Synthesis of glycerol carbonate from glycerol and CO2 over Cu-Zr complex oxide
-
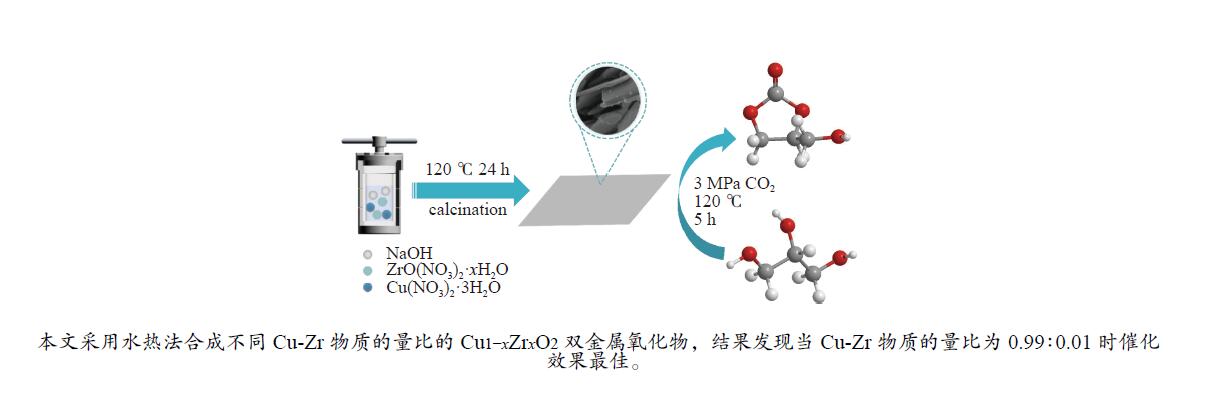
摘要: 采用水热法合成了一系列不同Cu-Zr物质的量比的Cu1−xZrxO2双金属氧化物,以此为催化剂,将生物柴油生产过程副产物甘油与温室气体CO2耦合反应制备精细化工产品碳酸甘油酯。结果表明,Zr掺杂量不同,催化剂对甘油羰基化反应效果呈现明显差距,最佳反应条件下,Cu0.99Zr0.01O2催化剂具有最佳催化性能,甘油转化率和碳酸甘油酯选择性分别达到64.1%和85.9%。并且发现与纯CuO和纯ZrO2相比,Cu1−xZrxO2复合氧化物在甘油与CO2耦合反应体系中表现出更强的催化活性,结合X射线粉末衍射(XRD)、扫描电子显微镜(SEM)、透射电子显微镜(TEM)、X射线光电子能谱(XPS)、N2吸附-脱附、程序升温还原(H2-TPR)、程序升温脱附(TPD)、傅里叶变换红外光谱(FT-IR)等表征手段,推测高活性与Zr在CuO表面的分散程度、催化剂表面氧物种含量及酸碱性位点数量有关。此外,为了研究催化剂的稳定性,以Cu0.99Zr0.01O2催化剂作为基准进行了循环性能测试,结果表明,经过六次循环后,甘油的转化率和碳酸甘油酯的选择性未发生明显变化,说明该催化剂稳定性良好。Abstract: A series of Cu1−xZrxO2 bimetallic oxides with different Cu-Zr molar ratios for glycerol carbonate synthesis from glycerol and CO2 were prepared by hydrothermal method. The results found that the performance was significantly affected by the Zr doping amounts. Under the optimal reaction conditions, the Cu0.99Zr0.01O2 catalyst had the best catalytic performance. The conversion of glycerol and the selectivity of glycerol carbonate reached 64.1% and 85.9%, respectively. Cu1−xZrxO2 complex oxide exhibited better activity than pure CuO and pure ZrO2. The structures, morphologies and surface properties of the catalysts were characterized by X-ray powder diffraction (XRD), Scanning electron microscopy (SEM), Transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), N2 adsorption and desorption, Temperature programmed reduction (H2-TPR), Temperature programmed desorption (TPD) and Fourier Transform Infrared Spectroscopy (FT-IR). It is speculated that the high activity is related to the degree of dispersion of Zr on the surface of CuO, the surface content of oxygen species and the number of acidic-basic sites. In addition, catalytic activity did not change significantly after six cycles, indicating the excellent stability of the catalyst.
-
Key words:
- glycerol /
- glycerol carbonate /
- carbon dioxide /
- complex oxide /
- 2-cyanopyridine
-
表 1 Cu1−xZrxO2样品表面氧种类含量
Table 1 Surface oxygen species contents of Cu1−xZrxO2 samples
Sample Surface oxygen species content/% OⅠ OⅡ OⅢ OⅠ/(OⅡ+OⅢ) CuO 53.64 33.03 13.33 1.16 Cu0.99Zr0.01O2 57.78 29.85 12.36 1.37 Cu0.95Zr0.05O2 54.9 30.14 14.90 1.22 Cu0.75Zr0.25O2 47.01 43.06 9.93 0.89 Cu0.60Zr0.40O2 34.78 38.91 26.31 0.53 Cu0.40Zr0.60O2 64.60 26.92 8.48 1.82 ZrO2 74.74 15.05 10.22 2.96 表 2 Cu1−xZrxO2催化剂的织构性质
Table 2 Texture properties of the Cu1−xZrxO2 catalyst
Sample SBET/(m2·g−1) Pore volume/
(cm3·g−1)Average pore size/nm CuO 8.2 0.027 12.9 Cu0.99Zr0.01O2 14.3 0.044 11.2 Cu0.95Zr0.05O2 18.3 0.067 12.1 Cu0.75Zr0.25O2 55.3 0.152 9.0 Cu0.60Zr0.40O2 59.5 0.128 7.5 Cu0.40Zr0.60O2 106.6 0.194 5.8 ZrO2 118.5 0.249 6.5 表 3 不同Zr含量Cu1−xZrxO2催化剂催化活性
Table 3 Evaluation of catalytic activity of the Cu1−xZrxO2 catalyst
Sample Conversion of glycerol/% Selectivity of GC/% Yield of GC/% Blank 34.3 11.7 4.0 CuO 67.9 73.9 50.2 Cu0.99Zr0.01O2 64.1 85.9 55.1 Cu0.95Zr0.05O2 61.1 85.2 52.1 Cu0.75Zr0.25O2 59.7 79.3 47.3 Cu0.60Zr0.40O2 65.6 74.4 48.8 Cu0.40Zr0.60O2 51.3 91.0 46.7 ZrO2 34.0 11.8 4.0 Reaction conditions: glycerol 0.92 g, 2-cyanopyridine 3.26 g, DMF 10 mL, 120 ℃, 5 h, p(CO2) = 3 MPa. -
[1] HÖÖK M, TANGANG X. Depletion of fossil fuels and anthropogenic climate change—A review[J]. Energy policy,2013,52:797−809. doi: 10.1016/j.enpol.2012.10.046 [2] WANG Y, NIU C, WANG D. Metallic nanocatalysts for electrochemical CO2 reduction in aqueous solutions[J]. J Colloid Interface Sci,2018,527:95−106. doi: 10.1016/j.jcis.2018.05.041 [3] HUANG C H, TAN C S. A review: CO2 utilization[J]. Aerosol Air Qual Res,2014,14(2):480−499. doi: 10.4209/aaqr.2013.10.0326 [4] KNUTSON T R, TULEYA R E. Impact of CO2-induced warming on simulated hurricane intensity and precipitation: Sensitivity to the choice of climate model and convective parameterization[J]. J Clim,2004,17(18):3477−3495. doi: 10.1175/1520-0442(2004)017<3477:IOCWOS>2.0.CO;2 [5] SONG L, JIANG Y X, ZHANG Z, et al. CO2=CO+: Recent advances in carbonylation of C-H bonds with CO2[J]. ChemComm,2020,56(60):8355−8367. [6] MATTIA D, JONES M D, O'BYRNE J P, et al. Towards carbon-neutral CO2 conversion to hydrocarbons[J]. ChemSusChem,2015,8(23):4064−4072. doi: 10.1002/cssc.201500739 [7] RAHPEYMA S S, RAHEB J. Microalgae biodiesel as a valuable alternative to fossil fuels[J]. Bioenergy Res,2019,12(4):958−965. doi: 10.1007/s12155-019-10033-6 [8] SINGH D, SHARMA D, SONI S L, et al. A review on feedstocks, production processes, and yield for different generations of biodiesel[J]. Fuel,2020,262:116553. doi: 10.1016/j.fuel.2019.116553 [9] MONTERIRO M R, KUGELMEIER C L, PINHEIRO R S, et al. Glycerol from biodiesel production: Technological paths for sustainability[J]. Renewable Sustainable Energy Rev,2018,88:109−122. doi: 10.1016/j.rser.2018.02.019 [10] SUN D, YAMADA Y, SATO S, et al. Glycerol hydrogenolysis into useful C3 chemicals[J]. Appl Catal B: Environ,2016,193:75−92. doi: 10.1016/j.apcatb.2016.04.013 [11] WU F, JIANG H, ZHU X, et al. Effect of tungsten species on selective hydrogenolysis of glycerol to 1, 3-propanediol[J]. ChemSusChem,2021,14(2):569−581. doi: 10.1002/cssc.202002405 [12] TORRES S, PALACIO R, LÓPEZ D. Support effect in Co3O4-based catalysts for selective partial oxidation of glycerol to lactic acid[J]. Appl Catal A: Gen,2021,621:118199. doi: 10.1016/j.apcata.2021.118199 [13] YAN H, SHEN Q, SUN Y, et al. Tailoring facets of α-Mn2O3 microcrystalline catalysts for enhanced selective oxidation of glycerol to glycolic acid[J]. ACS Catal,2021,11(11):6371−6383. doi: 10.1021/acscatal.1c01566 [14] YAN H, YAO S, ZHAO S, et al. Insight into the basic strength-dependent catalytic performance in aqueous phase oxidation of glycerol to glyceric acid[J]. Chem Eng Sci,2021,230:116191. doi: 10.1016/j.ces.2020.116191 [15] TAMOŠIĖŪNAS A, GIMŽAUSKAITĖ D, USCILA R, et al. Thermal arc plasma gasification of waste glycerol to syngas[J]. Appl Energy,2019,251:113306. doi: 10.1016/j.apenergy.2019.113306 [16] HU J, LI J, GU Y, et al. Oxidative carbonylation of glycerol to glycerol carbonate catalyzed by PdCl2 (phen)/KI[J]. Appl Catal A: Gen,2010,386(1/2):188−193. doi: 10.1016/j.apcata.2010.07.059 [17] CHRISTY S, NOSCHESE A, LOMELI-RODRIGUEZ M, et al. Recent progress in the synthesis and applications of glycerol carbonate[J]. Curr Opin Green Sustainable Chem,2018,14:99−107. doi: 10.1016/j.cogsc.2018.09.003 [18] ZHANG P, ZHU M, FAN M, et al. Rare earth-doped calcium-based magnetic catalysts for transesterification of glycerol to glycerol carbonate[J]. J Chin Chem Soc,2019,66(2):164−170. doi: 10.1002/jccs.201800164 [19] LI Y, LIU J, HE D. Catalytic synthesis of glycerol carbonate from biomass-based glycerol and dimethyl carbonate over Li-La2O3 catalysts[J]. Appl Catal A: Gen,2018,564:234−242. doi: 10.1016/j.apcata.2018.07.032 [20] SHUKLA K, SRIVASTAVA V C. Synthesis of organic carbonates from alcoholysis of urea: A review[J]. Catal Rev,2017,59(1):1−43. doi: 10.1080/01614940.2016.1263088 [21] WU Y, SONG X, CAI F, et al. Synthesis of glycerol carbonate from glycerol and diethyl carbonate over Ce-NiO catalyst: The role of multiphase Ni[J]. J Alloys Compd,2017,720:360−368. doi: 10.1016/j.jallcom.2017.05.292 [22] ARESTA M, DIBENEDETTO A, NOCITO F, et al. A study on the carboxylation of glycerol to glycerol carbonate with carbon dioxide: the role of the catalyst, solvent and reaction conditions[J]. J Mol Catal A: Chem,2006,257(1/2):149−153. doi: 10.1016/j.molcata.2006.05.021 [23] PARK C, NGUYEN-PHU H, SHIN E W. Glycerol carbonation with CO2 and La2O2CO3/ZnO catalysts prepared by two different methods: Preferred reaction route depending on crystalline structure[J]. Mol Catal,2017,435:99−109. doi: 10.1016/j.mcat.2017.03.025 [24] OCHOA-GÓMEZ J R, GÓMEZ-JIMÉNEZ-ABERASTURI O, RAMIREZ-LOPEZ C, et al. A brief review on industrial alternatives for the manufacturing of glycerol carbonate, a green chemical[J]. Org Process Res Dev,2012,16(3):389−399. doi: 10.1021/op200369v [25] SU X, LIN W, CHENG H, et al. Metal-free catalytic conversion of CO2 and glycerol to glycerol carbonate[J]. Green Chem,2017,19(7):1775−1781. doi: 10.1039/C7GC00260B [26] LI J, WAMG T. Chemical equilibrium of glycerol carbonate synthesis from glycerol[J]. J Chem Thermodyn,2011,43(5):731−736. doi: 10.1016/j.jct.2010.12.013 [27] VIEVILLE C, YOO J W, PELET S, et al. Synthesis of glycerol carbonate by direct carbonatation of glycerol in supercritical CO2 in the presence of zeolites and ion exchange resins[J]. Catal Lett,1998,56(4):245−247. doi: 10.1023/A:1019050205502 [28] DIBENEDETTO A, ANGELINI A, ARESTA M, et al. Converting wastes into added value products: from glycerol to glycerol carbonate, glycidol and epichlorohydrin using environmentally friendly synthetic routes[J]. Tetrahedron,2011,67(6):1308−1313. doi: 10.1016/j.tet.2010.11.070 [29] GEORGE J, PATEL Y, PILLAI S M, et al. Methanol assisted selective formation of 1, 2-glycerol carbonate from glycerol and carbon dioxide using nBu2SnO as a catalyst[J]. J Mol Catal A: Chem,2009,304(1/2):1−7. doi: 10.1016/j.molcata.2009.01.010 [30] LI H, JIAO X, LI L, et al. Synthesis of glycerol carbonate by direct carbonylation of glycerol with CO2 over solid catalysts derived from Zn/Al/La and Zn/Al/La/M (M=Li, Mg and Zr) hydrotalcites[J]. Catal Sci Technol,2015,5(2):989−1005. doi: 10.1039/C4CY01237B [31] LIU Z, LI B, QIAO F, et al. Catalytic performance of Li/Mg composites for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate[J]. ACS Omega,2022,7(6):5032−5038. doi: 10.1021/acsomega.1c05968 [32] DOSUNA-RODRÍGUEZ I, GAIGNEAUX E M. Glycerol acetylation catalysed by ion exchange resins[J]. Catal Today,2012,195(1):14−21. doi: 10.1016/j.cattod.2012.04.031 [33] LIU J, LI Y, ZHANG J, et al. Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters[J]. Appl Catal A: Gen,2016,513:9−18. doi: 10.1016/j.apcata.2015.12.030 [34] MA J, SONG J, LIU H, et al. One-pot conversion of CO2 and glycerol to value-added products using propylene oxide as the coupling agent[J]. Green Chem,2012,14(6):1743−1748. doi: 10.1039/c2gc35150a [35] AKHAVAN O, AZIMIRAD R, SAFA S, et al. CuO/Cu(OH)2 hierarchical nanostructures as bactericidal photocatalysts[J]. J Mater Chem A,2011,21(26):9634−9640. doi: 10.1039/c0jm04364h [36] BENJARAM M R, ATAULLAH K. Recent advances on TiO2-ZrO2 mixed oxides as catalysts and catalyst supports[J]. Catal Rev,2005,47:257−296. doi: 10.1081/CR-200057488 [37] LIU G, LIU J, LI W, et al. Aerobic oxidation of alcohols over Ru-Mn-Ce and Ru-Co-Ce catalysts: The effect of calcination temperature[J]. Appl Catal A: Gen,2017,535:77−84. doi: 10.1016/j.apcata.2017.02.006 [38] GANDHE A R, REBELLO J S, FIGUEIREDO J L, et al. Manganese oxide OMS-2 as an effective catalyst for total oxidation of ethyl acetate[J]. Appl Catal B: Environ,2007,72(1/2):129−135. doi: 10.1016/j.apcatb.2006.10.017 [39] KONDAWAR S E, POTDAR A S, RODE C V. Solvent-free carbonylation of glycerol with urea using metal loaded MCM-41 catalysts[J]. RSC Adv,2015,5(21):16452−16460. doi: 10.1039/C4RA11590B [40] ZHAO M, YAN H, LU R, et al. Insight into the selective oxidation mechanism of glycerol to 1, 3-dihydroxyacetone over AuCu-ZnO interface[J]. AIChE,2022,68(11):e17833. doi: 10.1002/aic.17833 [41] LI D, WANG Z, HUANG J, et al. Ultrafine CeO2 nanodots embedded in porous ZrO2 for efficient and sustainable chlorine recycle through hydrochloric acid catalytic oxidation[J]. ChemistrySelect,2020,5(40):12442−12449. doi: 10.1002/slct.202003184 [42] LIU B, LI C, ZHANG G, et al. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-doped CeO2 nanorods[J]. ACS Catal,2018,8(11):10446−10456. doi: 10.1021/acscatal.8b00415 [43] CLIMENT M J, CORMA A, DE FRUTOS P, et al. Chemicals from biomass: Synthesis of glycerol carbonate by transesterification and carbonylation with urea with hydrotalcite catalysts. The role of acid-base pairs[J]. J Catal,2010,269(1):140−149. doi: 10.1016/j.jcat.2009.11.001 [44] LIU P, DERCHI M, HENSEN E J M. Promotional effect of transition metal doping on the basicity and activity of calcined hydrotalcite catalysts for glycerol carbonate synthesis[J]. Appl Catal B: Environ,2014,144:135−143. doi: 10.1016/j.apcatb.2013.07.010 [45] ZHAO H, FANG K, ZHOU J, et al. Direct synthesis of methyl formate from syngas on Cu-Mn mixed oxide catalyst[J]. Int J Hydrog Energy,2016,41(21):8819−8828. doi: 10.1016/j.ijhydene.2016.03.149 [46] GAO Z, ZHOU Z, WANG M, et al. Highly dispersed Pd anchored on heteropolyacid modified ZrO2 for high efficient hydrodeoxygenation of lignin-derivatives[J]. Fuel,2023,334:126768. doi: 10.1016/j.fuel.2022.126768 [47] MEGHA, MONDAL K, GHANTY T K, et al. Adsorption and activation of CO2 on small-sized Cu-Zr bimetallic clusters[J]. J Phys Chem A,2021,125(12):2558−2572. doi: 10.1021/acs.jpca.1c00751 [48] HONDA M, TAMURA M, NAKAO K, et al. Direct cyclic carbonate synthesis from CO2 and diol over carboxylation/hydration cascade catalyst of CeO2 with 2-cyanopyridine[J]. ACS Catal,2014,4(6):1893−1896. doi: 10.1021/cs500301d [49] MA J, LIU J, ZHANG Z, et al. Mechanisms of ethylene glycol carbonylation with carbon dioxide[J]. Comput Theor Chem,2012,992:103−109. doi: 10.1016/j.comptc.2012.05.010 [50] DI COSIMO J I, DIEZ V K, XU M, et al. Structure and surface and catalytic properties of Mg-Al basic oxides[J]. J Catal,1998,178(2):499−510. doi: 10.1006/jcat.1998.2161 [51] ZHANG J, HE D. Surface properties of Cu/La2O3 and its catalytic performance in the synthesis of glycerol carbonate and monoacetin from glycerol and carbon dioxide[J]. J Colloid Interface Sci,2014,419:31−38. doi: 10.1016/j.jcis.2013.12.049 -





 下载:
下载: