Impact of B-site cations of MgX2O4 (X=Mn, Fe, Cr) spinels on the chemical looping oxidative dehydrogenation of ethane to ethylene
-
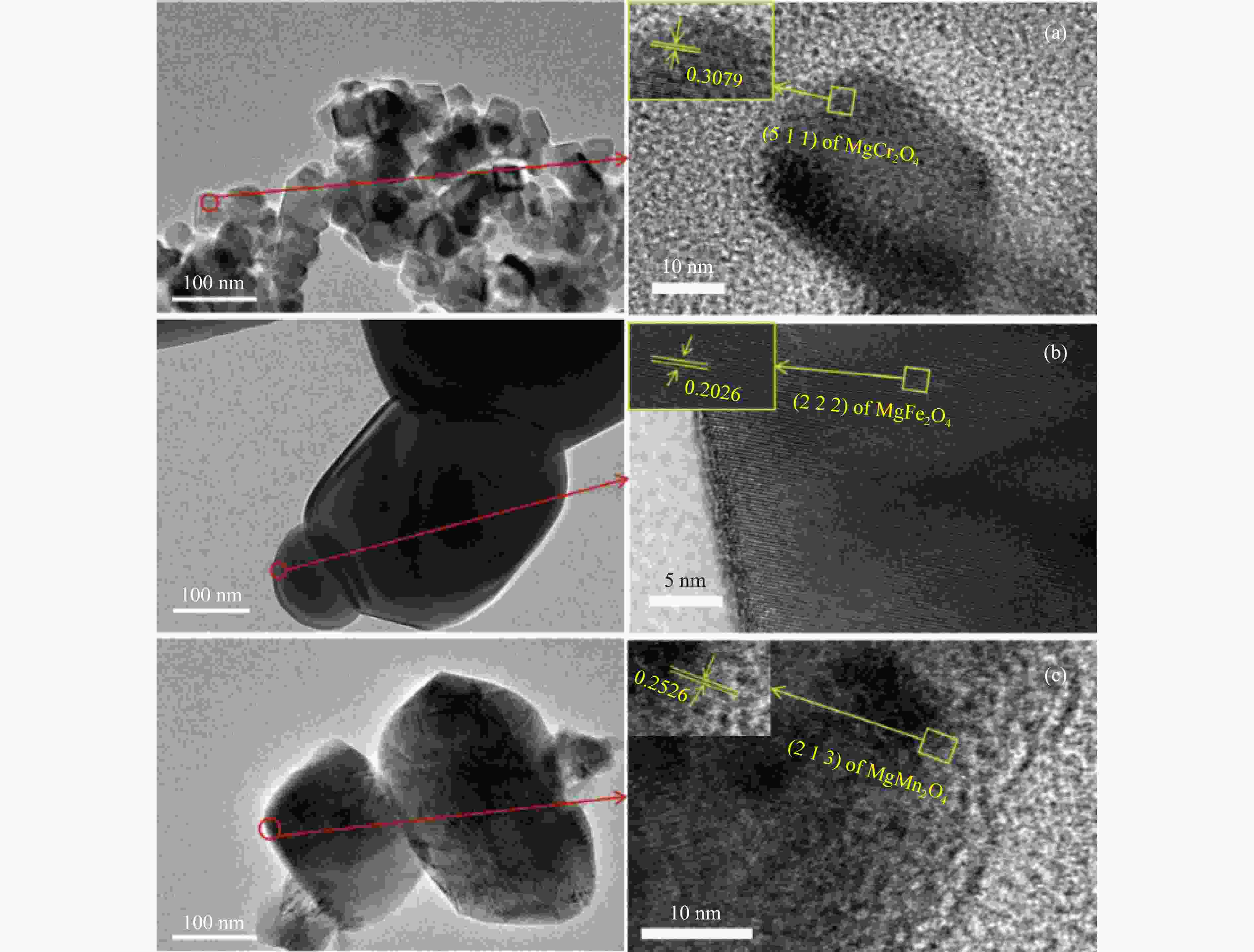
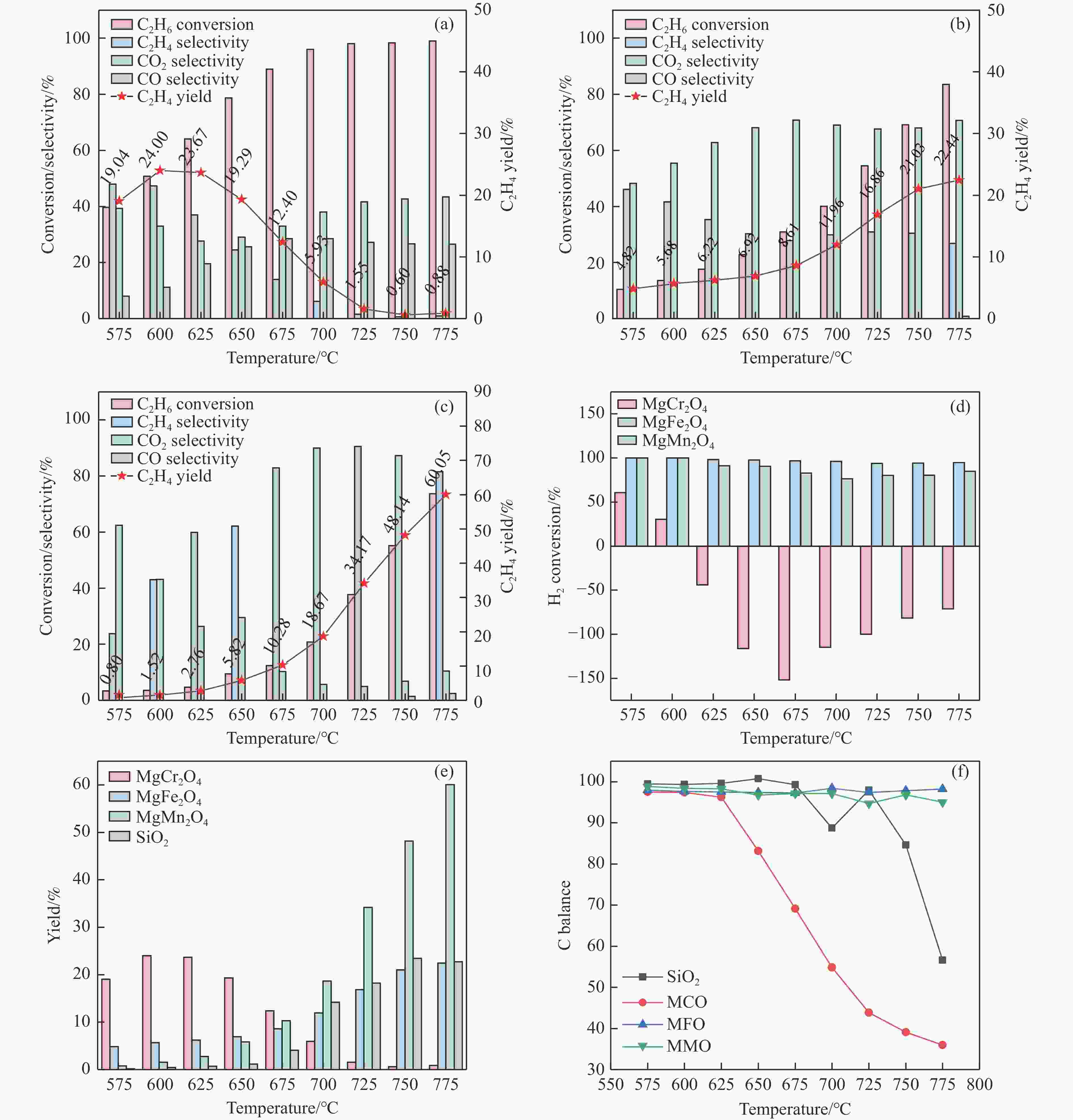
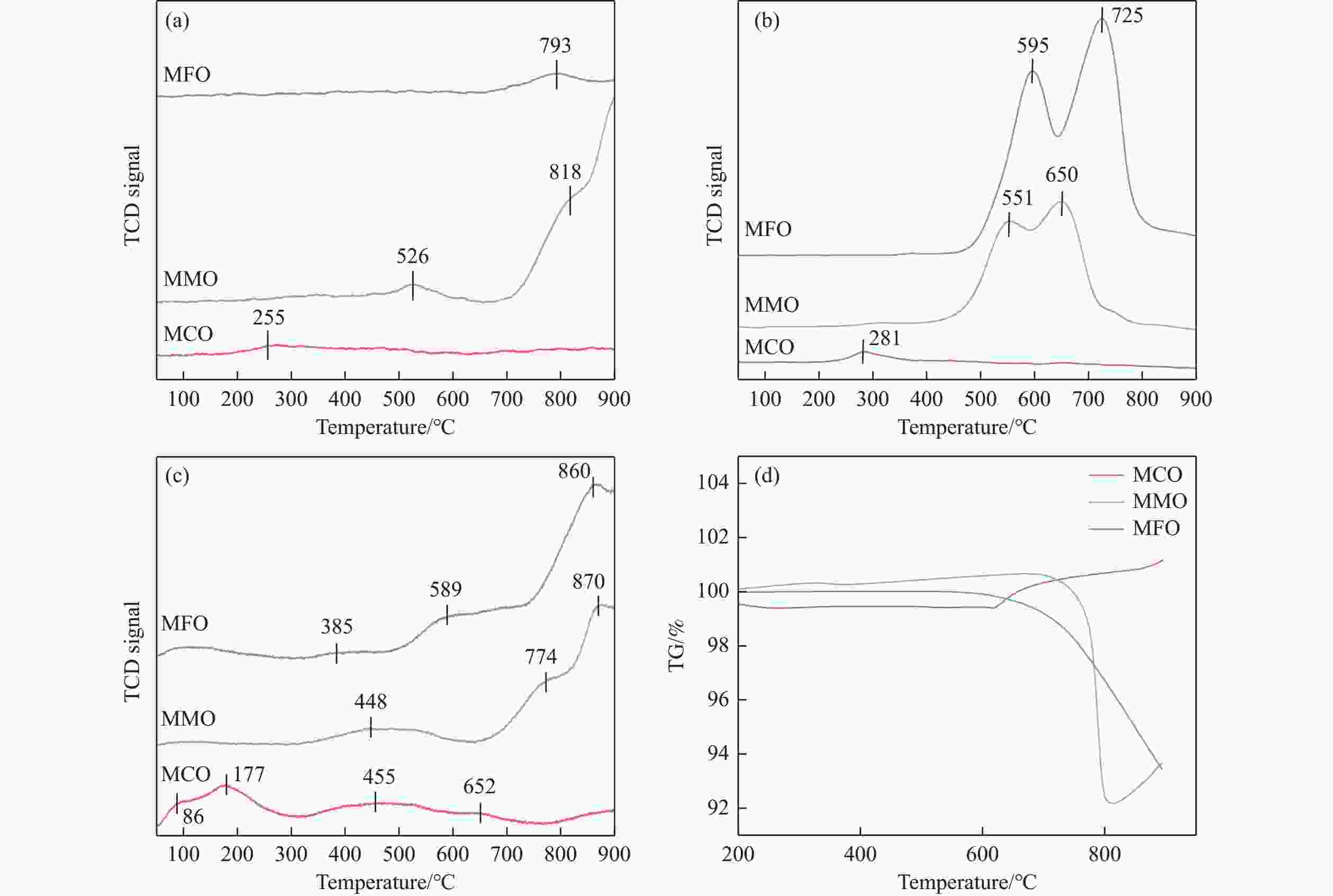
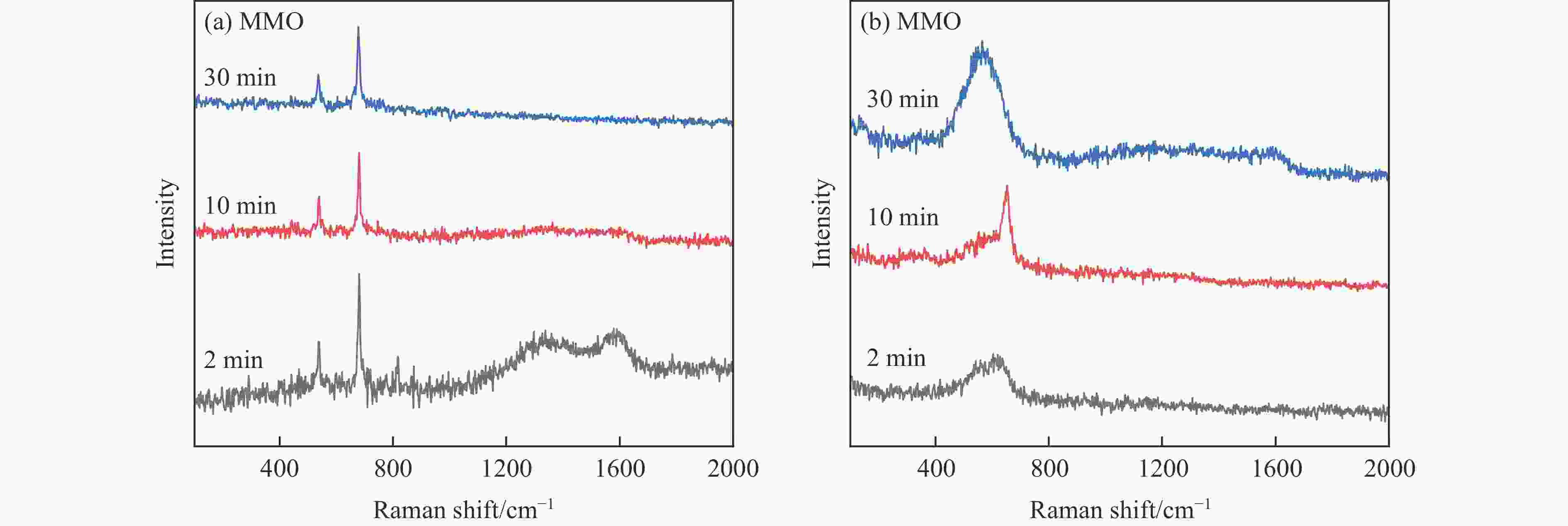
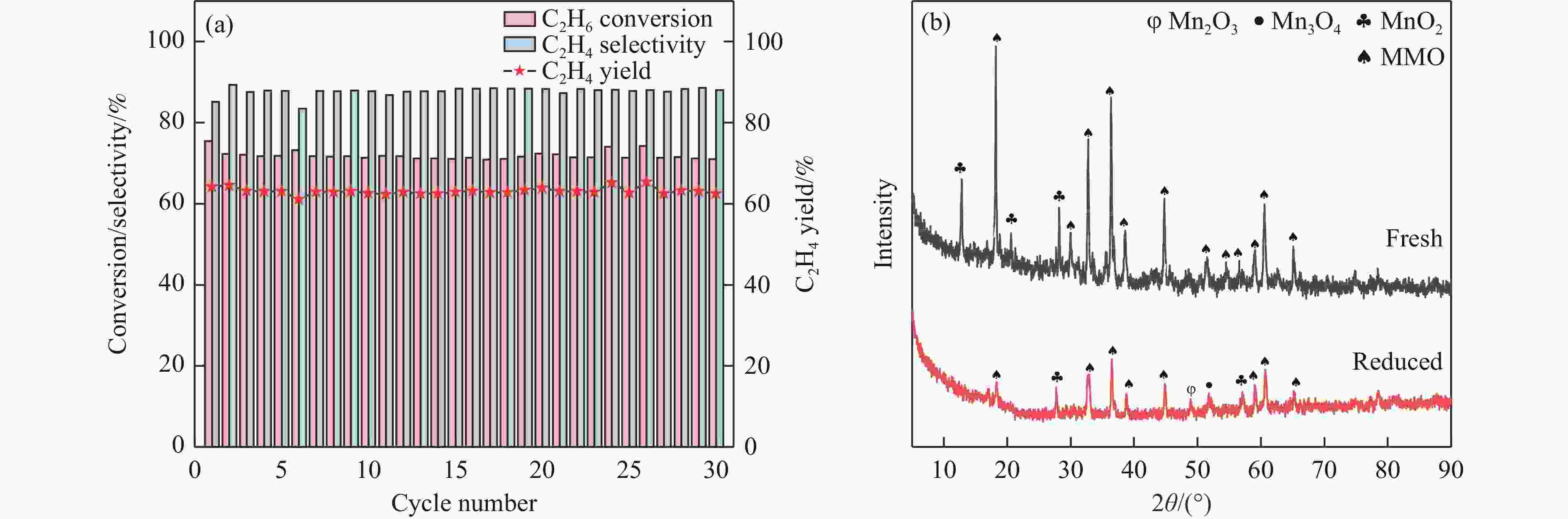
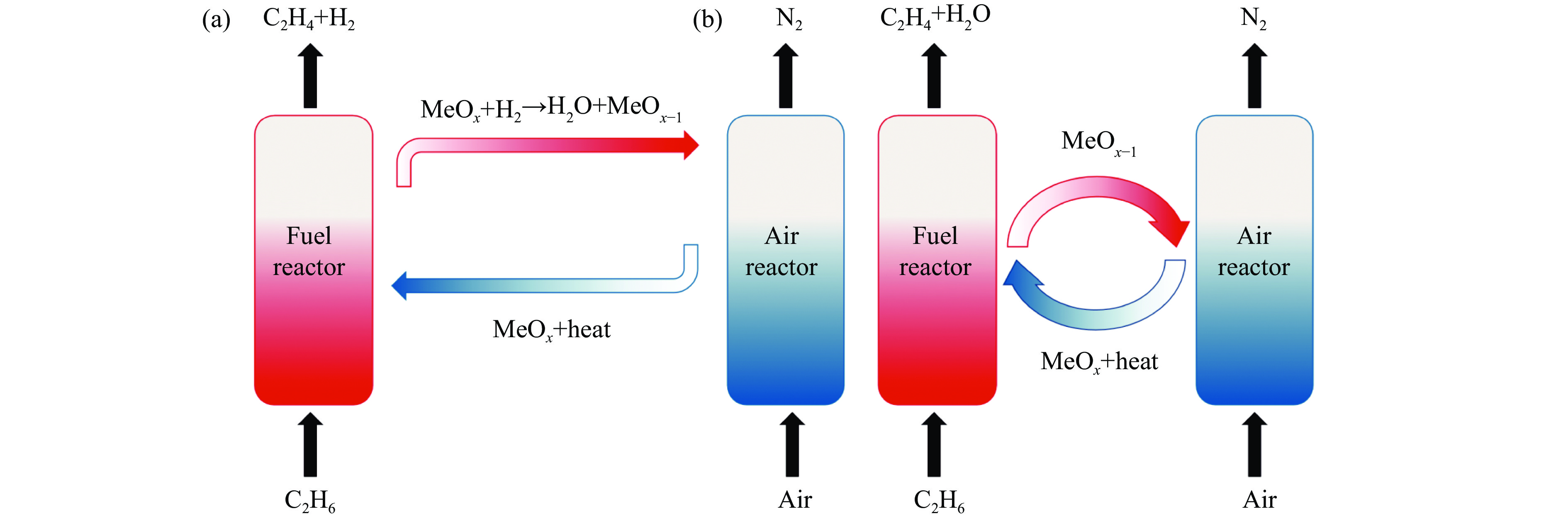
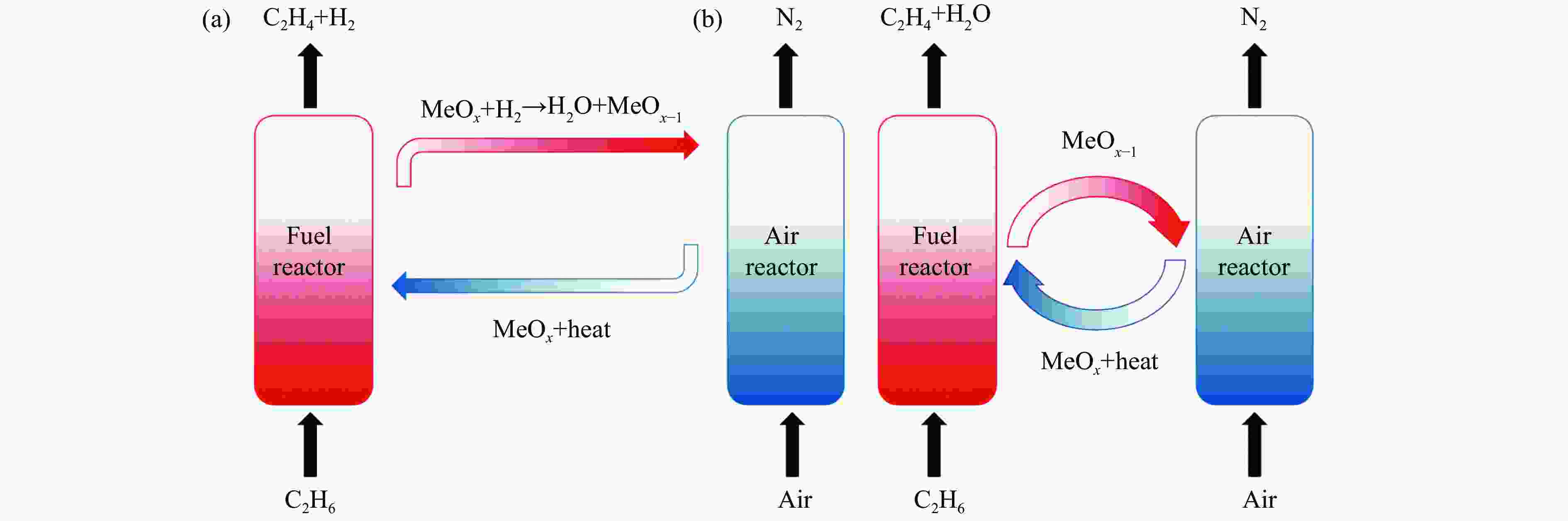
摘要: 化学链循环氧化脱氢技术(CL-ODH)是一个多功能的平台,它可以利用载氧体中晶格氧的选择性氧化这一特性,实现乙烷向乙烯的高值化转化。本研究探讨了MgX2O4(X=Cr、Fe或Mn)型尖晶石载氧体中B位元素对乙烷CL-ODH性能的影响。采用固定床和H2-TPR、O2-TPD、TG、原位拉曼、SEM、TEM等方法对MgX2O4尖晶石进行了性能测试和表征。结果表明,MgCr2O4仅释放微量表面吸附氧,更倾向于催化乙烷转化为焦炭和氢气。MgFe2O4通过提供更多的表面晶格氧,促进乙烷深度氧化成CO2。MgMn2O4载氧体在乙烷CL-ODH反应中能够释放出大量的体相晶格氧,它可以选择性燃烧氢气以推进反应正向进行,增加乙烯的选择性,实现了73.7%的乙烷转化率和81.46%的乙烯选择性。此外,MgMn2O4催化剂在乙烷CL-ODH反应中进行了30次的氧化还原循环实验,表现出稳定的反应性能,在整个循环测试中乙烯收率大约为62%。MgX2O4尖晶石氧化物中B位元素影响了其晶格氧的供应能力,从而影响了其在乙烷CL-ODH反应中的性能。
-
关键词:
- 乙烷CL-ODH /
- MgX2O4尖晶石型载氧体 /
- 乙烷生产乙烯 /
- MgMn2O4
Abstract: Chemical looping oxidative dehydrogenation (CL-ODH) provides a multifunctional conversion platform that can take advantage of the selective oxidation of lattice oxygen in oxygen carrier to achieve high-valued ethane to ethylene conversion. In this study, we explored the effect of B-site element in MgX2O4 (X=Cr, Fe, or Mn) spinel-type oxygen carriers on the performance of ethane CL-ODH. The properties test and characterization of MgX2O4 spinel were tested by fixed bed and H2-TPR, O2-TPD, TG, in-situ Raman, SEM, and TEM. The results showed that because MgCr2O4 only released a small amount of adsorbed surface oxygen, it tended to catalyze the conversion of ethane to coke and hydrogen. MgFe2O4 facilitated the deep oxidation of ethane into CO2 by providing more surface lattice oxygen. Meanwhile, since a significant amount of bulk lattice oxygen was released by the MgMn2O4 oxygen carrier, it could burn hydrogen in a targeted manner to advance the reaction and increased ethylene's selectivity. Thereby, MgMn2O4 achieved an ethane conversion of 73.7% with an ethylene selectivity of 81.46%. Furthermore, the MgMn2O4 catalyst demonstrated stable reactivity and an ethylene yield of about 62% in ethane CL-ODH over the 30 redox cycles. The screening tests indicated that the B-site elements in MgX2O4 spinel oxides could significantly influence their ability to supply lattice oxygen, thereby affecting their performance in ethane CL-ODH reaction.-
Key words:
- ethane CL-ODH /
- MgX2O4 spinel-type oxygen carrier /
- ethane to ethylene /
- MgMn2O4
-
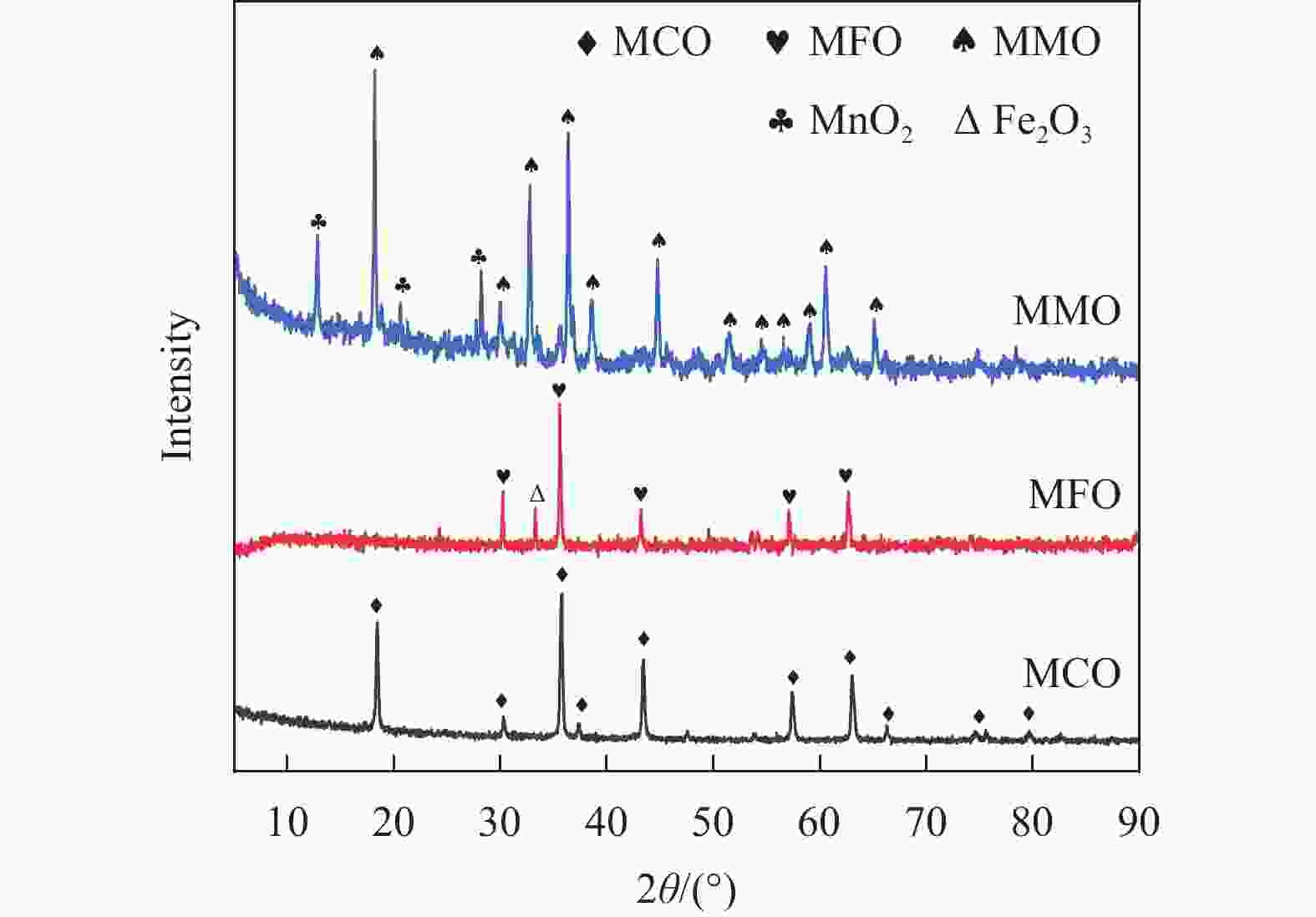
Table 1 Specific surface areas of fresh oxygen carriers of MCO, MFO and MMO
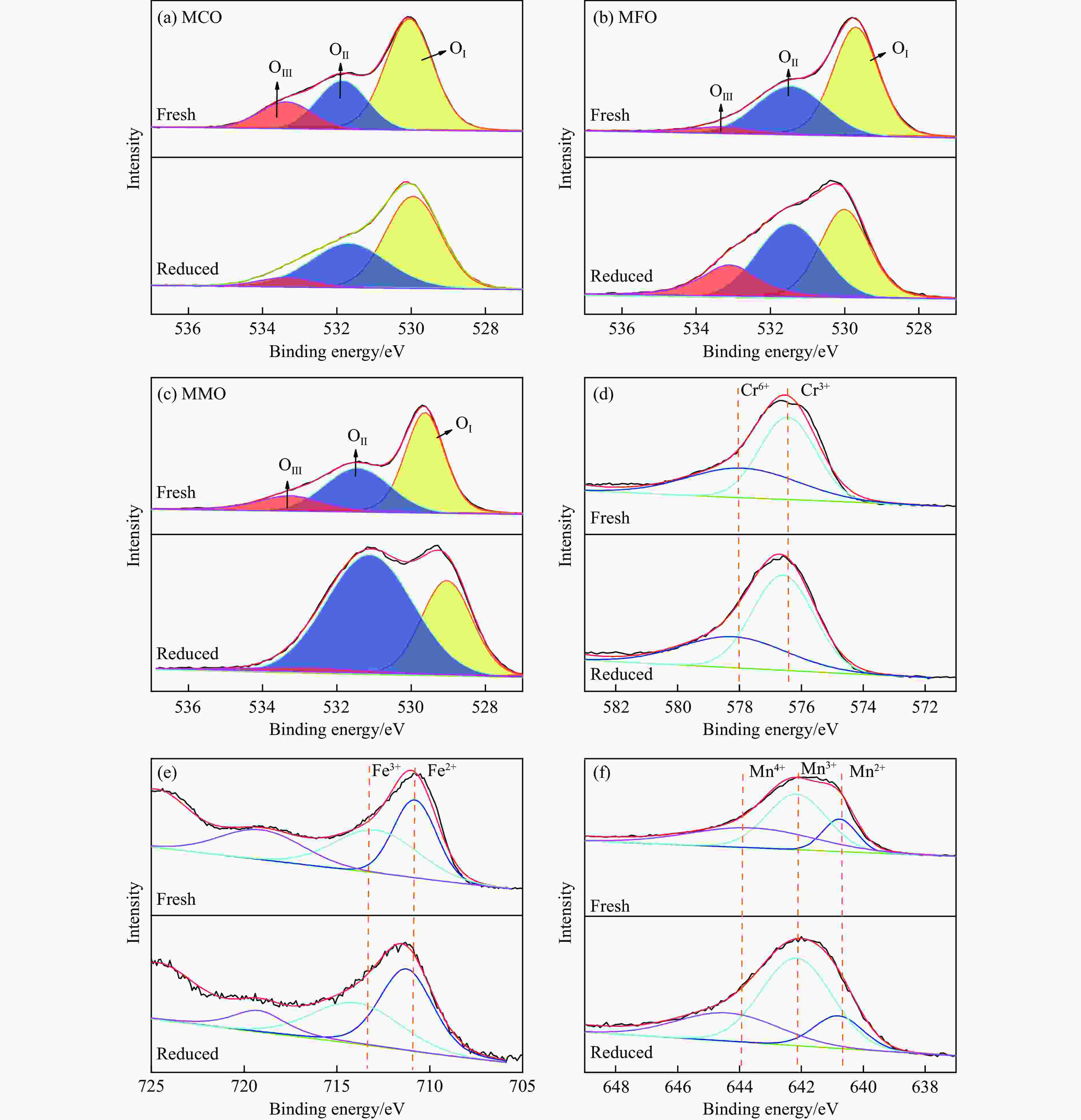
SBET/(m2·g−1) vpore/(cm3·g−1) Average pore diameter d/nm MgCr2O4 21.002 0.142 27.071 MgFe2O4 14.638 0.057 15.464 MgMn2O4 7.716 0.035 18.248 Table 2 Content changes of O 1s and B-position elements before and after reaction
OⅠ OⅡ OⅢ O(Ⅱ+Ⅲ)/ OⅠ Change of valence state of B-site ions fresh reduced fresh reduced fresh reduced fresh reduced fresh reduced MgCr2O4 59.26 54.61 25.78 39.38 14.96 6.01 0.69 0.83 Cr6+/Cr3+ 0.70 0.57 MgFe2O4 57.50 39.17 36.03 40.05 6.46 20.78 0.74 1.55 Fe3+/Fe2+ 1.07 0.76 MgMn2O4 50.71 30.81 34.12 63.00 15.16 6.19 0.97 2.24 Mn4+/(Mn3++Mn2+) 0.55 0.37 -
[1] BIKBAEVA V, NESTERENKO N, KONNOV S, et al. A low carbon route to ethylene: Ethane oxidative dehydrogenation with CO2 on embryonic zeolite supported Mo-carbide catalyst[J]. Appl Catal B: Environ, 2023, 320. [2] CANCINO-TREJO F, SANTES V, CARDENAS J A A, et al. Active Ni and Fe species on catalysts Ni/Al2O3 and NiFe/Al2O3 for the oxidative dehydrogenation (ODH) of ethane to ethylene assisted by CO2[J]. Chem Eng J Adv, 2022, 12. [3] KONG L, LI J, LIU Q, et al. Promoted catalytic performances of highly dispersed V-doped SBA-16 catalysts for oxidative dehydrogenation of ethane to ethylene[J]. J Energy Chem,2016,25(4):577−586. doi: 10.1016/j.jechem.2016.04.004 [4] RILEY C R, RIVA A D L, IBARRA I L, et al. Achieving high ethylene yield in non-oxidative ethane dehydrogenation[J]. Appl Catal A: Gen, 2021, 624. [5] SYOENS S H, OLAHOVA N, MUÑOA GANDARILLAS A E, et al. State-of-the-art of coke formation during steam cracking: Anti-coking surface technologies[J]. Ind Eng Chem Res,2018,57(48):16117−16136. doi: 10.1021/acs.iecr.8b03221 [6] SEKI H, SAITO H, TOKO K, et al. Effect of Ba addition to Ga-α-Al2O3 catalyst on structure and catalytic selectivity for dehydrogenation of ethane[J]. Appl Catal A: Gen,2019,581:23−30. doi: 10.1016/j.apcata.2019.05.008 [7] LI X, ZHOU Y, QIAO B, et al. Enhanced stability of Pt/Al2O3 modified by Zn promoter for catalytic dehydrogenation of ethane[J]. J Energy Chem,2020,51:14−20. doi: 10.1016/j.jechem.2020.03.045 [8] NAKAGAWA K, KAJITA C, OKUMURA K, et al. Role of carbon dioxide in the dehydrogenation of ethane over gallium-loaded catalysts[J]. J Catal,2001,203(1):87−93. doi: 10.1006/jcat.2001.3306 [9] LUONGO G, DONAT F, BORKk A H, et al. Highly selective oxidative dehydrogenation of ethane to ethylene via chemical looping with oxygen uncoupling through structural engineering of the oxygen carrier[J]. Adv Energy Mater, 2022, 12 (23). [10] PING L, ZHANG Y, WANG B, et al. Unraveling the surface state evolution of IrO2 in ethane chemical looping oxidative dehydrogenation[J]. ACS Catal,2023,13(2):1381−1399. doi: 10.1021/acscatal.2c05770 [11] HUANG X, YANG Z, QIU J, et al. Ethylene production over A/B-site doped BaCoO3 perovskite by chemical looping oxidative dehydrogenation of ethane[J]. Fuel, 2022, 327. [12] BRODY L, NEAL L, LIU J, et al. Autothermal chemical looping oxidative dehydrogenation of ethane: Redox catalyst performance, longevity, and process analysis[J]. Energy Fuels,2022,36(17):9736−9744. doi: 10.1021/acs.energyfuels.2c01293 [13] TIJANI M M, MOSTAFAVI E, MAHINPEY N. Process simulation and thermodynamic analysis of a chemical looping combustion system using methane as fuel and NiO as the oxygen carrier in a moving-bed reactor[J]. Chem Eng Process, 2019, 144. [14] TIAN X, ZHENG C, LI F, et al. Co and Mo Co-doped Fe2O3 for selective ethylene production via chemical looping oxidative dehydrogenation[J]. ACS Sustainable Chem Eng,2021,9(23):8002−8011. doi: 10.1021/acssuschemeng.1c02726 [15] CAI R, BRODY L, TIAN Y, et al. Numerical modeling of chemical looping oxidative dehydrogenation of ethane in parallel packed beds[J]. Chem Eng J , 2023, 469. [16] HARIBAL V P, NEAL L M, LI F. Oxidative dehydrogenation of ethane under a cyclic redox scheme-Process simulations and analysis[J]. Energy,2017,119:1024−1035. doi: 10.1016/j.energy.2016.11.039 [17] DE LAS OBRAS LOSCERTALES M, ABAD A, GARCÍA-LABIANO F, et al. Reaction kinetics of a NiO-based oxygen carrier with ethanol to be applied in chemical looping processes[J]. Fuel, 2023, 345. [18] YUSUF S, NEAL L M, LI F. Effect of promoters on manganese-containing mixed metal oxides for oxidative dehydrogenation of ethane via a cyclic redox scheme[J]. ACS Catal,2017,7(8):5163−5173. doi: 10.1021/acscatal.7b02004 [19] WANG J, LIANG X, XING Z et al. Ce-doped LaMnO3 redox catalysts for chemical looping oxidative dehydrogenation of ethane[J]. Catalysts, 2023, 13(1). [20] ELBADAWI A H, OSMAN M S, RAZZAKk S A, et al. VO x-Nb/La-γAl2O3 catalysts for oxidative dehydrogenation of ethane to ethylene[J]. J Taiwan Inst Chem Eng,2016,61:106−116. doi: 10.1016/j.jtice.2016.01.003 [21] TIAN X, ZHENG C, ZHAO H, et al. Ce-modified SrFeO3-for ethane oxidative dehydrogenation coupled with CO2 splitting via a chemical looping scheme[J]. Appl Catal B: Environ, 2022, 303. [22] GAO Y, NEAL L M, LI F. Li-promoted La xSr2− xFeO4−δ core-shell redox catalysts for oxidative dehydrogenation of ethane under a cyclic redox scheme[J]. ACS Catal,2016,6(11):7293−7302. doi: 10.1021/acscatal.6b01399 [23] YUSUF S, NEAL L, BAO Z, et al. Effects of sodium and tungsten promoters on Mg6MnO8-based core-shell redox catalysts for chemical looping—Oxidative dehydrogenation of ethane[J]. ACS Catal,2019,9(4):3174−3186. doi: 10.1021/acscatal.9b00164 [24] YUSUF S, NEAL L, HARIBAL V, et al. Manganese silicate based redox catalysts for greener ethylene production via chemical looping – Oxidative dehydrogenation of ethane[J]. Appl Catal B: Environ,2018,232:77−85. doi: 10.1016/j.apcatb.2018.03.037 [25] BORTOLOZZI J P, WEISS T, GUTIERREZ L B, et al. Comparison of Ni and Ni-Ce/Al2O3 catalysts in granulated and structured forms: Their possible use in the oxidative dehydrogenation of ethane reaction[J]. Chem Eng J,2014,246:343−352. doi: 10.1016/j.cej.2014.02.078 [26] JI X, LIU Y, LIU J, et al. Na2WO4-tuned manganese ore as a high-effective redox catalyst for selective hydrogen combustion in the presence of methane and benzene[J]. Appl Catal B: Environ, 2022, 307. [27] LI M, VAN VEEN A C. Selective production of ethylene via continuous oxidative dehydrogenation of ethane in (Dy2O3/MgO)-(Li-K) Cl composite membrane reactor[J]. Chem Eng J,2019,365:344−350. doi: 10.1016/j.cej.2018.12.106 [28] XIN C, WANG F, XU G Q. Tuning surface V5+ concentration in M1 phase MoVSbOx catalysts for ethylene production from ethane through oxidative dehydrogenation reaction[J]. Appl Catal A: Gen, 2021, 610. [29] QASIM M, AYOUB M, GHAZALI N A, et al. Recent advances and development of various oxygen carriers for the chemical looping combustion process: A review[J]. Ind Eng Chem Res,2021,60(24):8621−8641. doi: 10.1021/acs.iecr.1c01111 [30] MISHRA A, DUDEK R, GAFFNEY A, et al. Spinel oxides as coke-resistant supports for NiO-based oxygen carriers in chemical looping combustion of methane[J]. Catal Today, 2019. [31] WANG T, GAO Y, LIU Y, et al. Core-shell Na2WO4/CuMn2O4 oxygen carrier with high oxygen capacity for chemical looping oxidative dehydrogenation of ethane[J]. Fuel, 2021, 303. [32] LIU F, LIU J, LI Y, et al. Studies on the synergistically improved reactivity of spinel NiFe2O4 oxygen carrier for chemical-looping combustion[J]. Energy, 2022, 239. [33] ZHAO P, EHARA M, SATSUMA A, et al. Theoretical study of the propene combustion catalysis of chromite spinels: Reaction mechanism and relation between the activity and electronic structure of Spinels[J]. J Phys Chem C,2021,125(47):25983−26002. doi: 10.1021/acs.jpcc.1c06760 [34] HU J, ZHAO W, HU R, et al. Catalytic activity of spinel oxides MgCr2O4 and CoCr2O4 for methane combustion[J]. Mater Res Bull,2014,57:268−273. doi: 10.1016/j.materresbull.2014.06.001 [35] DE HOYOS-SIFUENTES D H, RESÉDIZ-HERNÁNDEZ P J, DÍAIZ-GUILLÉN J A, et al. Synthesis and characterization of MgFe2O4 nanoparticles and PEG-coated MgFe2O4 nanocomposite[J]. J Mater Res Technol,2022,18:3130−3142. doi: 10.1016/j.jmrt.2022.03.117 [36] ALAMDARI A, KARIMZADEH R, ABBASIZADEH S. Present state of the art of and outlook on oxidative dehydrogenation of ethane: Catalysts and mechanisms[J]. Rev Chem Eng,2021,37(4):481−532. doi: 10.1515/revce-2017-0109 [37] GAO Y, NEAL L, DING D et al. Recent advances in intensified ethylene production—A review[J]. ACS Catal,2019,9(9):8592−8621. doi: 10.1021/acscatal.9b02922 [38] DING W, ZHAO K, JIANG S, et al. Alkali-metal enhanced LaMnO3 perovskite oxides for chemical looping oxidative dehydrogenation of ethane[J]. Appl Catal A, 2021, 609. [39] GONG W, WANG T, WANG L et al. High-performance of CrOx/HZSM-5 catalyst on non-oxidative dehydrogenation of C2H6 to C2H4: Effect of supporting materials and associated mechanism[J]. Fuel Process. , 2022, 233. [40] MALLESWARA RAO T V, ZAHIDI E M, SAYARI A. Ethane dehydrogenation over pore-expanded mesoporous silica-supported chromium oxide: 2. Catalytic properties and nature of active sites[J]. J Mol Catal A Chem,2009,301(1/2):159−165. doi: 10.1016/j.molcata.2008.12.027 [41] YANH H, XU L, CHEN M, et al. Facilely fabricating highly dispersed Ni-based catalysts supported on mesoporous MFI nanosponge for CO2 methanation[J]. Microporous Mesoporous Mater. , 2020, 302. [42] AMINI E, REZAEI M, SADEGHINIA M. Low temperature CO oxidation over mesoporous CuFe2O4 nanopowders synthesized by a novel sol-gel method[J]. Chin J Catal,2013,34(9):1762−1767. doi: 10.1016/S1872-2067(12)60653-6 [43] SONG D, LIN Y, LI C, et al. Review on Migration and Transformation of Lattice Oxygen during Chemical Looping Conversion: Advances and Perspectives[J]. Energy Fuels,2023,37(8):5743−5756. doi: 10.1021/acs.energyfuels.3c00402 [44] SONG D, LIN Y, FANG S, et al. Unraveling the atomic interdiffusion mechanism of NiFe2O4 oxygen carriers during chemical looping CO2 conversion[J]. Carbon Ener, 2023, e493. [45] DOU J, FUNDERBURG J, YANG K, et al. CexZr1–xO2-supported CrOx catalysts for CO2-assisted oxidative dehydrogenation of propane—Probing the active sites and strategies for enhanced stability[J]. ACS Catal , 2023-10-22. [46] POST J E, MCKEOWN D A, HEANEY A P J. Raman spectroscopy study of manganese oxides: Layer structures[J]. Am Mineral,2021,106(3):351−366. doi: 10.2138/am-2021-7666 [47] BECHGAARD T K, SCANNELL G, HUANG L, et al. Structure of MgO/CaO sodium aluminosilicate glasses: Raman spectroscopy study[J]. J Non Cryst Solids,2017,470:145−151. doi: 10.1016/j.jnoncrysol.2017.05.014 -




 下载:
下载: