of toluene over Mn-Co-O/Ce0.65Zr0.35O2 mixed oxide catalysts[J]. Chem Pap,2018,72:161−173. doi: 10.1007/s11696-017-0267-8 [44] DENG L, HUANG C, KAN J, et al. Effect of coating modification of cordierite carrier on catalytic performance of supported NiMnO3 catalysts for VOCs combustion[J]. J Rare Earth,2018,36:265−272. doi: 10.1016/j.jre.2017.07.015 [45] LIU G, YUE R, JIA Y, et al. Catalytic oxidation of benzene over Ce-Mn oxides synthesized by flame spray pyrolysis[J]. Particuology,2013,11:454−459. doi: 10.1016/j.partic.2012.09.013 [46] LUO Y, ZHENG Y, ZUO J, et al. Insights into the high performance of Mn-Co oxides derived from metalorganic frameworks for total toluene oxidation[J]. J Hazard Mater,2018,349:119−127. doi: 10.1016/j.jhazmat.2018.01.053 [47] WANG T, SUN Y, ZHOU Y, et al. Identifying influential parameters of octahedrally coordinated cations in spinel ZnMnxCo2−xO4 oxides for the oxidation reaction[J]. ACS Catal,2018,8:8568−8577. doi: 10.1021/acscatal.8b02376 [48] LI S, MO S, WANG D, et al. Synergistic effect for promoted benzene oxidation over monolithic CoMnAlO catalysts derived from in situ supported LDH film[J]. Catal Today,2019,332:132−138. doi: 10.1016/j.cattod.2018.08.014 [49] CUO Z, WANG D, GONG Y, et al. A novel porous ceramic membrane supported monolithic Cu-doped Mn-Ce catalysts for benzene combustion[J]. Catalysts,2019,9:652. doi: 10.3390/catal9080652 [50] LI W, LIU H, MA X, et al. Fabrication of silica supported Mn-Ce benzene oxidation catalyst by a simple and environment-friendly oxalate approach[J]. J Porous Mater,2018,25:107−117. doi: 10.1007/s10934-017-0424-z [51] ZUO S, YANG P, WANG X. Efficient and environmentally friendly synthesis of AlFe-PILC-Supported MnCe catalysts for benzene combustion[J]. ACS Omega,2017,2:5179−5186. doi: 10.1021/acsomega.7b00592 [52] CUO Z, DENG Y, LI W, et al. Monolithic Mn/Ce-based catalyst of fibrous ceramic membrane for complete oxidation of benzene[J]. Appl Surf Sci,2018,456:594−601. doi: 10.1016/j.apsusc.2018.06.207 [53] CHEN J, CHEN X, YAN D, et al. A facile strategy of enhancing interaction between cerium and manganese oxides for catalytic removal of gaseous organic contaminants[J]. Appl Catal B: Environ,2019,250:396−407. doi: 10.1016/j.apcatb.2019.03.042 [54] ZANG M, ZHAO C, WANG Y, et al. Ceramic-monolith-supported La0.8Ce0.2MnO3 catalysts for toluene oxidation[J]. Mater Lett,2019,253:196−200. doi: 10.1016/j.matlet.2019.05.135 [55] ZANG M, ZHAO C, WANG Y, et al. Low temperature catalytic combustion of toluene over three-dimensionally ordered La0.8Ce0.2MnO3/cordierite catalysts[J]. Appl Surf Sci,2019,483:355−362. doi: 10.1016/j.apsusc.2019.03.320 [56] WANG S, LI T, CHENG X, et al. Regulating the concentration of dissolved oxygen to achieve the directional transformation of reactive oxygen species: A controllable oxidation process for ciprofloxacin degradation by calcined CuCoFe-LHD[J]. Water Res,2023,233:119744. doi: 10.1016/j.watres.2023.119744 [57] WANG S, ZHU J, LI T, et al. Oxygen vacancy-mediated CuCoFe/tartrate-LHD catalyst directly activates oxygen to produce superoxide radicals: Transformation of active species and implication for nitrobenzene degradation[J]. Environ Sci Technol,2022,56:7924−47934. doi: 10.1021/acs.est.2c00522 [58] CARRILLO A M, CARRIAZO J G. Cu and Co oxides supported on halloysite for the total oxidation of toluene[J]. Appl Catal B: Environ,2015,164:443−452. doi: 10.1016/j.apcatb.2014.09.027 [59] LI L, JING F, YAN J, et al. Highly effective self-propagating synthesis of CeO2-doped MnO2 catalysts for toluene catalytic combustion[J]. Catal Today,2017,297:167−172. doi: 10.1016/j.cattod.2017.04.053 [60] LI L, SONG L, FEI Z, et al. Effect of different supports on activity of Mn-Ce binary oxides catalysts for toluene combustion[J]. J Rare Earth,2020,40:645−651. [61] HUANG H, ZHANG C, WANG L, et al. Promotional effect of HZSM-5 on the catalytic oxidation of toluene over MnOx/HZSM-5 catalysts[J]. Catal Sci Technol,2016,6:4260−4270. doi: 10.1039/C5CY02011E [62] HUANG H, LING W, JIN L, et al. Support effect on catalytic activity of VOCs combustion over supported Cu-Mn-Ce catalysts[J]. J Rare Earths,2012,30(3):295−300. [63] LIU X, ZHOU K, WANG L, et al. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods[J]. J Am Chem Soc,2009,131:3140−3141. doi: 10.1021/ja808433d -





 下载:
下载: