Recent contributions of photoionization mass spectrometry in the study of typical solid fuel pyrolysis
-
摘要: 通过热解将固体燃料在热化学上转化为运输燃料和增值化学品是实现固体燃料高效利用的最为切实可行和兼顾经济性的途径之一。由于热解反应产物的复杂性,对固体燃料热解的中间产物和最终产物在分子水平上进行全面阐明对于理解热解反应机理至关重要,同时对提高热解过程的可持续性具有积极意义。光电离质谱(PIMS)技术被普遍认为是一种高度通用的过程分析技术,通过对热解气相产物中的离子进行实时检测和分析,为热解提供了实时的信息。为此,本工作综述了近年来PIMS技术在固体燃料(包括煤、生物质和固体推进剂)热解领域的应用,并对不同实验和模型的进展进行了概述。这些进展相互促进,有助于学者加深对热解固体燃料复杂过程的理解,并为未来深入研究热解机理提供有力支持。Abstract: Pyrolysis, an economically viable method, thermochemically converts solid fuel into transportation fuels and value-added chemicals, such as clean gas, liquid fuels, and chemicals, alongside undesirable by-products. Photoionization mass spectrometry (PIMS) is a versatile technique for real-time process analysis, offering ‘soft’ ionization for complex analytes, detecting and analyzing ions during in-situ pyrolysis. This review focuses on recent applications of PIMS during pyrolysis of solid fuels (i.e. coal, biomass and energetic materials). It summarizes studies on mass spectrometric analysis combined with different reactors and highlights the benefits othrough online PIMS as a diagnostic tool for in situ analysis. It provides an overview of interplay between experimental advancements and models and discusses future perspectives, potential applications in support of mechanistic studies.
-
Key words:
- pyrolysis /
- photoionization mass spectrometry /
- coal /
- biomass /
- solid propellants /
- on-line analysis
-
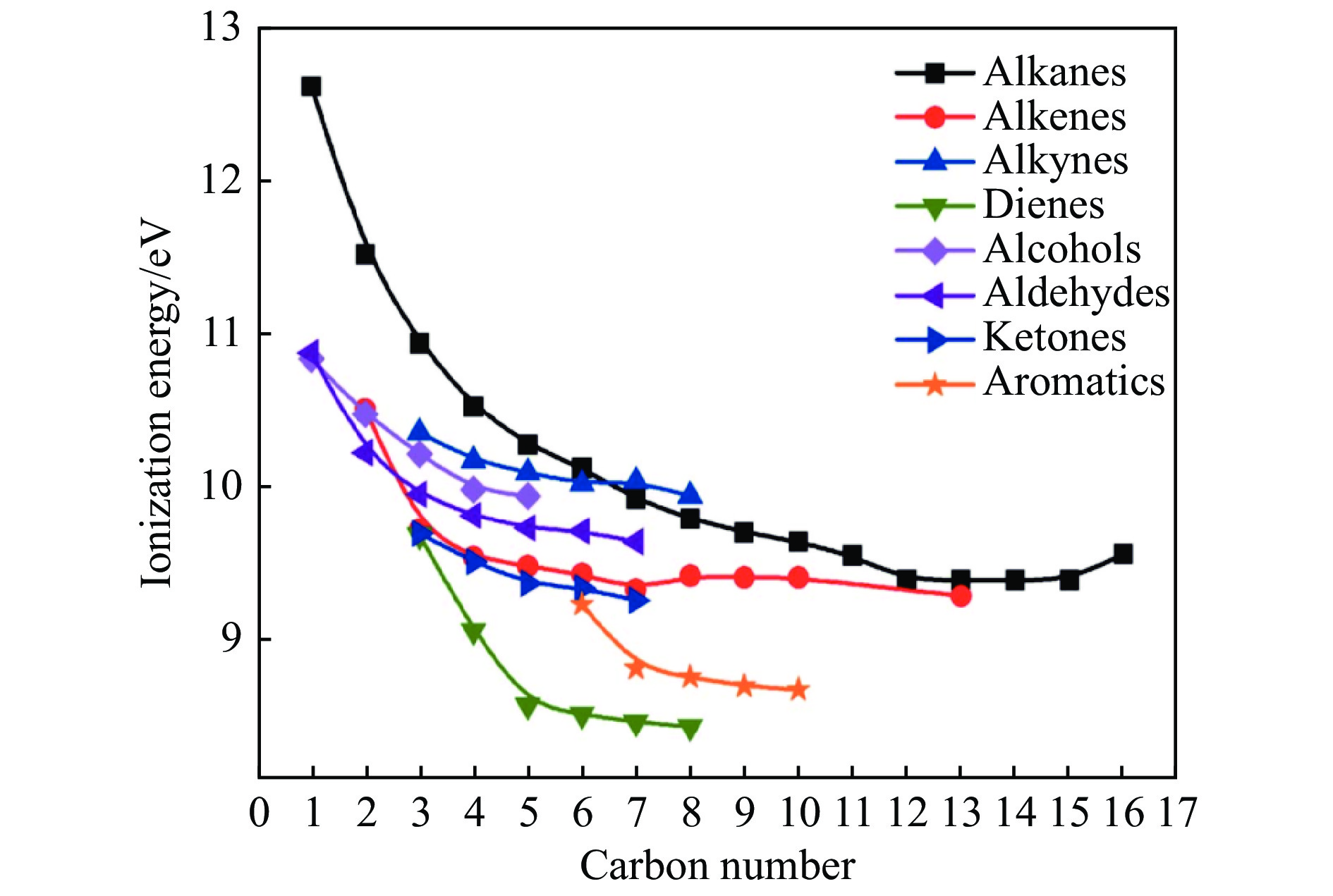
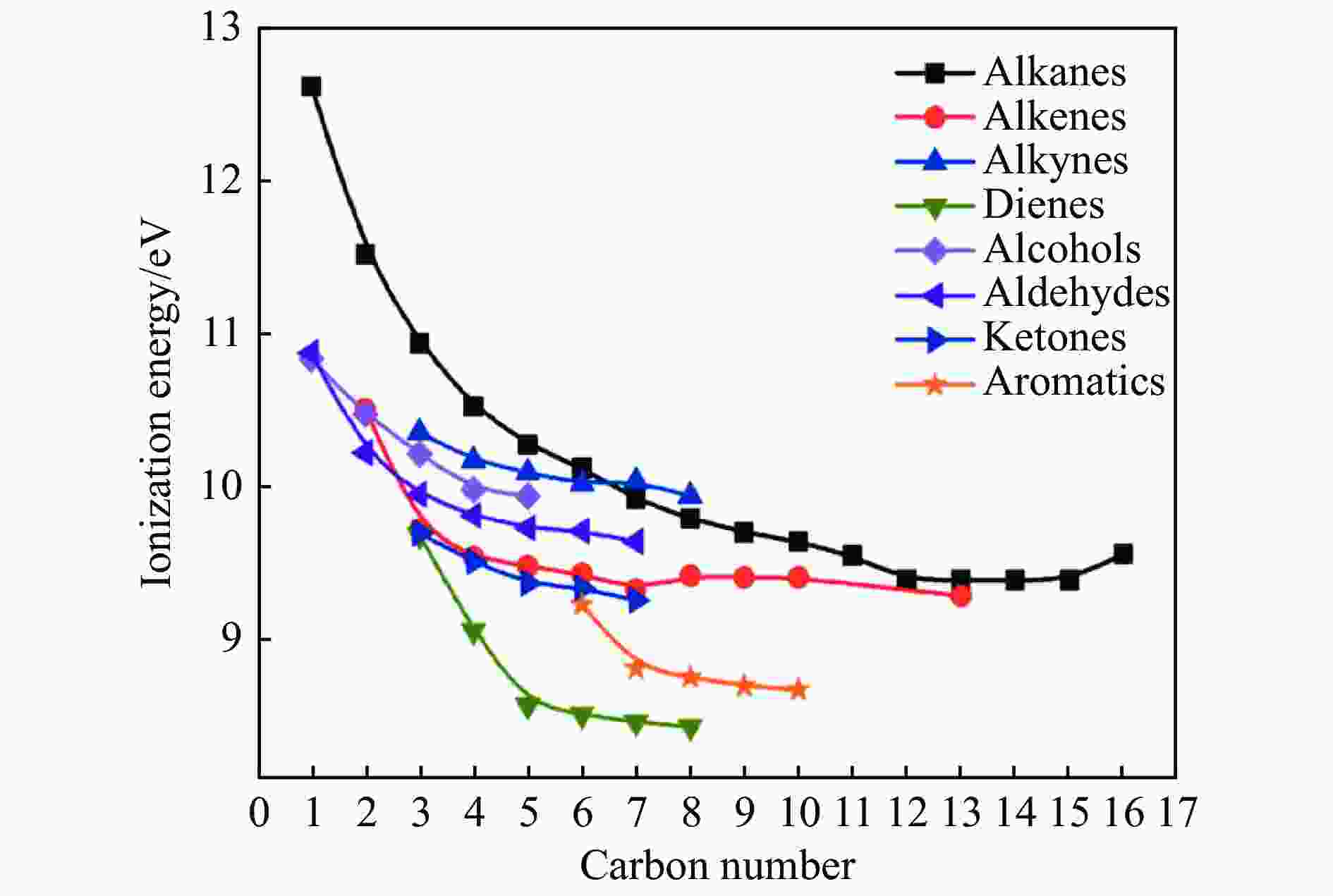
Figure 1 Energy of ionization for certain organic compounds[8] (with permission from International Union of Crystallography)
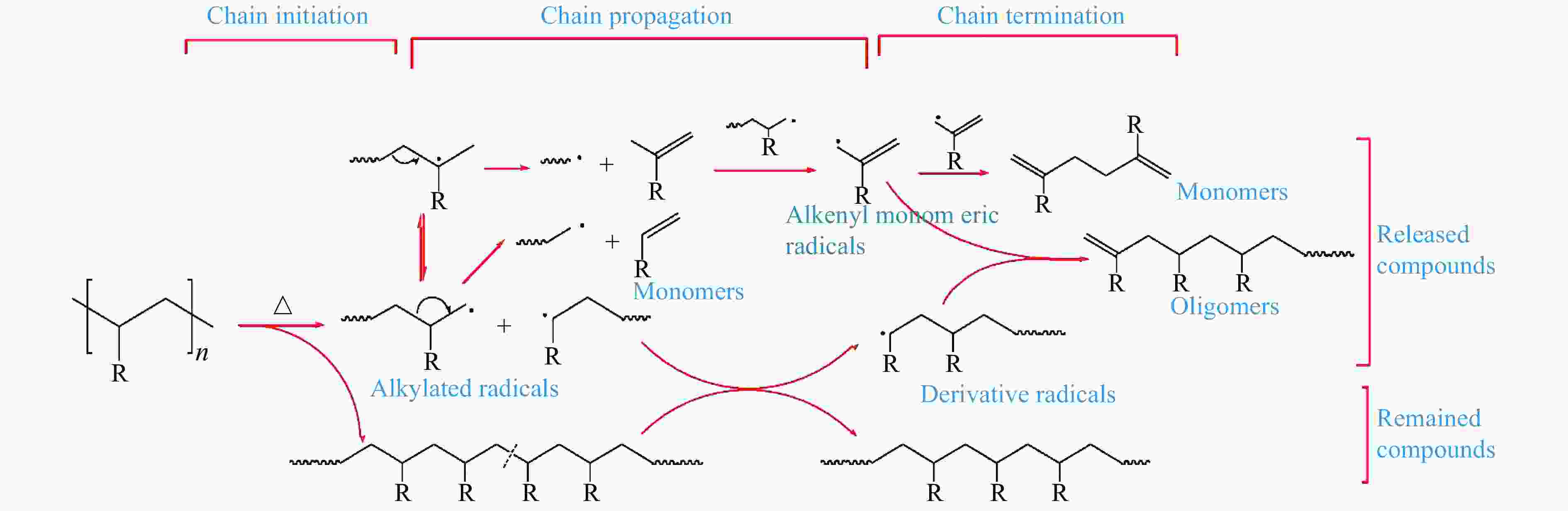
Figure 2 Proposed mechanism for the pyrolysis of model compounds featuring C−C bonds[38] (with permission from Elsevier)
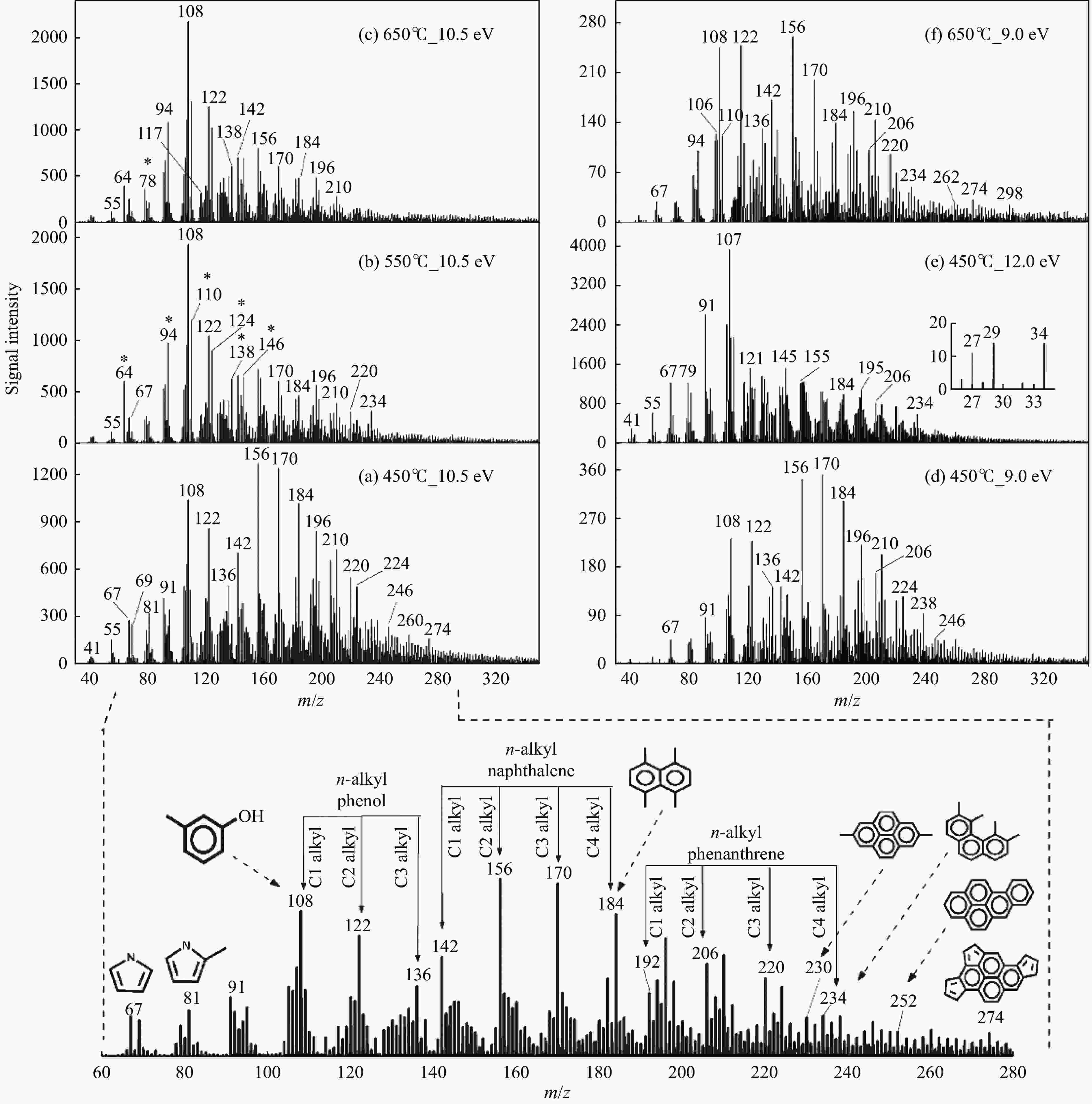
Figure 3 Mass spectra profiles of volatile products from Huainan coal pyrolysis[44] (with permission from Amer Chemical Soc)
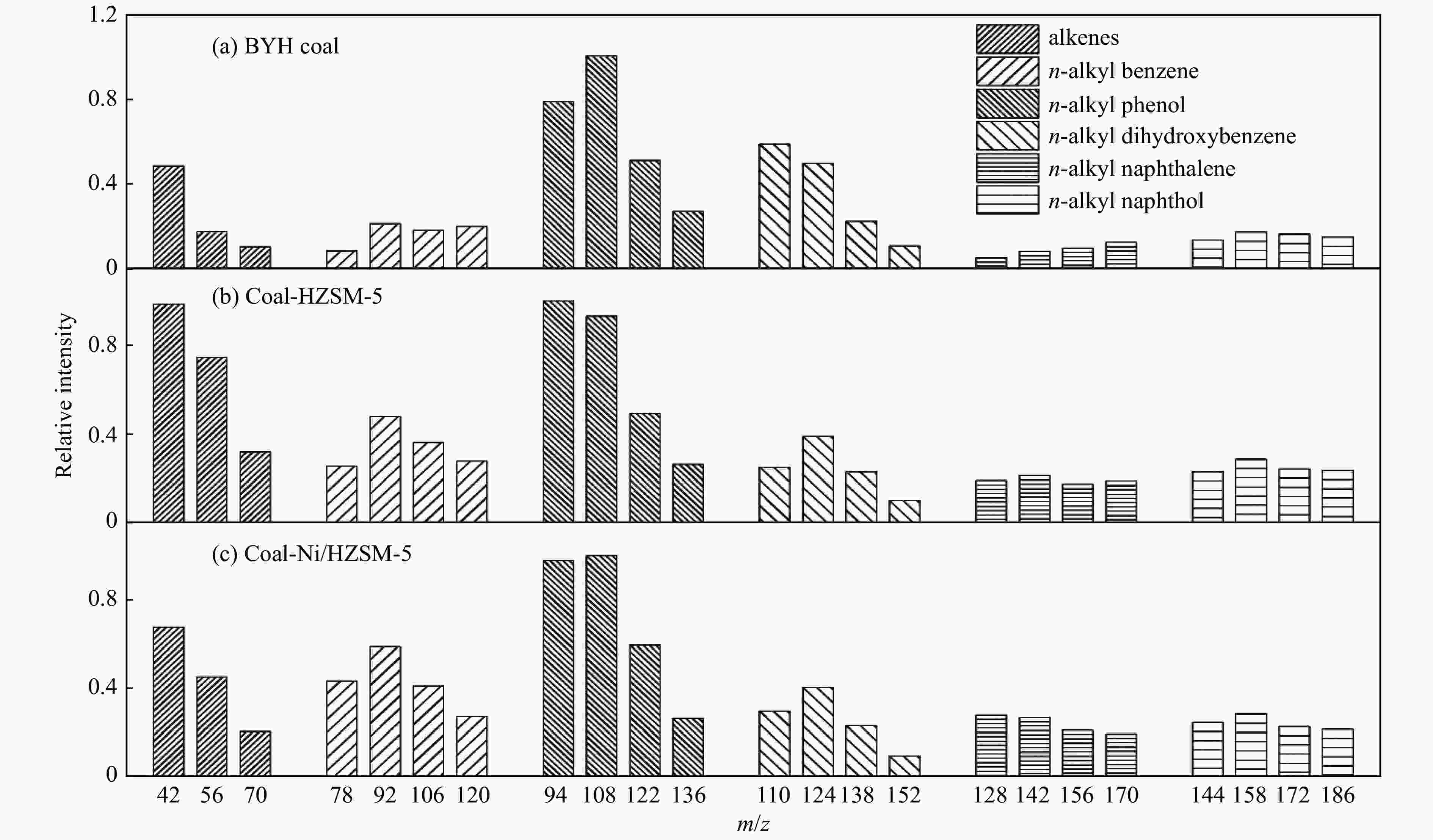
Figure 4 The influence of catalysts on the relative content of typical coal pyrolysis products[56] (with permission from Elsevier)
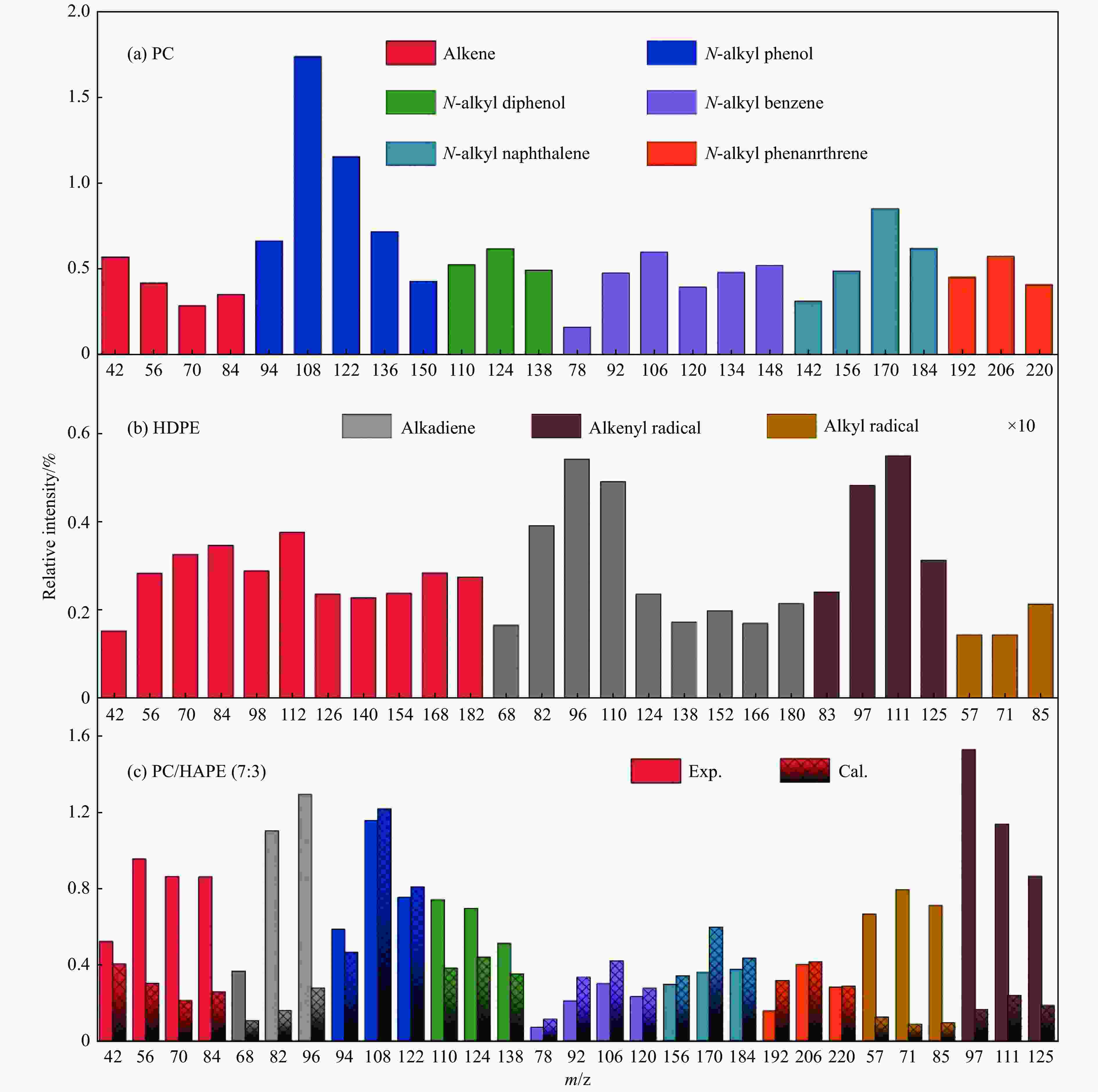
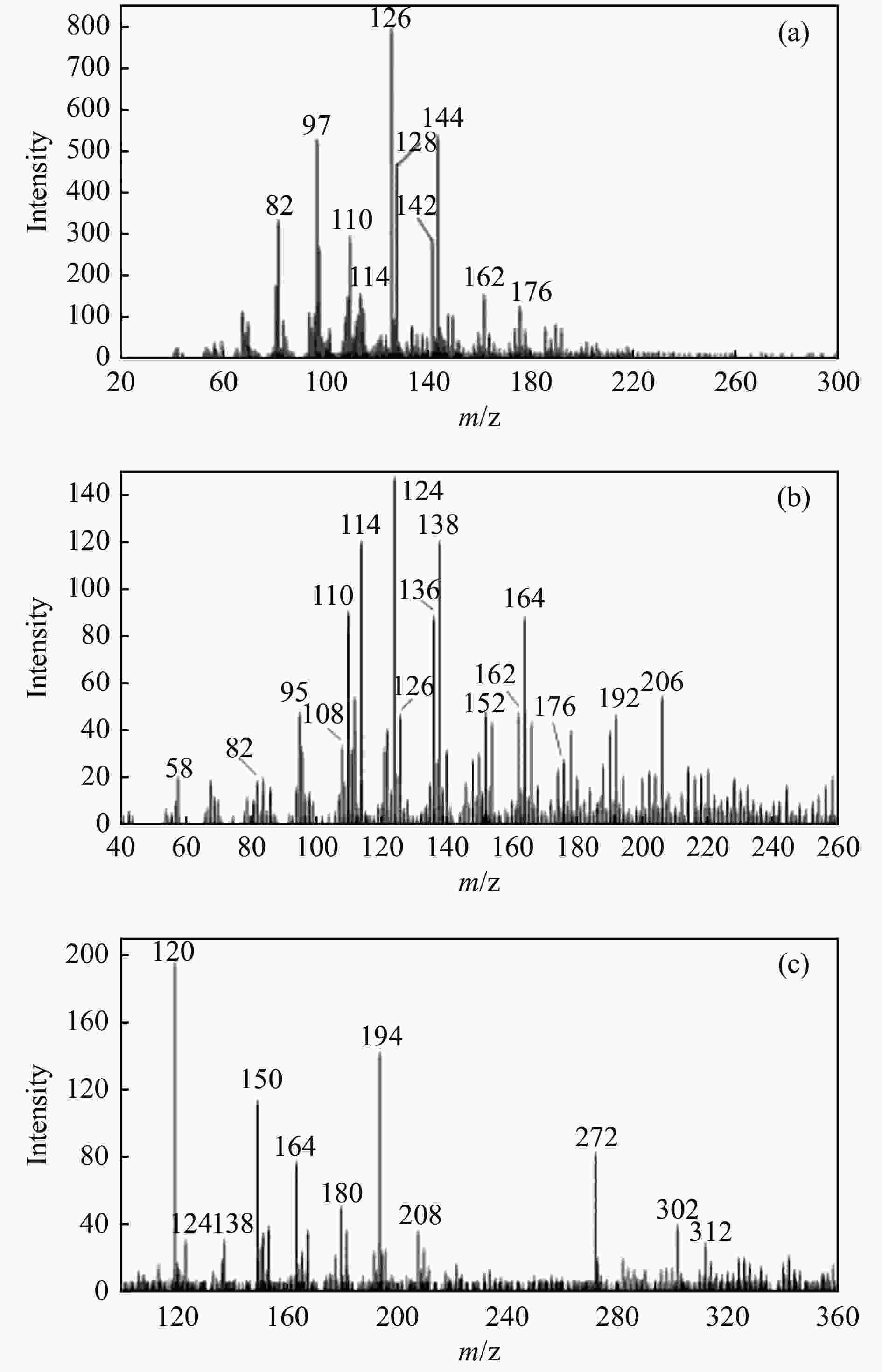
Figure 5 Relative content of primary volatiles from PC (a), HDPE (b) and their mixture PC/HDPE (7:3) pyrolysis (c)[61] (with permission from Elsevier)
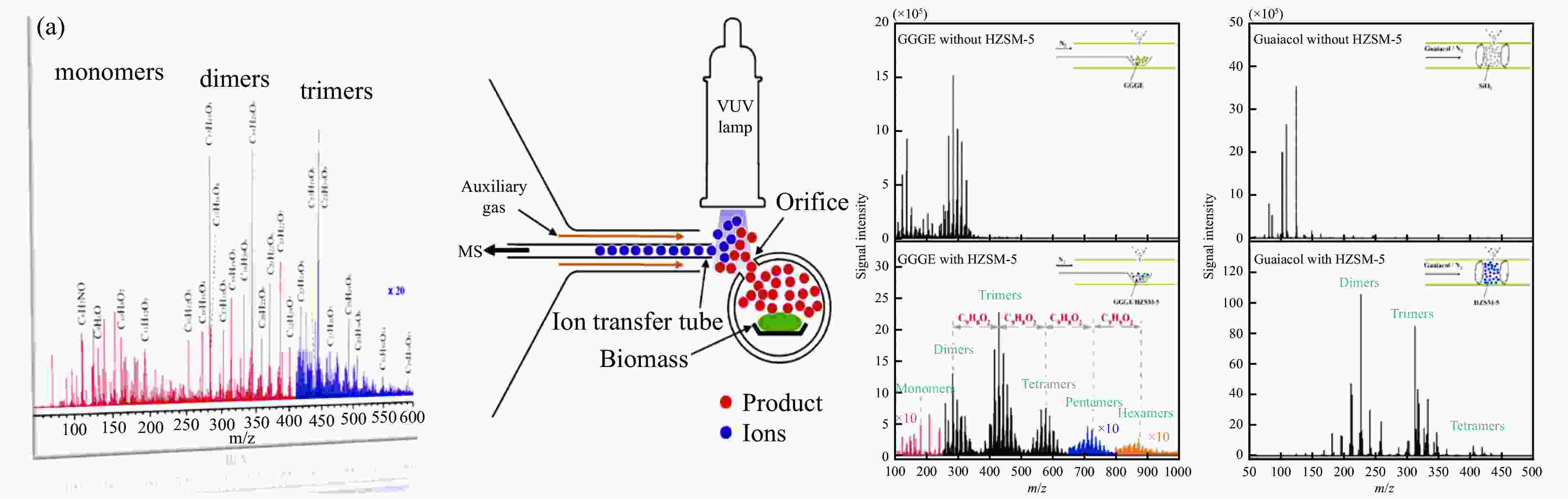
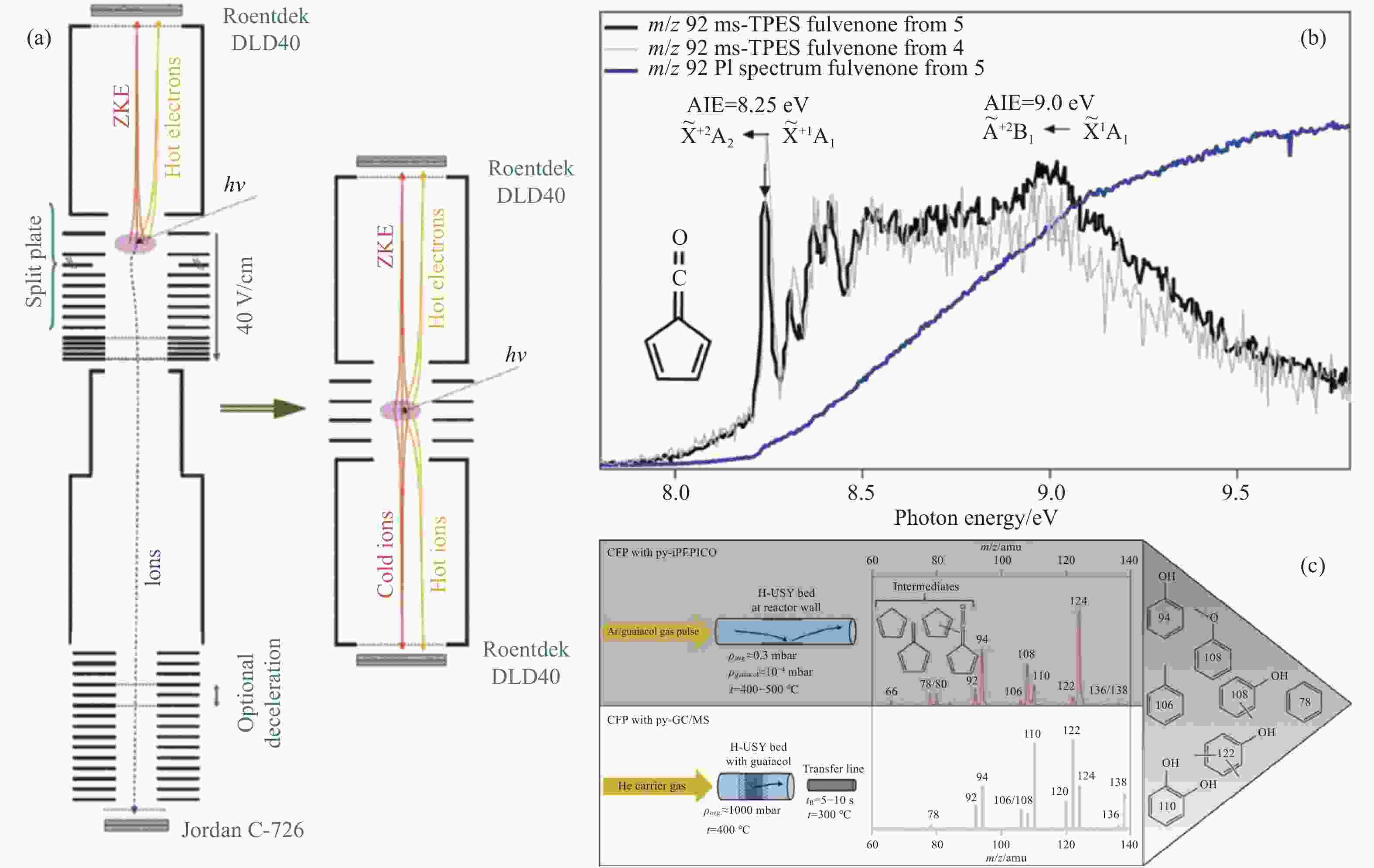
Figure 7 (a) Schematic representation of iPEPICO setup[72] (b) PI spectrum of fulvenone ketene from catalytic pyrolysis of 2-methoxy acetophenone[73] (c) Fast pyrolysis of guaiacol over H-USY and results from the mass spectrum of iPEPICO at 10.5 eV photon energy[69] (with permission from AIP Publishing, Wiley-V C H Verlag Gmbh and Nature Portfolio)
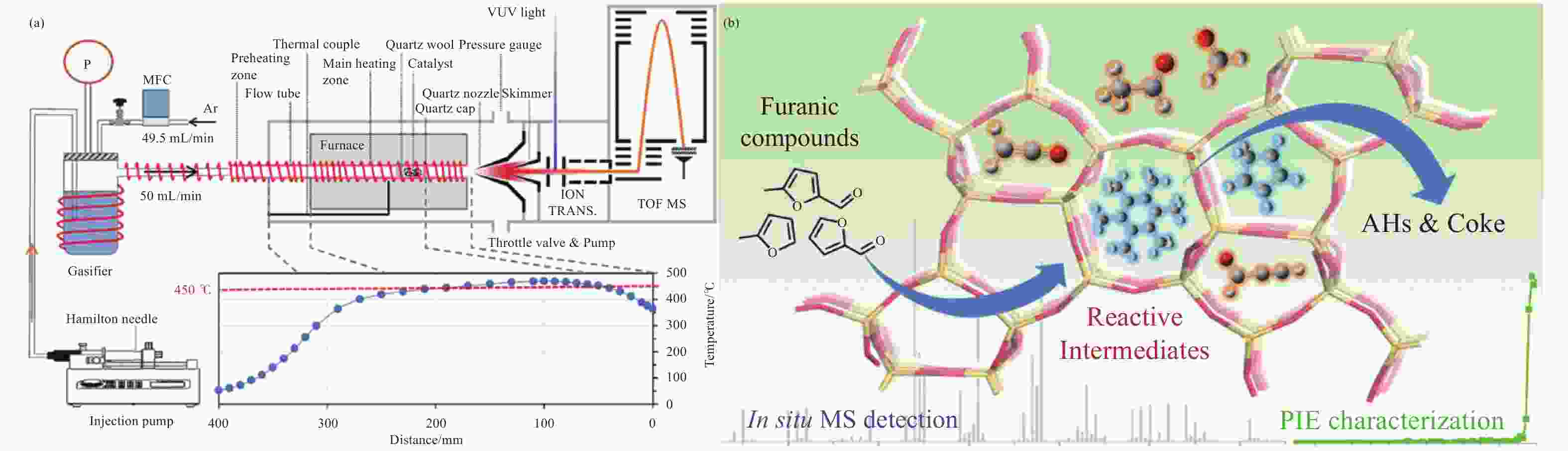
Figure 8 Pyrolysis of furanic compounds over zeolite catalyst with mass spectrometric analysis (a) Schematic diagram of experimental set-up (b) Proposed pathways[77] (with permission from Amer Chemical Soc)
Figure 9 The pyrolysis of major constituents at 350 °C(a): Microcrystalline cellulose; (b): Birch hemicellulose; (c): Miscanthus lignin (photon energy 9.5 eV)[78]. (with permission from Royal Soc Chemistry)
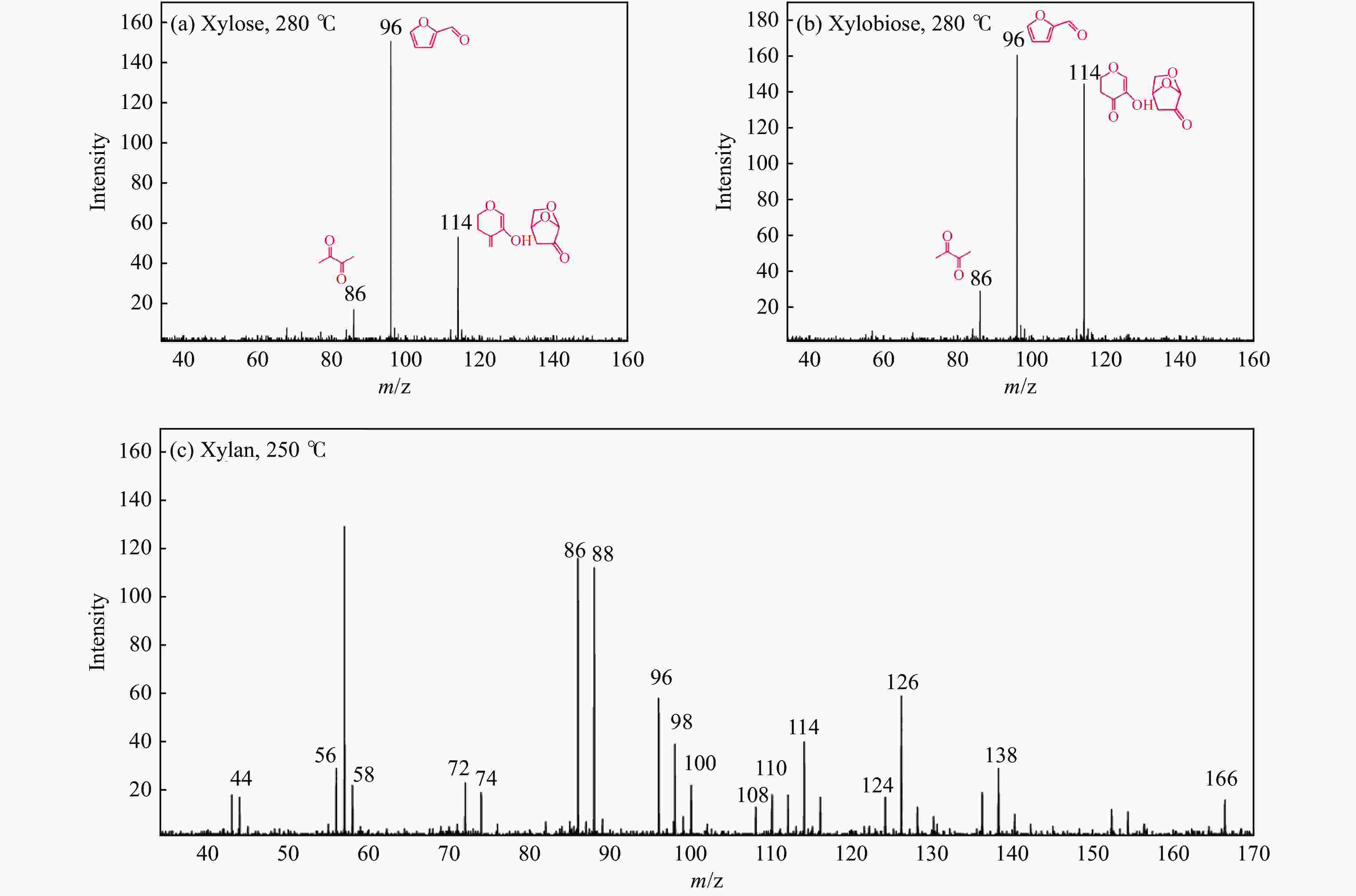
Figure 10 Typical pyrolysis products of hemicellulose model compounds (a) xylose at 280 °C; (b) xylobiose at 280 °C; (c) xylan at 250 °C[82] (with permission from Elsevier)
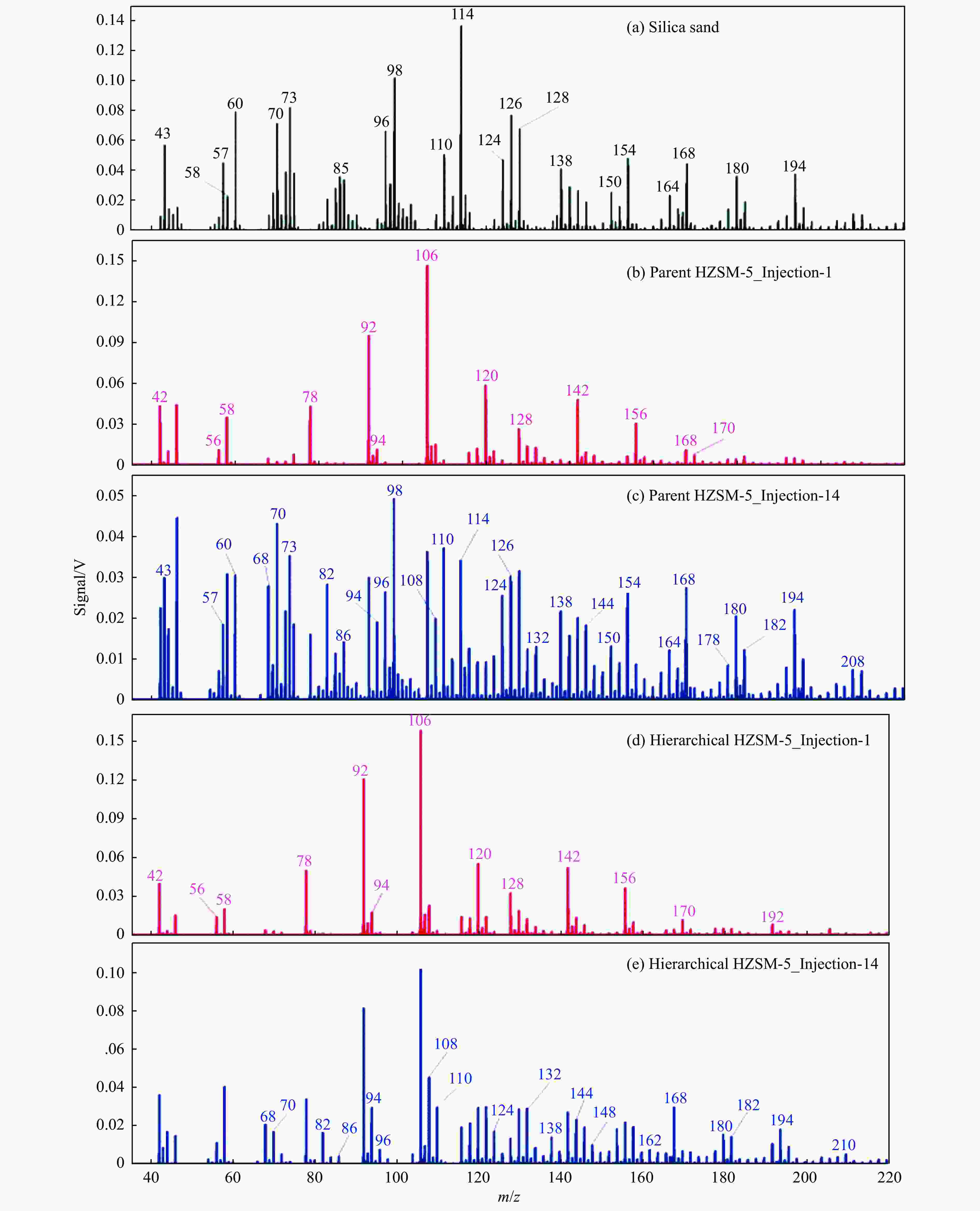
Figure 11 Comparison of zeolite catalysts with different structures for catalytic pyrolysis of Oka in a fluidized bed at 500 °C (a) blank test, (b) the first injected of the fresh HZSM-5,(c) the 14th injected of the coked HZSM-5, (d) the first injected of the fresh hierarchical HZSM-5, (e) the 14th injected of the coked hierarchical HZSM-5[94](with permission from Royal Soc Chemistry)
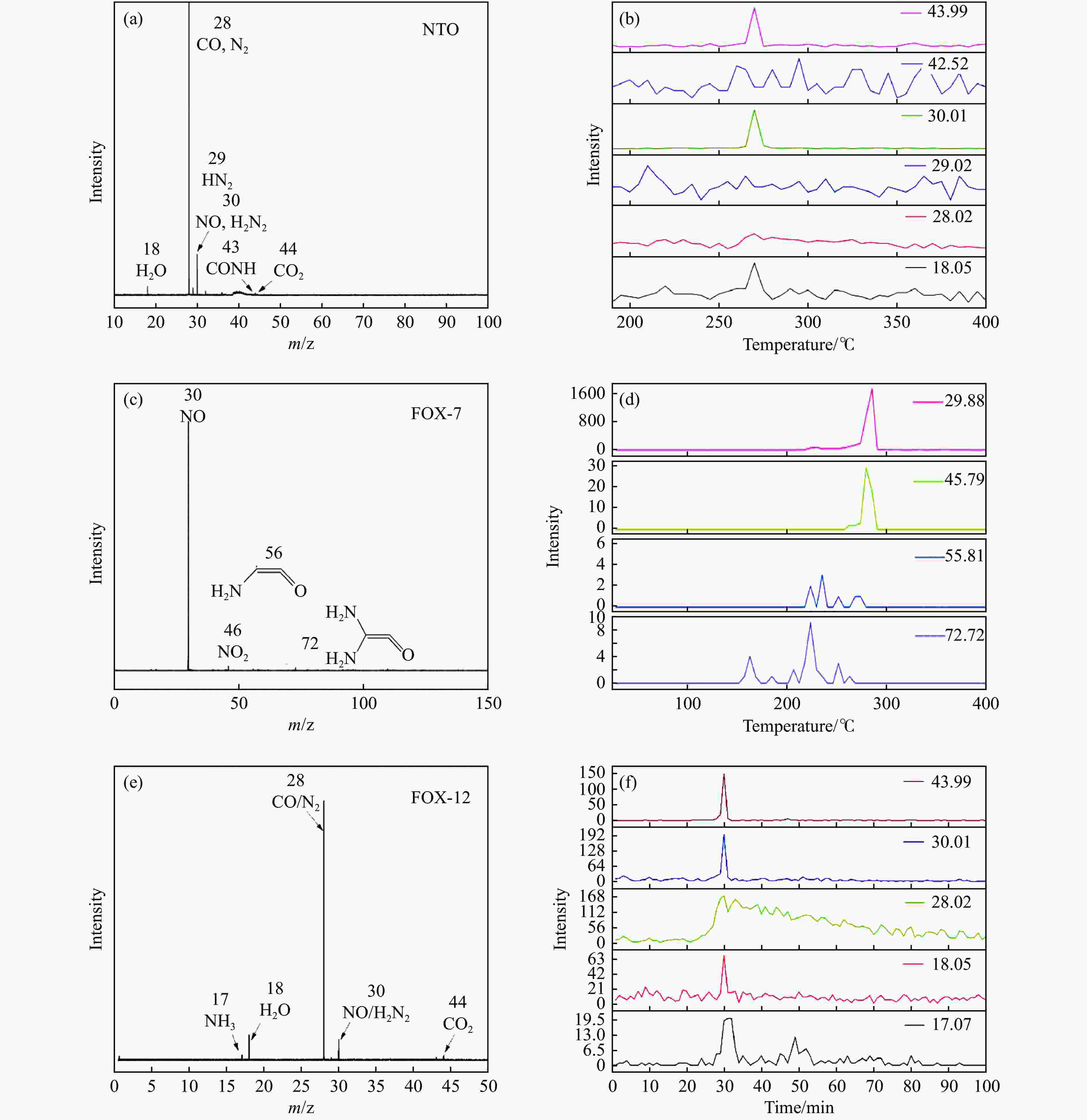
Figure 12 The results obtained by online PIMS in pyrolysis experiments: (a) the total intensity of different products of NTO; (b) Products evolution of NTO with temperature; (c) the total intensity of different products of FOX-7; (d) Products evolution of FOX-7 with temperature; (e) the total intensity of different products of FOX-12; (f) Products evolution of FOX-12 with temperature[108−109] (with permission from Royal Soc Chemistry and Elsevier)
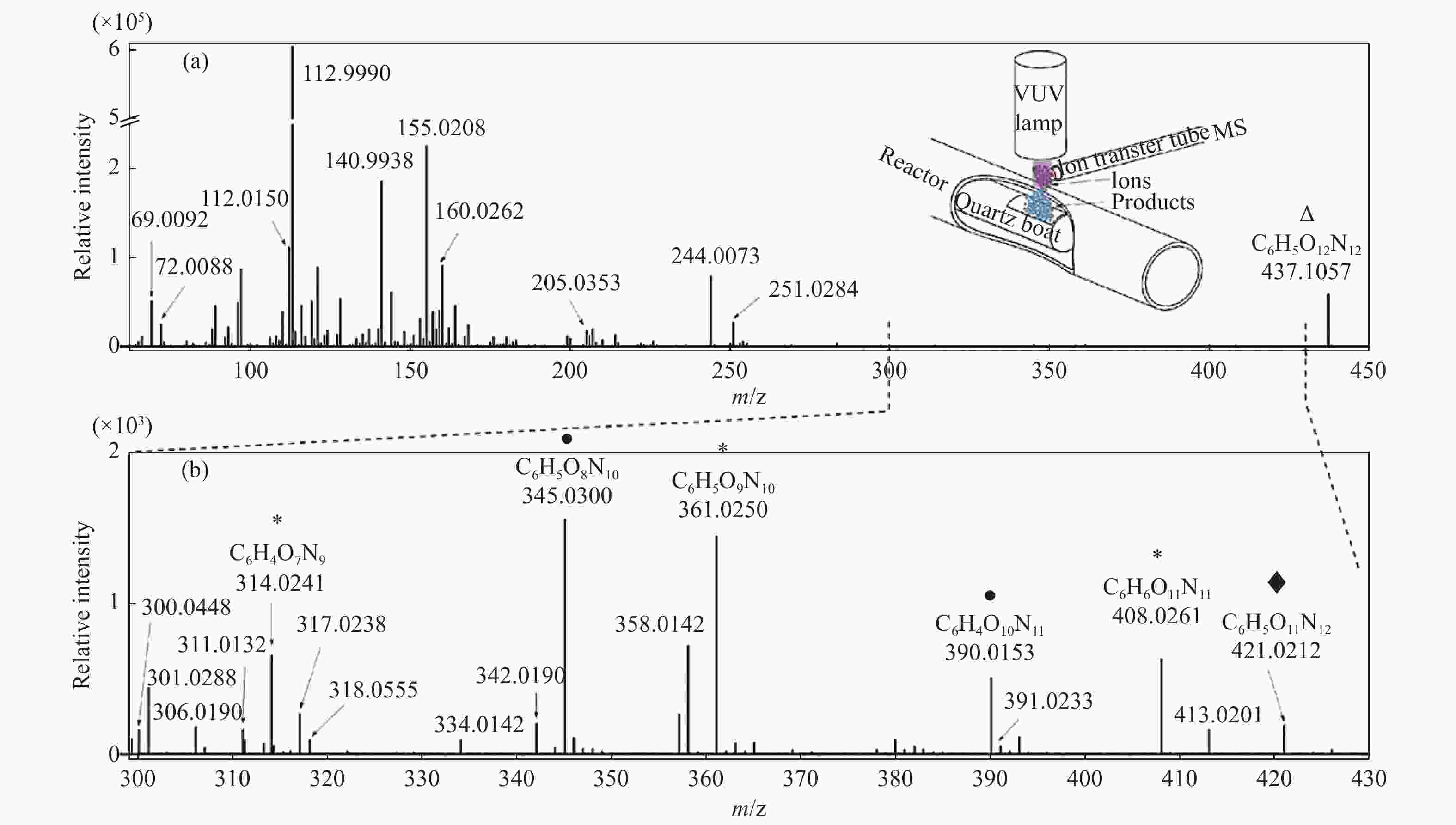
Figure 13 APPI HRMS in situ mass spectra of CL-20 decomposition products. (a) Full pyrolysis product spectrum, (b) enlarged spectrum in the m/z 300−430 range. The signal of the CL-20 molecule is represented by a down triangle symbol, while the products of the NO2 elimination, NO elimination, and O elimination reactions are represented by circles, stars, and diamonds, respectively[110] (with permission from Elsevier)
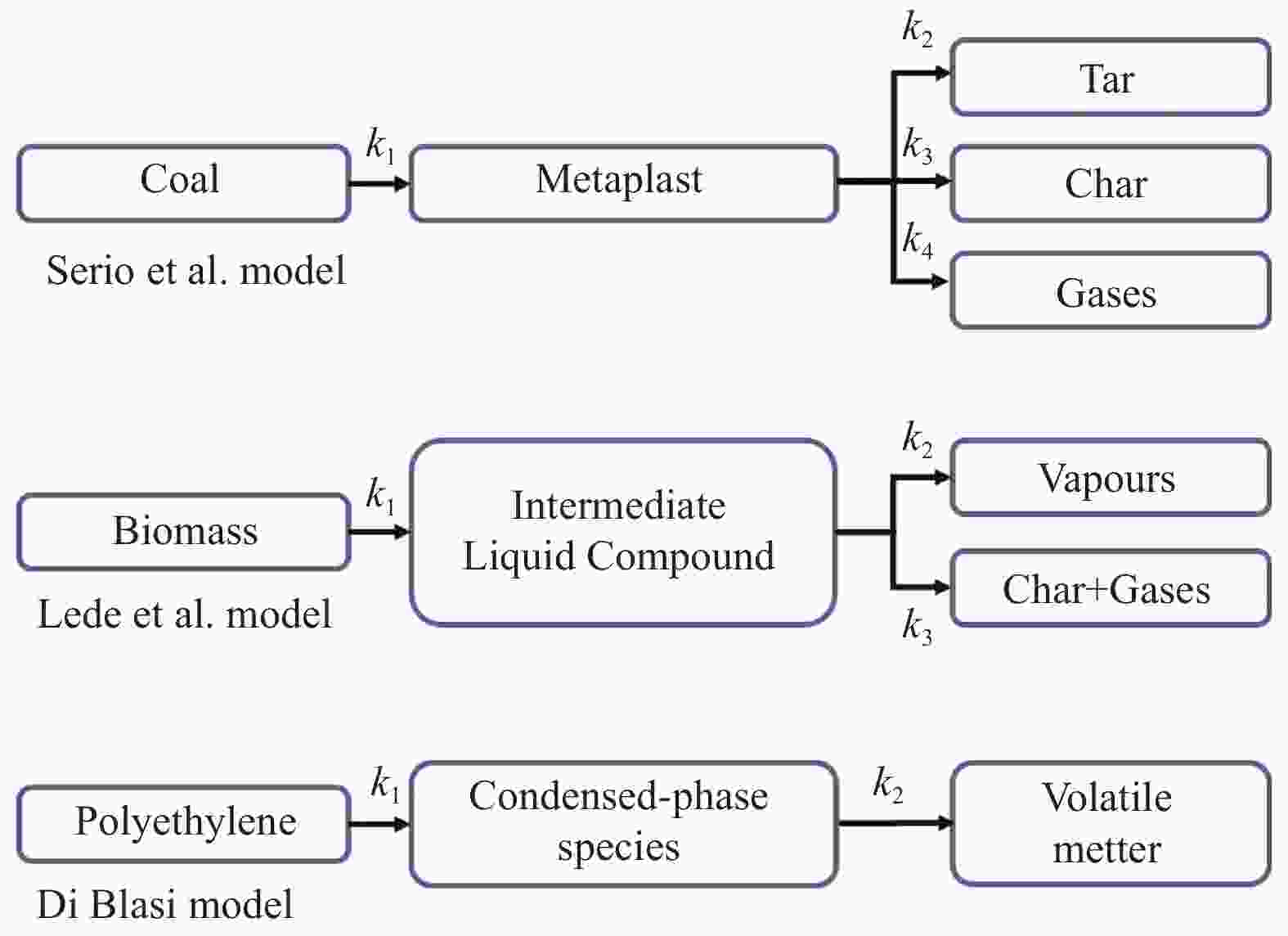
Figure 15 Tar, char and gases predicted by lumped kinetic models[125] (with permission from Taylor & Francis Inc)
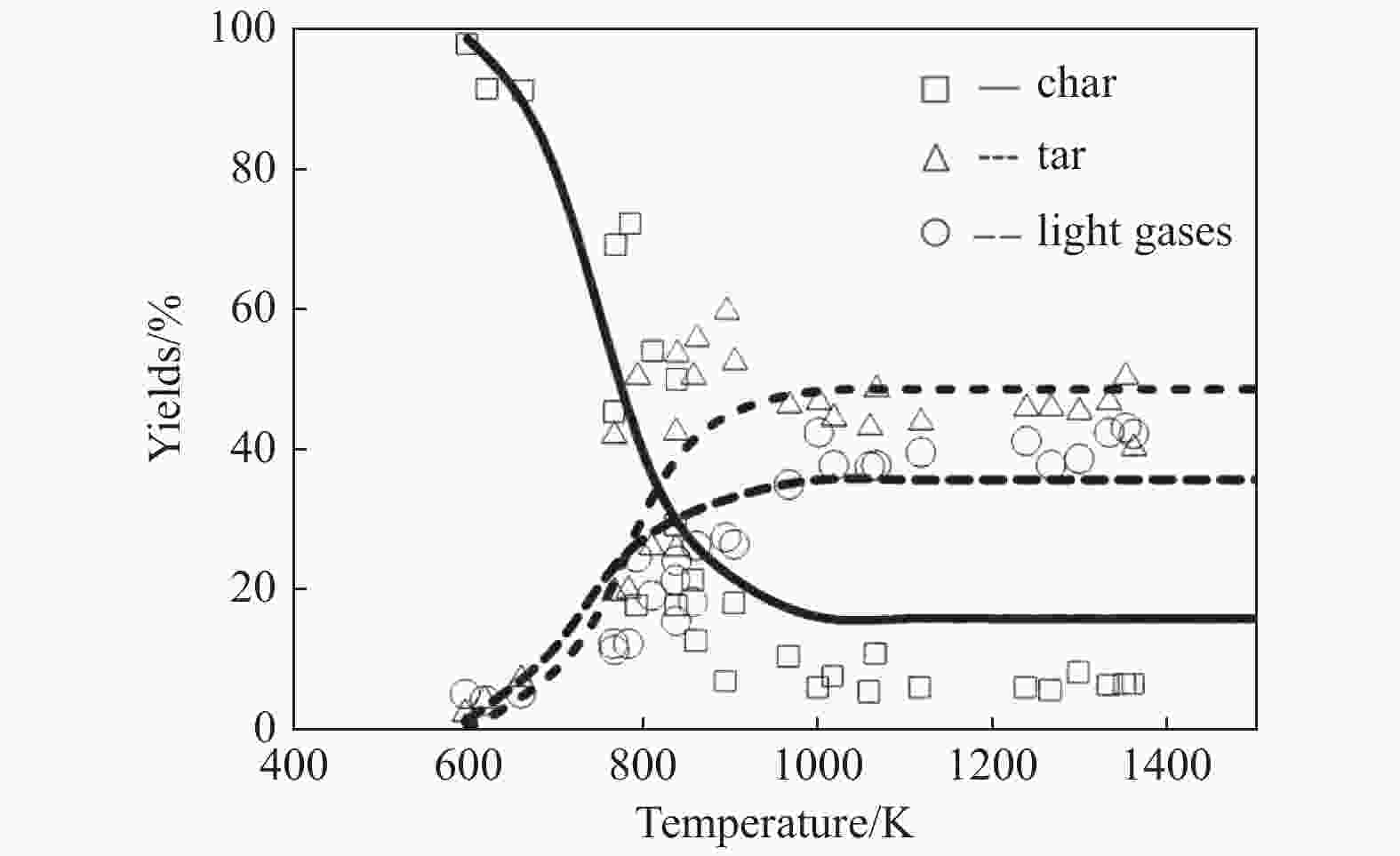
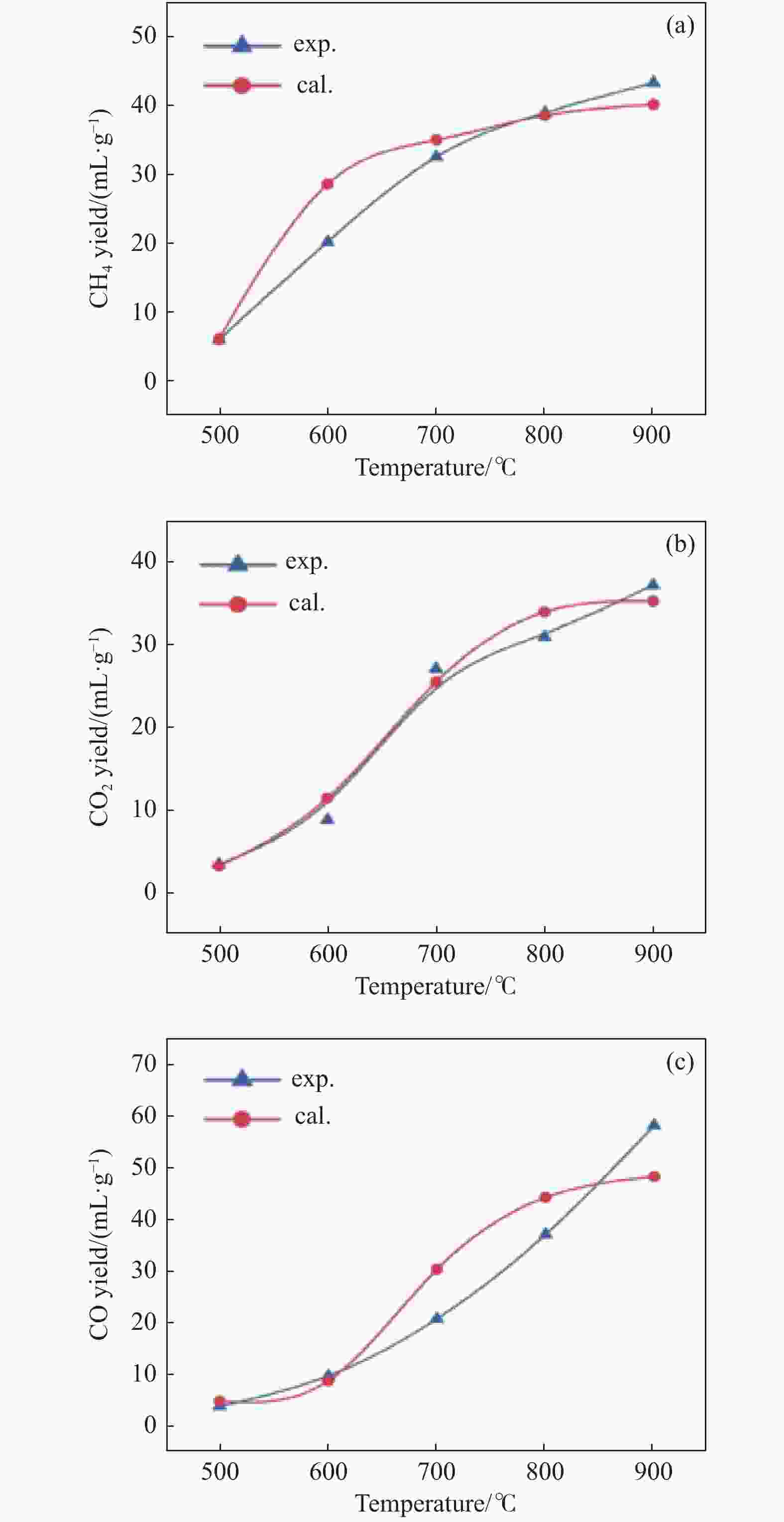
Figure 16 Expremental and simulated light gases yield by coal pyrolysis with the improved CPD model[130] (with permission from Royal Society of Chemistry)
Table 1 Main characteristics of various raw biomass catalytic pyrolysis with PIMS
Feedstocks Pyrolysis temperatures Methods Catalyst Key findings Ref. Reactor Light source Spruce/fir mixture, beech 300−560 °C TG VUV lamp − at 300 °C, there were mainly phenolic and furanic products, and the oxidation process of oxygenated compounds existed at high temperatures (>500 °C) [41] Beech, a mixture of spruce and fir, and coarse colza meal 250−500 °C TG VUV lamp − aliphatic hydrocarbons were found. alkaline biomass showed a strong signal of nitrogen-containing substances [22] Poplar 300–700 °C a tubular reactor SVUV − as a typical hardwood, the signal strength of pyrolysis products' syringyl subunits first increased and then decreased with the increase of temperature, while guaiacyl subunits continued to decrease [86] Micro-crystalline cellulose, xylan from birch, cellulose and lignin extracted from miscanthus and oak 350,450,
550 °Ca tubular reactor SVUV − a typical intermediate product of cellulose pyrolysis was found to be a possible precursor of the furanone-based species, and hydroxyacetaldehyde was the product of secondary reactions [78] Pine wood 300−700 °C a tubular reactor SVUV − as a typical softwood, polycyclic aromatic hydrocarbon (PAH) growth mechanism was demonstrated [87] Chrysophanol, emodin, rhein and aloe-emodin 373−973 K a PYR-2A pyrolyzer SVUV − the principal pyrolysis pathways for rhein involved the elimination reactions of CO, CO2 and HCOOH [88] Miscanthus, Douglas fir and oak 400 or 500 °C MFBRa VUV lamp − typical pyrolysis products of miscanthus, Douglas fir and oak were 4-vinylphenol, 4-methylguaiacol and 2,6-dimethoxy-4-(2-propenyl)-phenol [89] Miscanthus, oak and Douglas fir 200−500 °C a fixed-bed reactor VUV lamp − the most critical parameter affecting the chemical mechanisms during pyrolysis was the presence of inorganic constituents within the native biomass [83] Heartwood, sapwood, and bark (from Douglas fir and oak) 500 °C MFBRa Laser − the variation in pyrolysis products can largely be attributed to the mineral content as a primary factor [90] Douglas and oak 400 or 500 °C fixed bed reactor and MFBRa VUV lamp − the temporal evolution of key tar is indicated during both slow and fast pyrolysis conditions [91] Elm 500−700 °C MFBRa SVUV − the main factor impacting the change of primary tar during secondary reactions was the reaction temperature. At temperatures above 700 °C, the aerosols were primarily composed of large PAHs with over three rings [92] Elm 500−700 °C MFBRa aPPI − in secondary reactions, the primary mechanisms for transforming heavy compounds were deoxygenation and aromatization [93] Oak 500 or 600 °C MFBRa laser hierarchical zeolite desilicated zeolite was better than microporous zeolite for producing single aromatic compounds and was more stable when coke deposits formed [94] Xylan 300 °C a homemade tubular furnace SVUV Na2CO3 and K2CO3 alkali metal ions encouraged the creation of both char and lighter substances [23] Oka 500 °C MFBRa FT-ICR with ESI and APPI sources microporous and
hierarchical zeolitesthe mesopores HZSM-5 catalyst increased aromaticity and reduced oxygen-contenting products [95] Nannochloropsis, Spirulina, and Sargasso 500 °C a double micro-fixed-bed reactor SVUV HZSM-5 zeolite for algae, the product of monocyclic aromatic hydrocarbons was mainly derived from protein [96] Cellulose and polyethylene 50−700 °C TG VUV lamp HZSM-5 the co-feeding approach resulted in a notable enhancement in the generation of aromatic compounds [97] Cellulose and polyethylene 50−700 °C TG VUV lamp MgO the utilization of the MgO catalyst exhibited the capability to enhance the cellulose pyrolysis process and facilitate the cleavage of C–C bonds in polyethylene (PE) [98] Bamboo sawdust and polyethylene 50−650 °C fixed bed reactor VUV lamp − MgO catalyst can promote the Diels-Alder reaction in co-feeding pyrolysis, thus promoting aromatics production [99] Lignin and polyethylene 550 °C TG VUV lamp Cu-modified HZSM-5 Cu2O has better dehydrogenation activity and CuO has better selectivity of monocyclic aromatic hydrocarbons [40] a: A microfluidized bed reactor (MFBR). -
[1] WANG Y, ZHU Y, ZHOU Z, et al. Pyrolysis study on solid fuels: From conventional analytical methods to synchrotron vacuum ultraviolet photoionization mass spectrometry[J]. Energy Fuels,2016,30(3):1534−1543. doi: 10.1021/acs.energyfuels.5b02234 [2] CAO Y, CASENAS B, PAN W. Investigation of chemical looping combustion by solid fuels. 2. Redox reaction kinetics and product characterization with coal, biomass, and solid waste as solid fuels and CuO as an oxygen carrier[J]. Energy Fuels,2006,20(5):1845−1854. doi: 10.1021/ef050424k [3] GUO M, SONG W, BUHAIN J. Bioenergy and biofuels: History, status, and perspective[J]. Renewable Sustainable Energy Rev,2015,42:712−725. doi: 10.1016/j.rser.2014.10.013 [4] KAN T, STREZOV V, EVANS T. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters[J]. Renewable Sustainable Energy Rev,2016,57:1126−1140. doi: 10.1016/j.rser.2015.12.185 [5] HEMBERGER P, VAN BOKHOVEN J, PEREZ-RAMIREZ J, et al. New analytical tools for advanced mechanistic studies in catalysis: photoionization and photoelectron photoion coincidence spectroscopy[J]. Catal Sci Technol,2020,10(7):1975−1990. doi: 10.1039/C9CY02587A [6] KLAMPFL C, HIMMELSBACH M. Direct ionization methods in mass spectrometry: An overview[J]. Anal Chim Acta,2015,890:44−59. doi: 10.1016/j.aca.2015.07.012 [7] KANAUJIA P, SHARMA Y, AGRAWAL U, et al. Analytical approaches to characterizing pyrolysis oil from biomass[J]. TrAC-Trends Anal Chem,2013,42:125−136. doi: 10.1016/j.trac.2012.09.009 [8] ZHOU Z, DU X, YANG J, et al. The vacuum ultraviolet beamline/endstations at NSRL dedicated to combustion research[J]. J Synchrotron Radiat,2016,23:1035−1045. doi: 10.1107/S1600577516005816 [9] QI F. Combustion chemistry probed by synchrotron VUV photoionization mass spectrometry[J]. Proc Combust Inst,2013,34:33−63. doi: 10.1016/j.proci.2012.09.002 [10] NIYONSABA E, MANHEIM J, YERABOLU R, et al. Recent advances in petroleum analysis by mass spectrometry[J]. Anal Chem,2019,91(1):156−177. doi: 10.1021/acs.analchem.8b05258 [11] HEADLEY J, PERU K, BARROW M. Advances in mass spectrometric characterization of naphthenic acids fraction compounds in oil sands environmental samples and crude oil-A review[J]. Mass Spectrom Rev,2016,35(2):311−328. doi: 10.1002/mas.21472 [12] LETOURNEAU D, VOLMER D. Mass spectrometry-based methods for the advanced characterization and structural analysis of lignin: A review[J]. Mass Spectrom Rev,2023,42(1):144−188. doi: 10.1002/mas.21716 [13] RUGER C, TIEMANN O, NEUMANN A, et al. Review on evolved gas analysis mass spectrometry with soft photoionization for the chemical description of petroleum, petroleum-derived materials, and alternative feedstocks[J]. Energy Fuels,2021,35(22):18308−18332. doi: 10.1021/acs.energyfuels.1c02720 [14] LIU P, ZHUANG H, QIAN Y, et al. Recent advances in mass spectrometric studies on the reaction process of biomass pyrolysis[J]. Fuel Process Technol,2022,237:107473. doi: 10.1016/j.fuproc.2022.107473 [15] ZHOU Z, YANG J, YUAN W, et al. Probing combustion and catalysis intermediates by synchrotron vacuum ultraviolet photoionization molecular-beam mass spectrometry: Recent progress and future opportunities[J]. Phys Chem Chem Phys,2022,24(36):21567−21577. doi: 10.1039/D2CP02899A [16] DANG M, LIU R, DONG F, et al. Vacuum ultraviolet photoionization on-line mass spectrometry: Instrumentation developments and applications[J]. TrAC-Trends Anal Chem,2022,149:116542. doi: 10.1016/j.trac.2022.116542 [17] ESCHNER M, ZIMMERMANN R. Determination of photoionization cross-sections of different organic molecules using gas chromatography coupled to single-photon ionization (SPI) time-of-flight mass spectrometry (TOF-MS) with an electron-beam-pumped rare gas excimer light source (EBEL): I[J]. Appl Spectrosc,2011,65(7):806−816. doi: 10.1366/11-06233 [18] FENDT A, STREIBEL T, SKLORZ M, et al. On-line process analysis of biomass flash pyrolysis gases enabled by soft photoionization mass spectrometry[J]. Energy Fuels,2012,26(1):701−711. doi: 10.1021/ef2012613 [19] FENDT A, GEISSLER R, STREIBEL T, et al. Hyphenation of two simultaneously employed soft photo ionization mass spectrometers with thermal analysis of biomass and biochar[J]. Thermochim Acta,2013,551:155−163. doi: 10.1016/j.tca.2012.10.002 [20] MUHLBERGER F, HAFNER K, KAESDORF S, et al. Comprehensive on-line characterization of complex gas mixtures by quasi-simultaneous resonance-enhanced multiphoton ionization, vacuum-UV single-photon ionization, and electron impact ionization in a time-of-flight mass spectrometer: Setup and instrument characterization[J]. Anal Chem,2004,76(22):6753−6764. doi: 10.1021/ac049535r [21] BROWN A, DAYTON D, NIMLOS M, et al. Characterization of biomass pyrolysis vapors with molecular beam, single photon ionization time-of-flight mass spectrometry[J]. Chemosphere,2001,42(5/7):663−669. doi: 10.1016/S0045-6535(00)00240-X [22] STREIBEL T, FENDT A, GEISSLER R, et al. Thermal analysis/mass spectrometry using soft photo-ionisation for the investigation of biomass and mineral oils[J]. J Therm Anal Calorim,2009,97(2):615−619. doi: 10.1007/s10973-008-9769-5 [23] LI Y, WANG J, CHEN X, et al. Catalytic pyrolysis of xylan over alkali metal salts as revealed by synchrotron vacuum ultraviolet photoionization mass spectrometry[J]. J Anal Appl Pyrolysis,2018,135:94−100. doi: 10.1016/j.jaap.2018.09.014 [24] ZHAO L, KAISER R, XU B, et al. Pyrene synthesis in circumstellar envelopes and its role in the formation of 2D nanostructures[J]. Nat Astron,2018,2(5):413−419. doi: 10.1038/s41550-018-0399-y [25] YUAN W, LI Y, QI F. Challenges and perspectives of combustion chemistry research[J]. Sci China Chem,2017,60(11):1391−1401. doi: 10.1007/s11426-017-9066-9 [26] JIAO F, LI J, PAN X, et al. Selective conversion of syngas to light olefins[J]. Science,2016,351(6277):1065−1068. doi: 10.1126/science.aaf1835 [27] PAN Y, ZHANG L, GUO H, et al. Photoionisation and photodissociation studies of nonvolatile organic molecules by synchrotron VUV photoionisation mass spectrometry and theoretical calculations[J]. Int Rev Phys Chem,2010,29(2):369−401. doi: 10.1080/01442351003668697 [28] BUTCHER D. Vacuum ultraviolet radiation for single-photoionization mass spectrometry: A review[J]. Microchem J,1999,62(3):354−362. doi: 10.1006/mchj.1999.1745 [29] ROBB D, COVEY T, BRUINS A. Atmospheric pressure photoionisation: An ionization method for liquid chromatography-mass spectrometry[J]. Anal Chem,2000,72(15):3653−3659. doi: 10.1021/ac0001636 [30] HANOLD K, FISCHER S, CORMIA P, et al. Atmospheric pressure photoionization. 1. General properties for LC/MS[J]. Anal Chem,2004,76(10):2842−2851. doi: 10.1021/ac035442i [31] KAUPPILA T, SYAGE J, BENTER T. Recent developments in atmospheric pressure photoionization-mass spectrometry[J]. Mass Spectrom Rev,2017,36(3):423−449. doi: 10.1002/mas.21477 [32] THOMAS M, CHAN H, LOZANO D, et al. Solvent and flow rate effects on the observed compositional profiles and the relative intensities of radical and protonated species in atmospheric pressure photoionization mass spectrometry [J] Anal Chem, 2022, 94 (12): 4954-4960. [33] ROBB D, BLADES M. State-of-the-art in atmospheric pressure photoionization for LC/MS[J]. Anal Chim Acta,2008,627(1):34−49. doi: 10.1016/j.aca.2008.05.077 [34] TERRIER P, DESMAZIERES B, TORTAJADA J, et al. Apci/Appi for synthetic polymer analysis[J]. Mass Spectrom Rev,2011,30(5):854−874. doi: 10.1002/mas.20302 [35] BRITT P, BUCHANAN A. Thermolysis of surface-attached 1, 4-diphenylbutane: the role of hydrogen-transfer reactions on the surface in determining the product distribution[J]. J Org Chem,1991,56(21):6132−6140. doi: 10.1021/jo00021a032 [36] LI G, LI L, JIN L, et al. Experimental and theoretical investigation on three α, ω-diarylalkane pyrolysis[J]. Energy Fuels,2014,28(11):6905−6910. doi: 10.1021/ef502012b [37] LI G, LI L, JIN L, et al. Methyl substitution effect in pyrolysis of coal-based model compound isomers[J]. Fuel Process Technol,2018,178:371−378. doi: 10.1016/j.fuproc.2018.07.020 [38] ZHOU Y, LI L, JIN L, et al. Pyrolytic behavior of coal-related model compounds connected with C-C bridged linkages by in-situ pyrolysis vacuum ultraviolet photoionization mass spectrometry[J]. Fuel,2019,241:533−541. doi: 10.1016/j.fuel.2018.12.046 [39] ZOLLER D, JOHNSTON M, TOMIC J, et al. Thermogravimetry-photoionization mass spectrometry of different rank coals[J]. Energy Fuels,1999,13(5):1097−1104. doi: 10.1021/ef990069w [40] TSUJI N, NISHIFUJI M, HAYASH S. The measurement of various molecules of pyrolysis gas of coal by using VUV-SPI-TOFMS[J]. Hyperfine Interact,2013,216(1-3):127−131. doi: 10.1007/s10751-013-0822-9 [41] STREIBEL T, GEISSLER R, SARAJI-BOZORGZAD M, et al. Evolved gas analysis (EGA) in TG and DSC with single photon ionisation mass spectrometry (SPI-MS): molecular organic signatures from pyrolysis of soft and hard wood, coal, crude oil and ABS polymer[J]. J Therm Anal Calorim,2009,96(3):795−804. doi: 10.1007/s10973-009-0035-2 [42] SHI Z, JIN L, ZHOU Y, et al. Online analysis of initial volatile products of Shenhua coal and its macerals with pyrolysis vacuum ultraviolet photoionization mass spectrometry[J]. Fuel Process Technol,2017,163:67−74. doi: 10.1016/j.fuproc.2017.04.006 [43] COOL T, MCILROY A, QI F, et al. Photoionization mass spectrometer for studies of flame chemistry with a synchrotron light source[J]. Rev Sci Instrum,2005,76(9):094102. doi: 10.1063/1.2010307 [44] JIA L, WENG J, WANG Y, et al. Online analysis of volatile products from Bituminous coal pyrolysis with synchrotron vacuum ultraviolet photoionization mass spectrometry[J]. Energy Fuels,2013,27(2):694−701. doi: 10.1021/ef301670y [45] ZHU Y, WEN W, LI Y, et al. Pyrolysis study of Huainan coal with different particle sizes using TG analysis and online Py-PI-TOF MS[J]. J Energy Inst,2020,93(1):405−414. doi: 10.1016/j.joei.2019.01.016 [46] ZHENG M, PAN Y, WANG Z, et al. Capturing the dynamic profiles of products in Hailaer brown coal pyrolysis with reactive molecular simulations and experiments[J]. Fuel,2020,268:117290. doi: 10.1016/j.fuel.2020.117290 [47] LI G, ZHANG S, JIN L, et al. In-situ analysis of volatile products from lignite pyrolysis with pyrolysis-vacuum ultraviolet photoionization and electron impact mass spectrometry[J]. Fuel Process Technol,2015,133:232−236. doi: 10.1016/j.fuproc.2015.02.016 [48] MORGAN T, KANDIYOTI R. Pyrolysis of coals and biomass: Analysis of thermal breakdown and its products[J]. Chem Rev,2014,114(3):1547−1607. doi: 10.1021/cr400194p [49] . KONG X, BAI Y, YAN L, et al. Catalytic upgrading of coal gaseous tar over Y-type zeolites [J]. Fuel, 2016, 180 205-210. [50] GAO M, LV P, YANG Z, et al. Effects of Ca/Na compounds on coal gasification reactivity and char characteristics in H2O/CO2 mixtures[J]. Fuel,2017,206:107−116. doi: 10.1016/j.fuel.2017.05.079 [51] ZOU X, YAO J, YANG X, et al. Catalytic effects of metal chlorides on the pyrolysis of lignite[J]. Energy Fuels,2007,21(2):619. doi: 10.1021/ef060477h [52] BAN Y, JIN L, LIU F, et al. Pyrolysis behaviors of model compounds with representative oxygen-containing functional groups in coal over calcium[J]. Fuel,2022,310:122247. doi: 10.1016/j.fuel.2021.122247 [53] ZHOU Y, LI G, JIN L, et al. In situ analysis of catalytic effect of calcium nitrate on Shenmu coal pyrolysis with pyrolysis vacuum ultraviolet photoionization mass spectrometry[J]. Energy Fuels,2018,32(2):1061−1069. doi: 10.1021/acs.energyfuels.7b02385 [54] BAN Y, JIN L, ZHU J, et al. Insights into effect of Ca(OH)2 on pyrolysis behaviors and products distribution of Hongshaquan coal[J]. Fuel,2022,307:121791. doi: 10.1016/j.fuel.2021.121791 [55] ZHU Y, CHEN X, WANG Y, et al. Online study on the catalytic pyrolysis of Bituminous coal over HUSY and HZSM-5 with photoionization time-of-flight mass spectrometry[J]. Energy Fuels,2016,30(3):1598−1604. doi: 10.1021/acs.energyfuels.5b02286 [56] SHI Z, JIN L, ZHOU Y, et al. In-situ analysis of catalytic pyrolysis of Baiyinhua coal with pyrolysis time-of-flight mass spectrometry[J]. Fuel,2018,227:386−393. doi: 10.1016/j.fuel.2018.04.109 [57] GOUWS S, CARRIER M, BUNT J, et al. Co-pyrolysis of coal and raw/torrefied biomass: A review on chemistry, kinetics and implementation[J]. Renewable Sustainable Energy Rev,2021,135:110189. doi: 10.1016/j.rser.2020.110189 [58] ELLIS N, MASNADI M, ROBERTS D, et al. Mineral matter interactions during co-pyrolysis of coal and biomass and their impact on intrinsic char co-gasification reactivity[J]. Chem Eng J,2015,279:402−408. doi: 10.1016/j.cej.2015.05.057 [59] ZHOU Z, LIU C, CHEN X, et al. On-line photoionization mass spectrometric study of lignin and lignite co-pyrolysis: Insight into the synergetic effect[J]. J Anal Appl Pyrolysis,2019,137:285−292. doi: 10.1016/j.jaap.2018.12.009 [60] WENG J, LIU Y, ZHU Y, et al. Online study on the co-pyrolysis of coal and corn with vacuum ultraviolet photoionization mass spectrometry[J]. Bioresour Technol,2017,244:125−131. doi: 10.1016/j.biortech.2017.07.128 [61] WU Y, ZHU J, WANG Y, et al. Insight into co-pyrolysis interactions of Pingshuo coal and high-density polyethylene via in-situ Py-TOF-MS and EPR[J]. Fuel,2021,303:121199. doi: 10.1016/j.fuel.2021.121199 [62] ZHUANG H, CHEN L, XU Q, et al. Measurement and summary of photoionization data for biomass-derived compounds[J]. Rapid Commun Mass Spectrom,2022,36(23):9412. doi: 10.1002/rcm.9412 [63] REALE S, DI TULLIO A, SPRETI N, et al. Mass spectrometry in the biosynthetic and structural investigation of lignins[J]. Mass Spectrom Rev,2004,23(2):87−126. doi: 10.1002/mas.10072 [64] ASMADI M, KAWAMOTO H, SAKA S. The effects of combining guaiacol and syringol on their pyrolysis[J]. Holzforschung,2012,66(3):323−330. [65] LIU C, ZHANG Y, HUANG X. Study of guaiacol pyrolysis mechanism based on density function theory[J]. Fuel Process Technol,2014,123:159−165. doi: 10.1016/j.fuproc.2014.01.002 [66] LIU C, YE L, YUAN W, et al. Investigation on pyrolysis mechanism of guaiacol as lignin model compound at atmospheric pressure[J]. Fuel,2018,232:632−638. doi: 10.1016/j.fuel.2018.05.162 [67] CHEN X, ZHU L, CUI C, et al. In situ atmospheric pressure photoionization mass spectrometric monitoring of initial pyrolysis products of biomass in real time[J]. Anal Chem,2020,92(1):603−606. doi: 10.1021/acs.analchem.9b05200 [68] LIU C, CHEN X, LIU X, et al. Evidence of a phenolic pool as a key intermediate for zeolite-catalyzed lignin pyrolysis[J]. Angew Chem Int Ed.,2021,60(5):2643−2647. doi: 10.1002/anie.202011937 [69] HEMBERGER P, CUSTODIS V, BODI A, et al. Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis[J]. Nat Commun,2017,8:15946. doi: 10.1038/ncomms15946 [70] PAN Z, PUENTE-URBINA A, BODI A, et al. Isomer-dependent catalytic pyrolysis mechanism of the lignin model compounds catechol, resorcinol and hydroquinone[J]. Chem Sci,2021,12(9):3161−3169. doi: 10.1039/D1SC00654A [71] PAN Z, PUENTE-URBINA A, BATOOL S, et al. Tuning the zeolite acidity enables selectivity control by suppressing ketene formation in lignin catalytic pyrolysis[J]. Nat Commun,2023,14(1):4512. doi: 10.1038/s41467-023-40179-z [72] BODI A, HEMBERGER P, GERBER T, et al. A new double imaging velocity focusing coincidence experiment: i(2)PEPICO[J]. Rev Sci Instrum,2012,83(8):083105. doi: 10.1063/1.4742769 [73] HEMBERGER P, PAN Z, BODI A, et al. The threshold photoelectron spectrum of fulvenone: A reactive ketene derivative in lignin valorization[J]. Chemphyschem,2020,21(19):2217−2222. doi: 10.1002/cphc.202000477 [74] LIU P, HUANG J, YANG K, et al. Exploring the reaction chemistry of biomass upgrading over HZSM-5 catalyst through model compounds[J]. Fuel,2022,312:122874. doi: 10.1016/j.fuel.2021.122874 [75] CHENG Y, HUBER G. Chemistry of furan conversion into aromatics and olefins over HZSM-5: A model biomass conversion reaction[J]. Acs Catal,2011,1(6):611−628. doi: 10.1021/cs200103j [76] GRACA I, COMPAROT J, LAFORGE S, et al. Influence of phenol addition on the H-ZSM-5 zeolite catalytic properties during methylcyclohexane transformation[J]. Energy Fuels,2009,23(9):4224−4230. doi: 10.1021/ef9003472 [77] LIU P, SHAO W, YANG Z, et al. In situ mass spectrometric analysis on zeolite-catalyzed pyrolysis of furanic compounds: The role of reactive intermediates[J]. ACS Catal,2023,13(18):12227−12237. doi: 10.1021/acscatal.3c01948 [78] DUFOUR A, WENG J, JIA L, et al. Revealing the chemistry of biomass pyrolysis by means of tunable synchrotron photoionisation-mass spectrometry[J]. RSC Adv,2013,3(14):4786−4792. doi: 10.1039/c3ra40486b [79] LEDE J. Cellulose pyrolysis kinetics: An historical review on the existence and role of intermediate active cellulose[J]. J Anal Appl Pyrolysis,2012,94:17−32. doi: 10.1016/j.jaap.2011.12.019 [80] GHODAKE G, SHINDE S, KADAM A, et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy[J]. J Cleaner Prod,2021,297:126645. doi: 10.1016/j.jclepro.2021.126645 [81] JARVIS M, HAAS T, DONOHOE B, et al. Elucidation of biomass pyrolysis products using a laminar entrained flow reactor and char particle imaging[J]. Energy Fuels, 2011, 25 324−336. [82] DAI G, WANG G, WANG K, et al. Mechanism study of hemicellulose pyrolysis by combining in-situ DRIFT, TGA-PIMS and theoretical calculation[J]. Proc Combust Inst,2021,38(3):4241−4249. doi: 10.1016/j.proci.2020.06.196 [83] . LE BRECH Y, JIA L, CISSE S, et al. Mechanisms of biomass pyrolysis studied reactor with advanced gas analysis by combining a fixed bed [J]. J Anal Appl Pyrolysis, 2016, 117 334-346. [84] FISCHER G, PRIELER S, VAN VELTHUIZEN H. Biomass potentials of miscanthus, willow and poplar: results and policy implications for Eastern Europe, Northern and Central Asia[J]. Biomass Bioenergy,2005,28(2):119−132. doi: 10.1016/j.biombioe.2004.08.013 [85] DANG M, LIU R, DONG F, et al. Vacuum ultraviolet photoionization on-line mass spectrometry: instrumentation developments and applications[J]. TrAC, Trends Anal Chem,2022,149:116542. doi: 10.1016/j.trac.2022.116542 [86] WENG J, JIA L, WANG Y, et al. Pyrolysis study of poplar biomass by tunable synchrotron vacuum ultraviolet photoionization mass spectrometry[J]. Proc Combust Inst,2013,34:2347−2354. doi: 10.1016/j.proci.2012.05.077 [87] WENG J, JIA L, SUN S, et al. On-line product analysis of pine wood pyrolysis using synchrotron vacuum ultraviolet photoionization mass spectrometry[J]. Anal Bioanal Chem,2013,405(22):7097−7105. doi: 10.1007/s00216-012-6516-3 [88] JIA L, YANG J, ZHANG L, et al. Experimental and theoretical studies of pyrolysis of chrysophanol and its derivatives[J]. J Anal Appl Pyrolysis,2013,100:237−244. doi: 10.1016/j.jaap.2013.01.002 [89] JIA L, LE-BRECH Y, SHRESTHA B, et al. Fast pyrolysis in a microfluidized bed reactor: Effect of biomass properties and operating conditions on volatiles composition as analyzed by online single photoionization mass spectrometry[J]. Energy Fuels,2015,29(11):7364−7374. doi: 10.1021/acs.energyfuels.5b01803 [90] JIA L, BUENDIA-KANDIA F, DUMARCAY S, et al. Fast pyrolysis of heartwood, sapwood, and bark: A complementary application of online photoionization mass spectrometry andconventional pyrolysis gas chromatography/mass spectrometry[J]. Energy Fuels,2017,31(4):4078−4089. doi: 10.1021/acs.energyfuels.7b00110 [91] JIA L, DUFOUR A, LE BRECH Y, et al. On-line analysis of primary tars from biomass pyrolysis by single photoionization mass spectrometry: Experiments and detailed modelling[J]. Chem Eng J,2017,313:270−282. doi: 10.1016/j.cej.2016.12.021 [92] YANG K, WANG J, HUANG J, et al. Understanding the homogeneous reactions of primary tar from biomass pyrolysis by means of photoionization mass spectrometry[J]. Energy Fuels,2020,34(10):12678−12687. doi: 10.1021/acs.energyfuels.0c02178 [93] YANG K, HUANG J, DONG B, et al. Secondary reactions of primary rar from biomass pyrolysis: Characterization of heavy products by FT-ICR MS[J]. Energy Fuels,2021,35(16):13191−13199. doi: 10.1021/acs.energyfuels.1c01723 [94] JIA L, RAAD M, HAMIEH S, et al. Catalytic fast pyrolysis of biomass: superior selectivity of hierarchical zeolites to aromatics[J]. Green Chem,2017,19(22):5442−5459. doi: 10.1039/C7GC02309J [95] HERTZOG J, CARRE V, JIA L, et al. Catalytic fast pyrolysis of biomass over microporous and hierarchical zeolites: Characterization of heavy products[J]. ACS Sustainable Chem Eng,2018,6(4):4717−4728. doi: 10.1021/acssuschemeng.7b03837 [96] JIA L, CAO C, CHENG Z, et al. Ex situ catalytic pyrolysis of algal biomass in a double microfixed-bed reactor: Catalyst deactivation and its coking behavior[J]. Energy Fuels,2020,34(2):1918−1928. doi: 10.1021/acs.energyfuels.9b04024 [97] ZHOU Z, CHEN X, WANG Y, et al. Online photoionization mass spectrometric evaluation of catalytic co-pyrolysis of cellulose and polyethylene over HZSM-5[J]. Bioresour Technol,2019,275:130−137. doi: 10.1016/j.biortech.2018.12.045 [98] WENG J, CUI C, ZHOU Z, et al. Online investigation on catalytic co-pyrolysis of cellulose and polyethylene over magnesium oxide by advanced mass spectrometry[J]. Bioresour Technol,2021,338:125560. doi: 10.1016/j.biortech.2021.125560 [99] YANG K, WU K, LI F, et al. Investigation on the co-pyrolysis of bamboo sawdust and low-density polyethylene via online photoionization mass spectrometry and machine learning methods[J]. Fuel Process Technol,2023,240:107579. doi: 10.1016/j.fuproc.2022.107579 [100] GAMLIEL D, CHO H, FAN W, et al. On the effectiveness of tailored mesoporous MFI zeolites for biomass catalytic fast pyrolysis[J]. Appl Catal A: Gen,2016,522:109−119. doi: 10.1016/j.apcata.2016.04.026 [101] CHIAVERINI M, HARTING G, LU Y, et al. Pyrolysis behavior of hybrid-rocket solid fuels under rapid heating conditions[J]. J Propul Power,1999,15(6):888−895. doi: 10.2514/2.5512 [102] LAN G, LI J, ZHANG G, et al. Thermal decomposition mechanism study of 3-nitro-1, 2, 4-triazol-5-one (NTO): Combined TG-FTIR-MS techniques and ReaxFF reactive molecular dynamics simulations[J]. Fuel,2021,295:120655. doi: 10.1016/j.fuel.2021.120655 [103] ZHOU L, PIEKIEL N, CHOWDHURY S, et al. T-Jump/time-of-flight mass spectrometry for time-resolved analysis of energetic materials[J]. Rapid Commun Mass Sp,2009,23(1):194−202. doi: 10.1002/rcm.3815 [104] SIRACH R, DAVE P. 3-Nitro-1, 2, 4-triazol-5-one (NTO): High explosive insensitive energetic material[J]. Chem Heterocycl Compd,2021,57(7-8):720−730. doi: 10.1007/s10593-021-02973-9 [105] LARINA L. Tautomerism and structure of azoles: Nuclear magnetic resonance spectroscopy[J]. Adv Heterocycl Chem,2018,124:233−321. [106] WANG J, JIN S, CHEN S, et al. Molecular dynamic simulations for FOX-7 and FOX-7 based PBXs[J]. J Mol Model,2018,24(7):145. doi: 10.1007/s00894-018-3687-7 [107] TRZCINSKI W, BELAADA A. 1, 1-diamino-2, 2-dinitroethene (DADNE, FOX-7) - properties and formulations (a review)[J]. Cent Eur J Energy Mater,2016,13(2):527−544. doi: 10.22211/cejem/65000 [108] JIANG L, FU X, ZHOU Z, et al. Study of the thermal decomposition mechanism of FOX-7 by molecular dynamics simulation and online photoionization mass spectrometry[J]. RSC Adv,2020,10(36):21147−21157. doi: 10.1039/D0RA03443F [109] JIANG L, FU X, FAN X, et al. Study on N-guanylurea-dinitramide (GUDN) decomposition using theoretical simulations, online photoionization mass spectrometry and TG-DSC-IR-MS experiments[J]. Combust Flame,2021,229:111406. doi: 10.1016/j.combustflame.2021.111406 [110] ZHU Y, ZHOU Z, YE L, et al. Direct mass spectrometric observation and reaction mechanism of gas-phase initial intermediates during CL-20 decomposition[J]. Combust Flame,2022,241:112095. doi: 10.1016/j.combustflame.2022.112095 [111] DE JONG W, PIRONE A, WOJTOWICZ M. Pyrolysis of Miscanthus Giganteus and wood pellets: TG-FTIR analysis and reaction kinetics[J]. Fuel,2003,82(9):1139−1147. doi: 10.1016/S0016-2361(02)00419-2 [112] LV G, WU S. Analytical pyrolysis studies of corn stalk and its three main components by TG-MS and Py-GC/MS[J]. J Anal Appl Pyrolysis,2012,97:11−18. doi: 10.1016/j.jaap.2012.04.010 [113] WANG S, LIN H, RU B, et al. Comparison of the pyrolysis behavior of pyrolytic lignin and milled wood lignin by using TG-FTIR analysis[J]. J Anal Appl Pyrolysis,2014,108:78−85. doi: 10.1016/j.jaap.2014.05.014 [114] YANG X, ZHAO Y, LI R, et al. A modified kinetic analysis method of cellulose pyrolysis based on TG–FTIR technique[J]. Thermochim Acta,2018,665:20−27. doi: 10.1016/j.tca.2018.05.008 [115] DAI G, WANG G, WANG K, et al. Mechanism study of hemicellulose pyrolysis by combining in-situ DRIFT, TGA-PIMS and theoretical calculation[J]. Proc Combust Inst,2021,38(3):4241−4249. doi: 10.1016/j.proci.2020.06.196 [116] KRISTANTO J, AZIS M, PURWONO S. Multi-distribution activation energy model on slow pyrolysis of cellulose and lignin in TGA/DSC[J]. Heliyon,2021,7(7):8. [117] YANG X, ZHAO Y, LI W, et al. Unveiling the pyrolysis mechanisms of hemicellulose: Experimental and theoretical studies[J]. Energy Fuels,2019,33(5):4352−4360. doi: 10.1021/acs.energyfuels.9b00482 [118] WANG S, DAI G, YANG H, et al. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review[J]. Prog Energy Combust Sci,2017,62:33−86. doi: 10.1016/j.pecs.2017.05.004 [119] CAI J, WU W, LIU R. An overview of distributed activation energy model and its application in the pyrolysis of lignocellulosic biomass[J]. Renewable Sustainable Energy Rev,2014,36:236−246. doi: 10.1016/j.rser.2014.04.052 [120] DONSKOI E, MCELWAIN D. Optimization of coal pyrolysis modeling[J]. Combust Flame,2000,122(3):359−367. doi: 10.1016/S0010-2180(00)00115-2 [121] ULLOA C, GORDON A, GARCÍA X. Distribution of activation energy model applied to the rapid pyrolysis of coal blends[J]. J Anal Appl Pyrolysis,2004,71(2):465−483. doi: 10.1016/S0165-2370(03)00132-3 [122] SERIO M, HAMBLEN D, MARKHAM J, et al. Kinetics of volatile product evolution in coal pyrolysis-experiment and theory[J]. Energy Fuels,1987,1(2):138−152. doi: 10.1021/ef00002a002 [123] DIBLASI C. Linear pyrolysis of cellulosic and plastic waste[J]. J Anal Appl Pyrolysis,1997,40(1):463−479. [124] LÉDÉ J, BLANCHARD F, BOUTIN O. Radiant flash pyrolysis of cellulose pellets: Products and mechanisms involved in transient and steady state conditions[J]. Fuel,2002,81(10):1269−1279. doi: 10.1016/S0016-2361(02)00039-X [125] SHENG C, AZEVEDO J. Modeling biomass devolatilization using the chemical percolation devolatilization model for the main components[J]. Proc Combust Inst,2002,29:407−414. doi: 10.1016/S1540-7489(02)80054-2 [126] RANZI E, DENTE M, GOLDANIGA A, et al. Lumping procedures in detailed kinetic modeling of gasification, pyrolysis, partial oxidation and combustion of hydrocarbon mixtures[J]. Prog Energy Combust Sci,2001,27(1):99−139. doi: 10.1016/S0360-1285(00)00013-7 [127] RANZI E, CUOCI A, FARAVELLI T, et al. Chemical kinetics of biomass pyrolysis[J]. Energy Fuels,2008,22(6):4292−4300. doi: 10.1021/ef800551t [128] RANZI E, CORBETTA M, MANENTI F, et al. Kinetic modeling of the thermal degradation and combustion of biomass[J]. Chem Eng Sci,2014,110:2−12. doi: 10.1016/j.ces.2013.08.014 [129] FLETCHER T, KERSTEIN A, PUGMIRE R, et al. A chemical percolation model for devolatilization: Summary[J]. J Chem Inf Model,2013,53(9):1689−1699. [130] TONG L, FANG M, LI H, et al. Pyrolysis of a typical low-rank coal: application and modification of the chemical percolation devolatilization model[J]. RSC Adv.,2021,11(29):17993−18002. doi: 10.1039/D1RA01981C [131] NIKSA S. Predicting the rapid devolatilization of diverse forms of biomass with bio-FLASHCHAIN[J]. Proc Combust Inst,2000,28:2727−2733. doi: 10.1016/S0082-0784(00)80693-1 [132] YOU Y, WANG X, HAN X, et al. Kerogen pyrolysis model based on its chemical structure for predicting product evolution[J]. Fuel,2019,246:149−159. doi: 10.1016/j.fuel.2019.02.075 [133] HORTON S, ZHANG Y, MOHR R, et al. Implementation of a molecular-level kinetic model for plasma-arc municipal solid waste gasification[J]. Energy Fuels,2016,30(10):7904−7915. doi: 10.1021/acs.energyfuels.6b00899 -




 下载:
下载: