Study on the catalytic performance of fe in situ modified small crystallite silicalite-1 zeolite in chichibabin condensation reaction
-
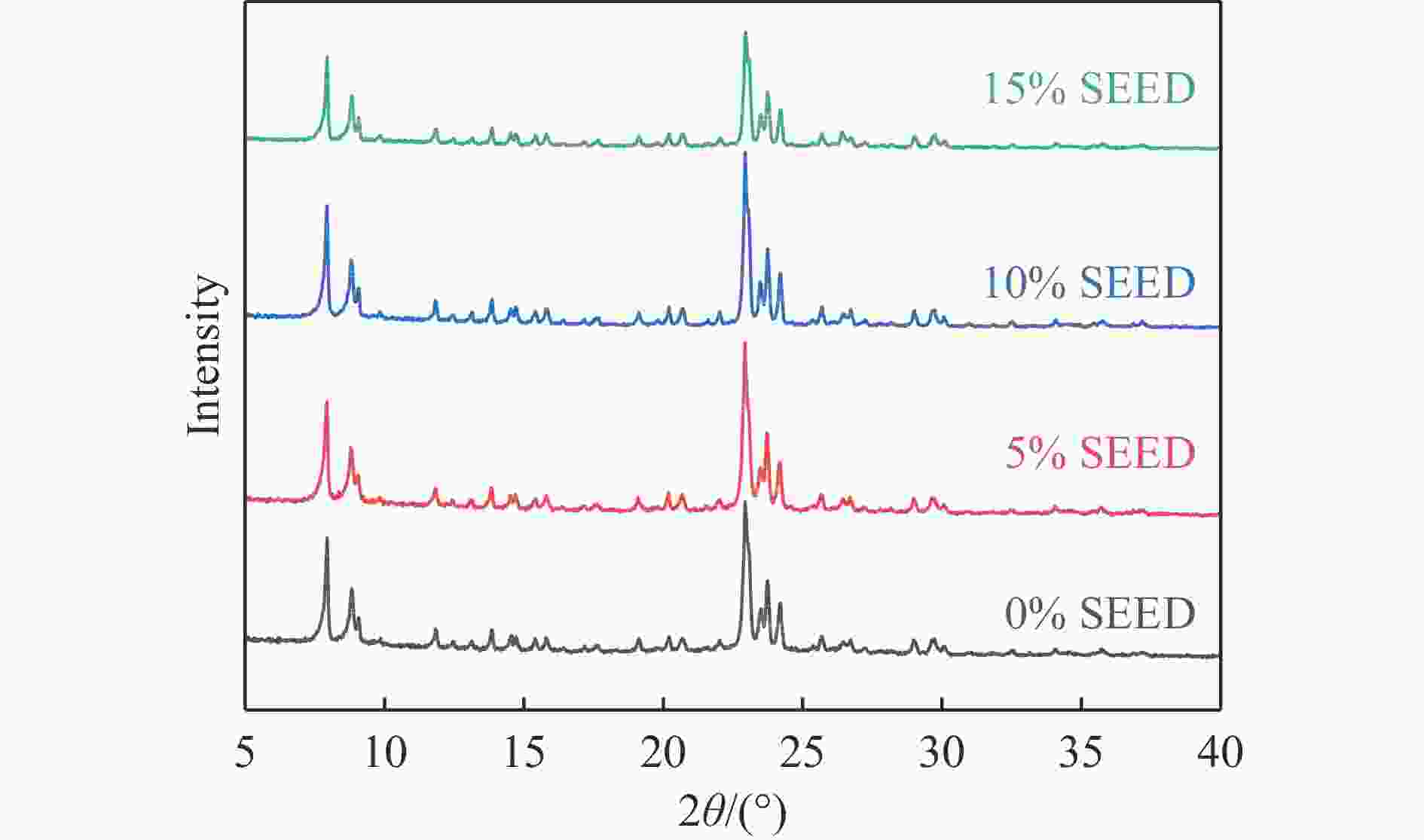
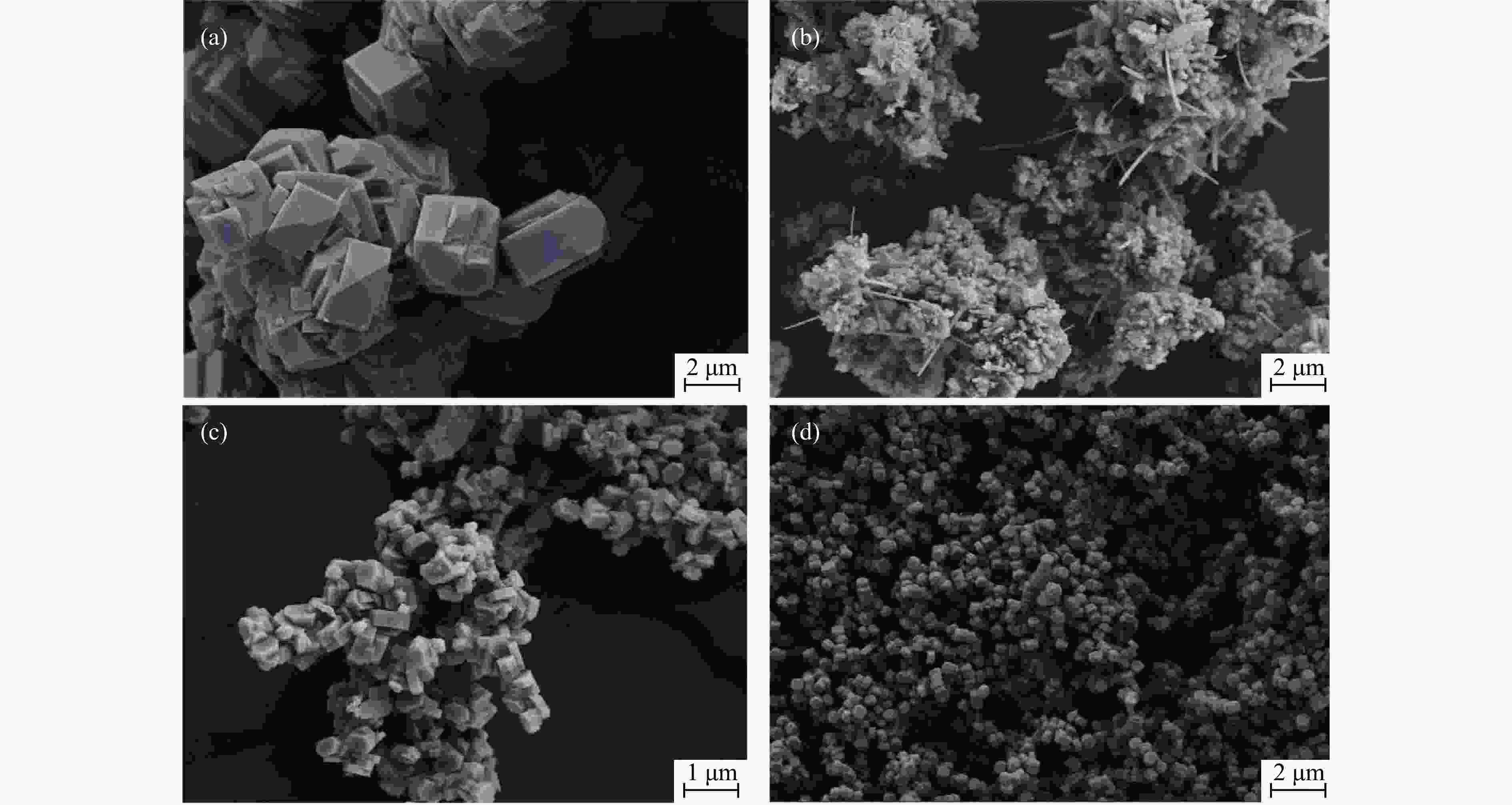
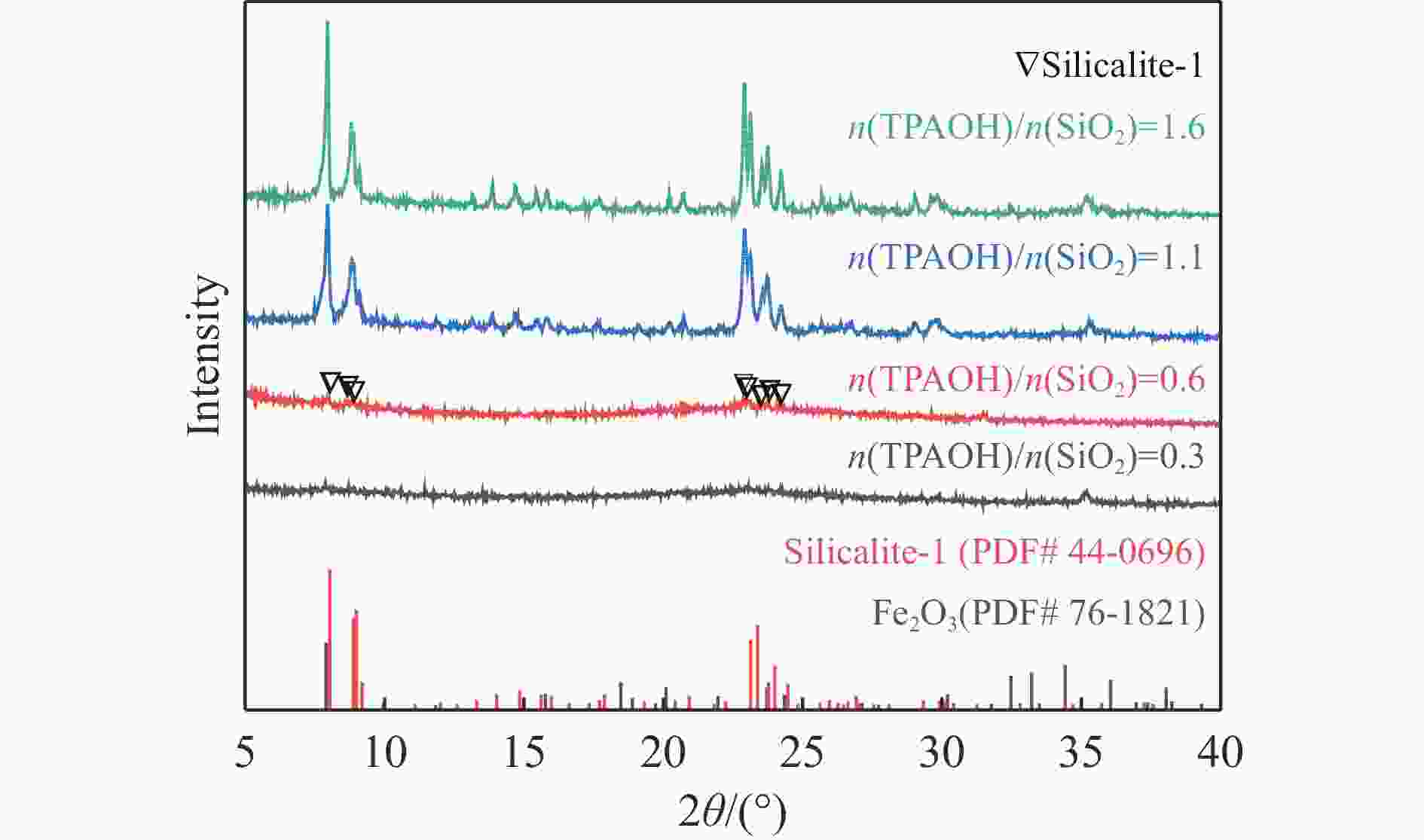
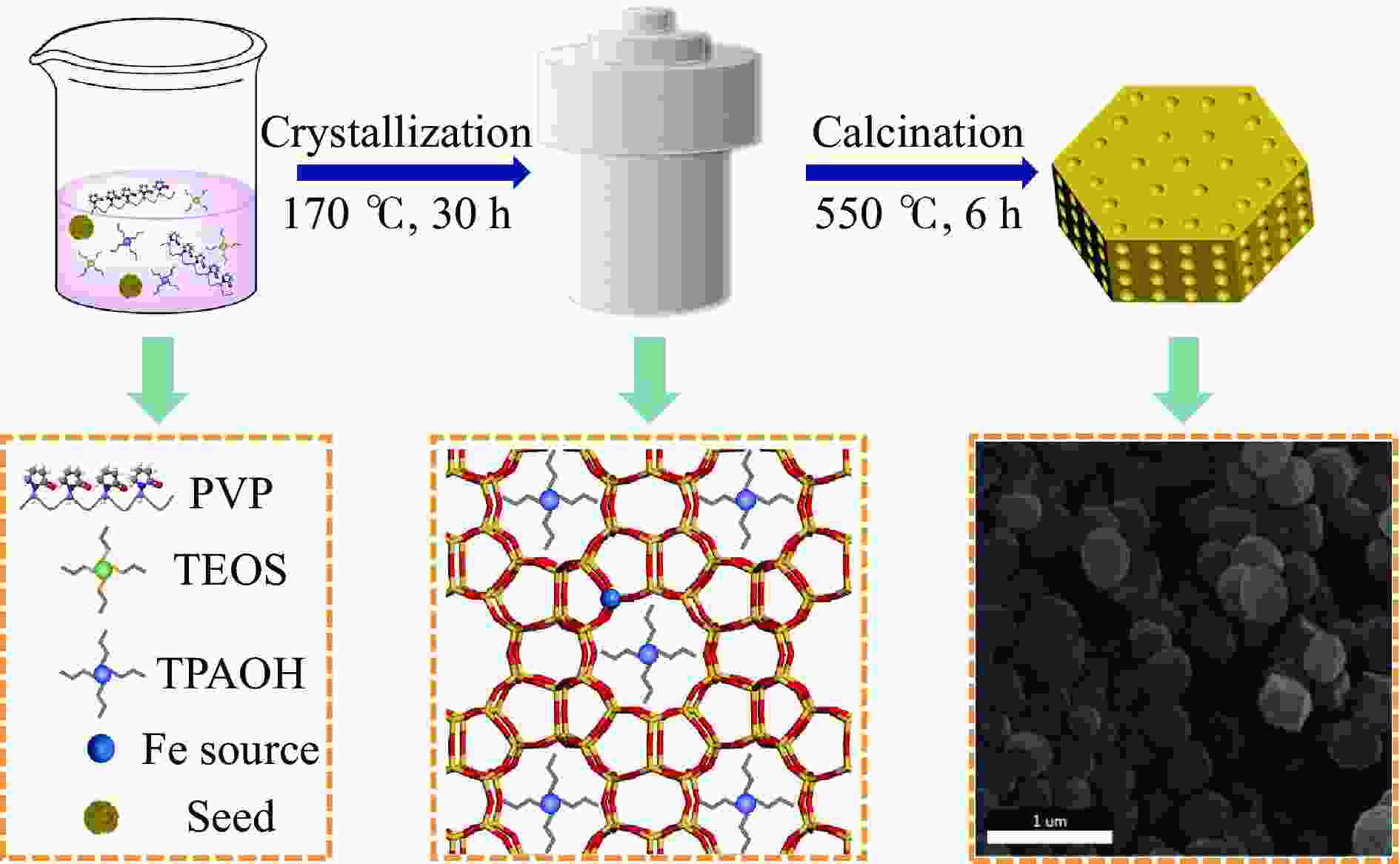
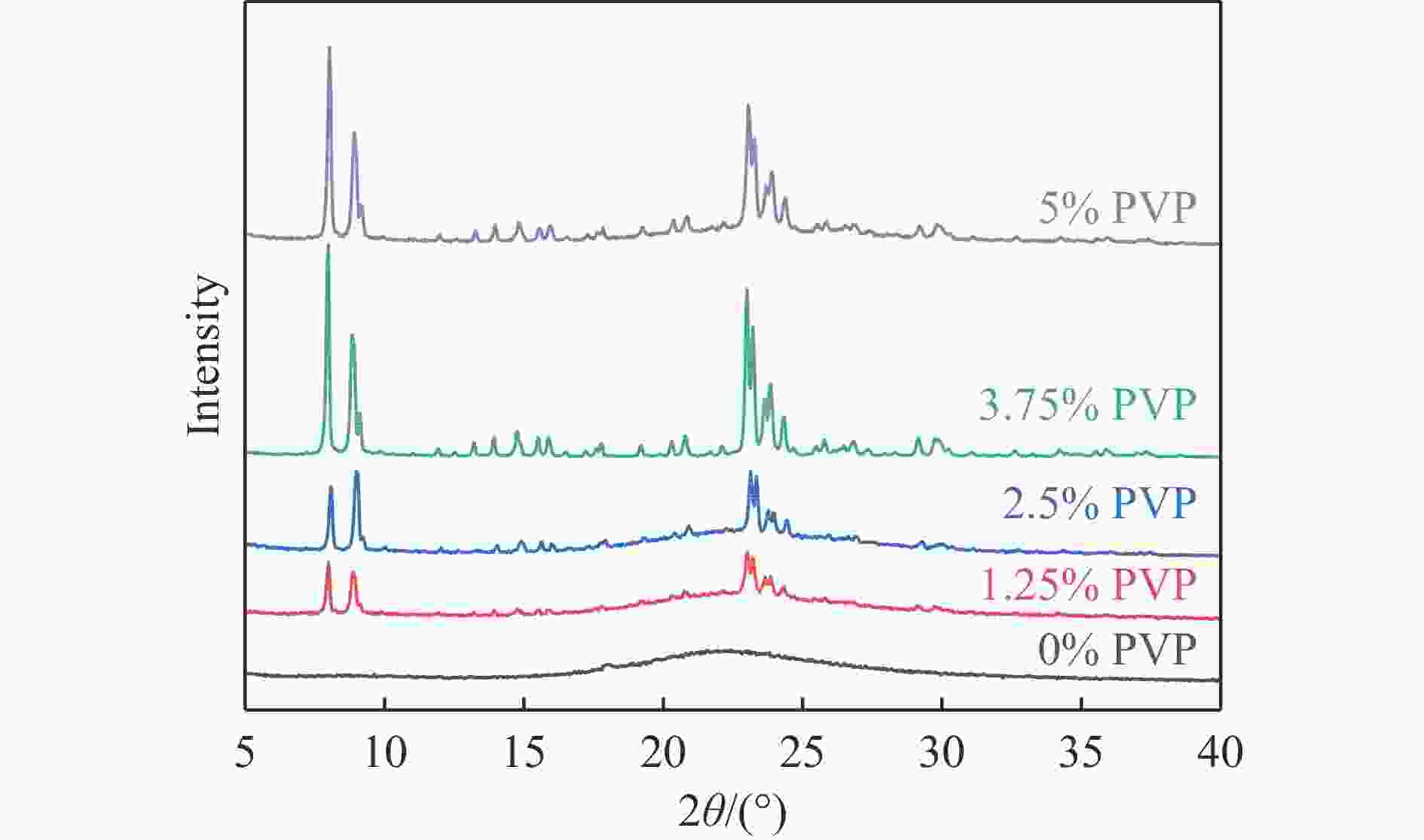
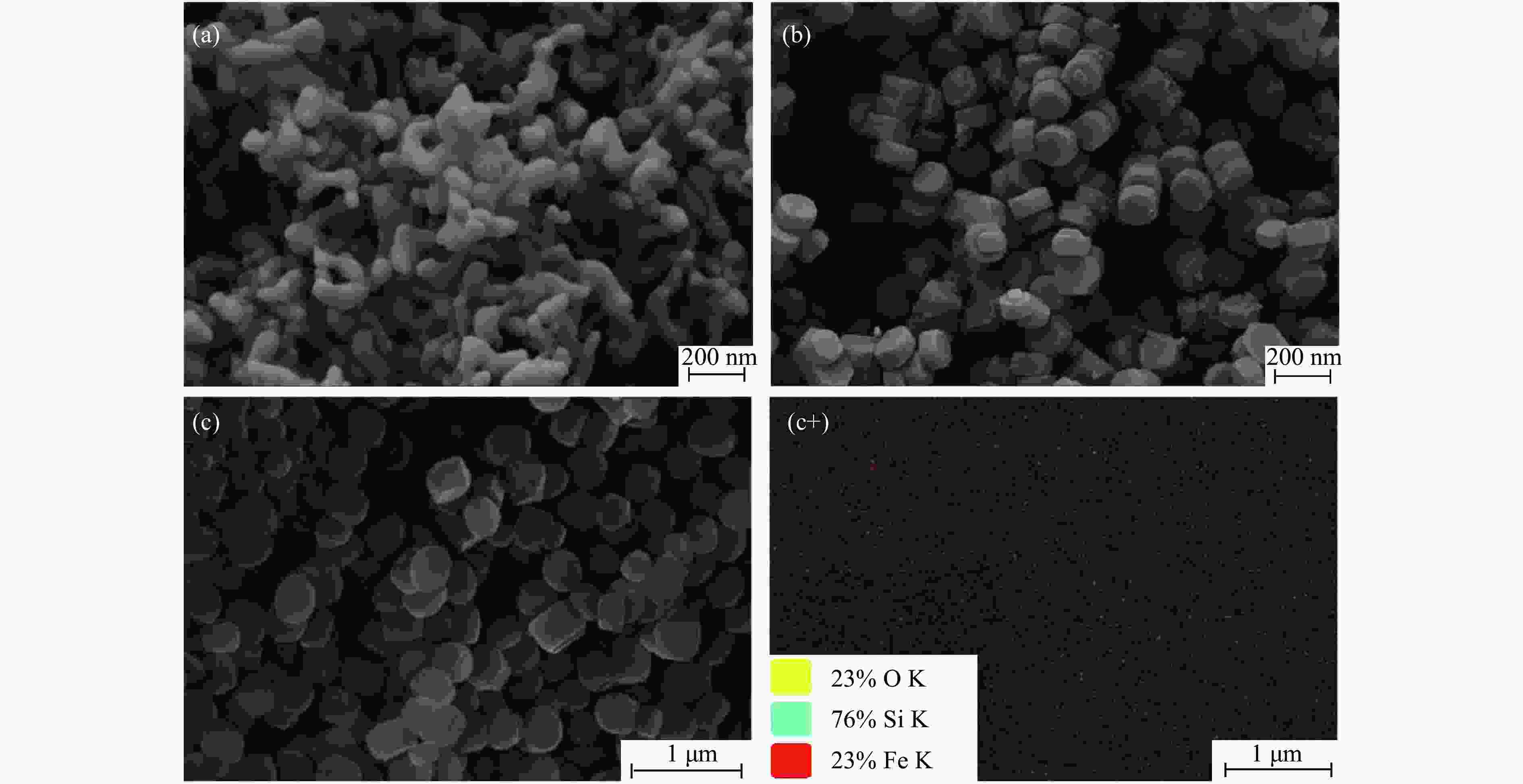
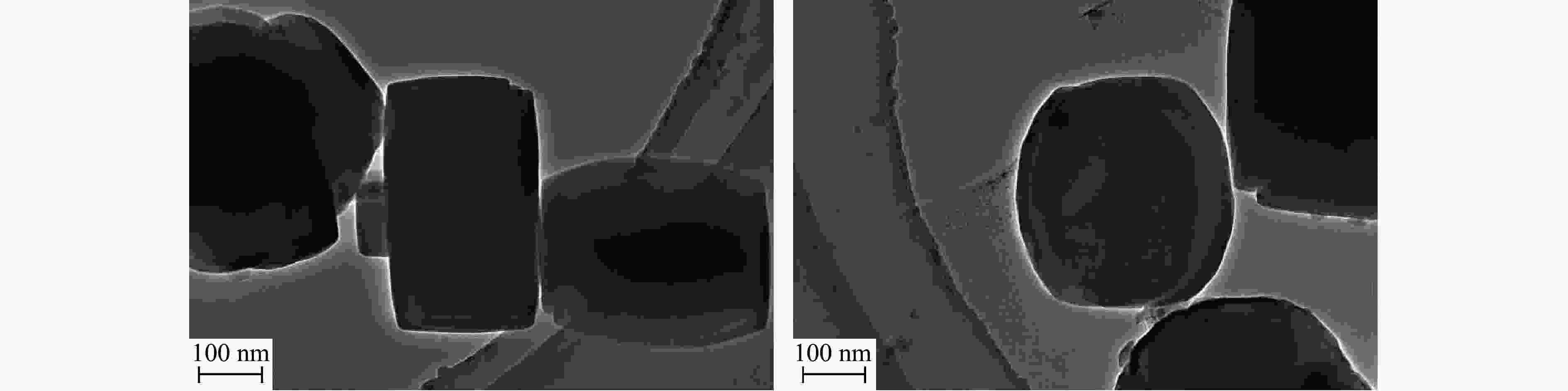
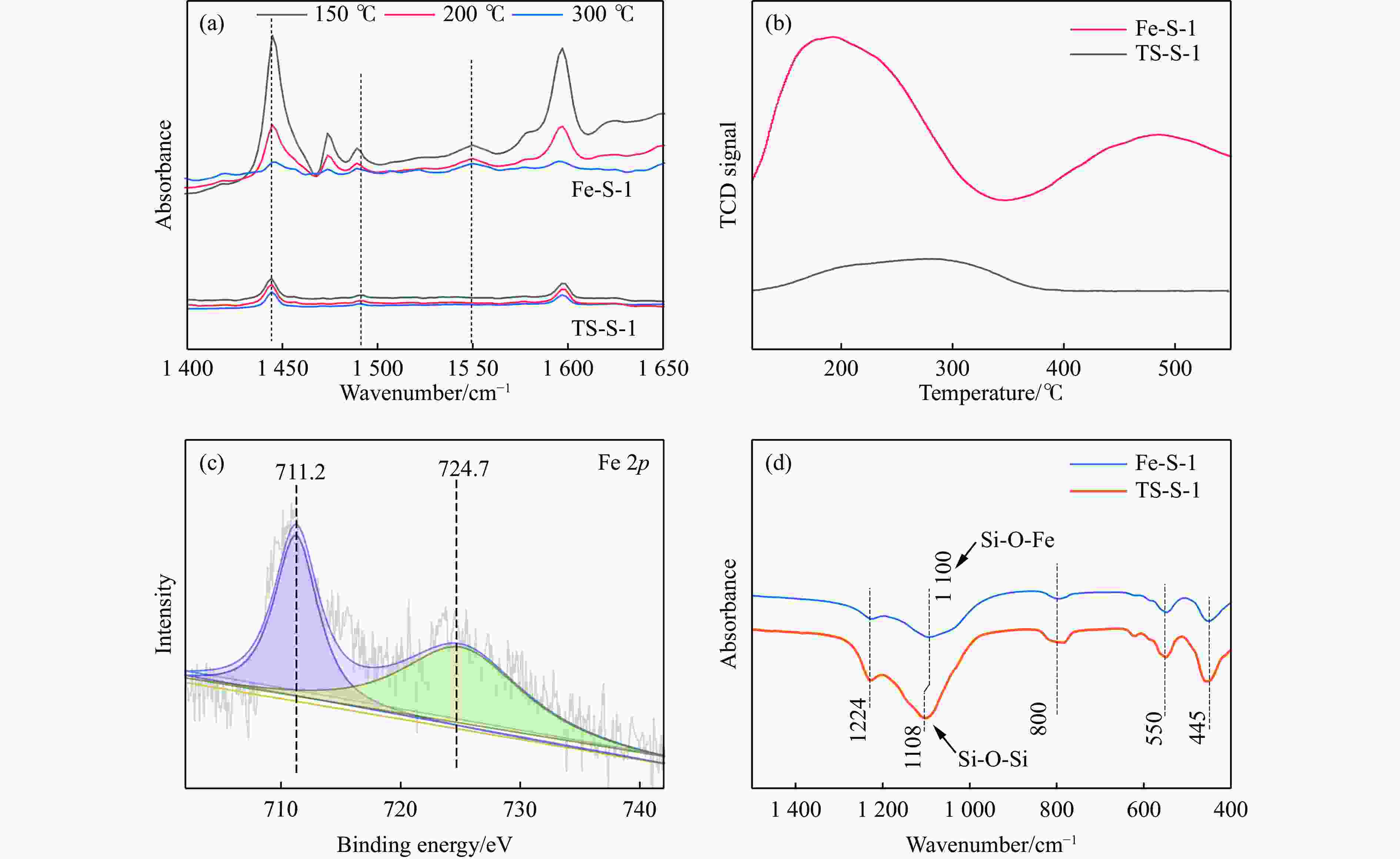
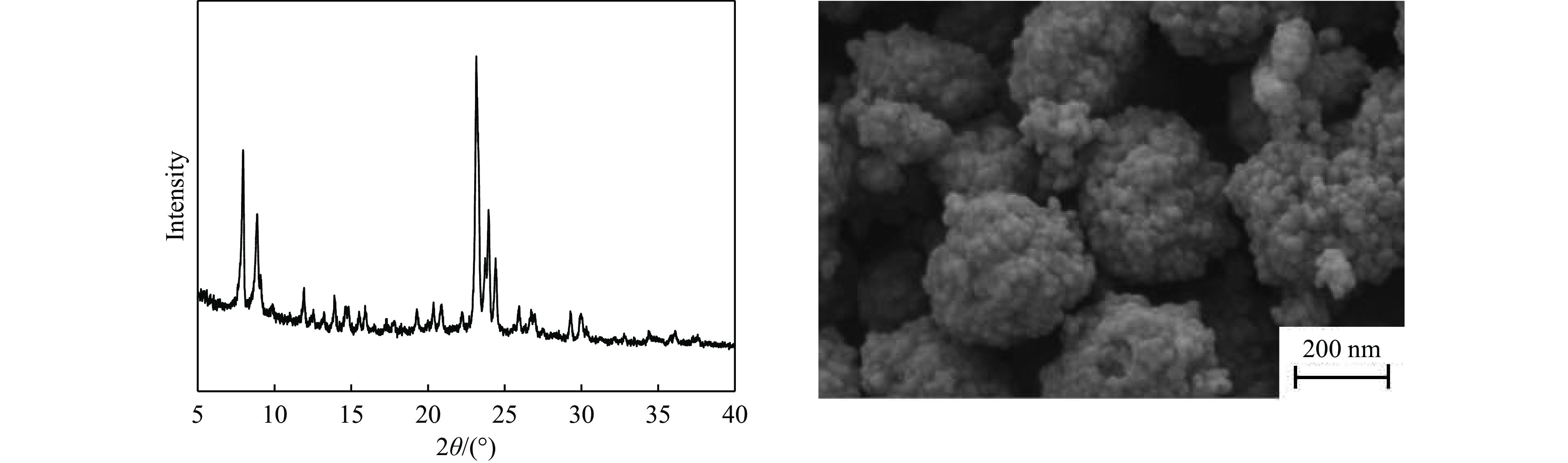
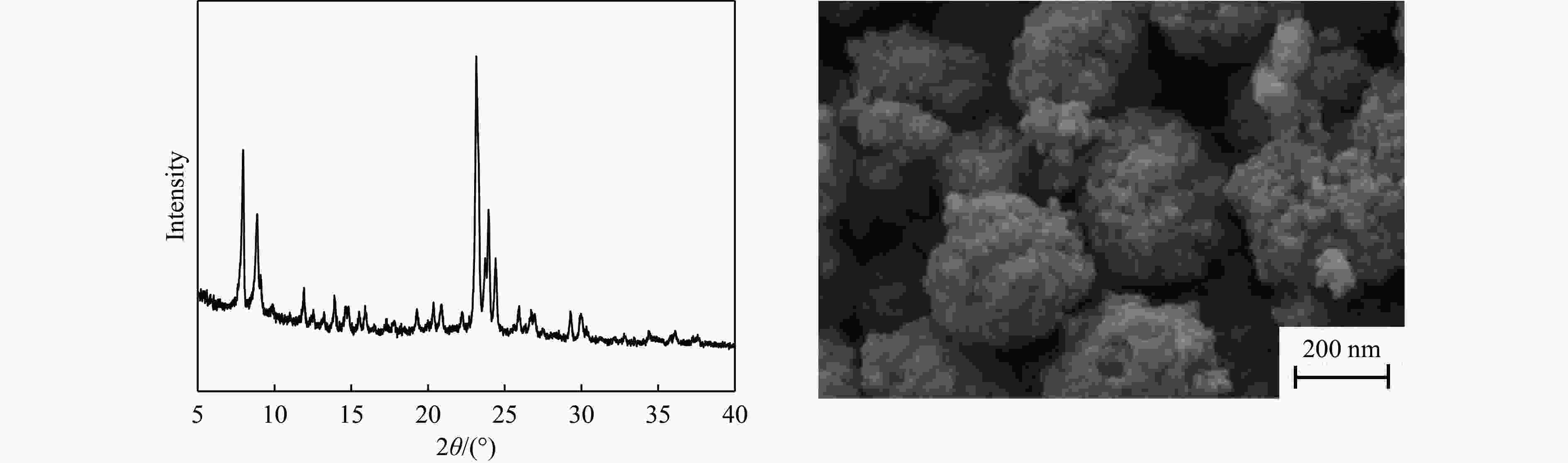
摘要: 吡啶及其衍生物统称为吡啶碱,其广泛应用于农药、医药等领域。Chichibabin醛氨缩合反应是目前工业制取吡啶碱最广泛的路线。目前,使用最广泛的ZSM-5分子筛受制于硅铝骨架结构的不稳定性,高活性反应周期较短(5 h),针对这一问题,本研究选用热稳定性、水热稳定性优异的Silicalite-1分子筛,使用聚乙烯吡咯烷酮(PVP)作为胶体分散剂,在水热合成分子筛的过程中向骨架中引入Fe,结合XRD、SEM、TG、BET、NH3-TPD、Py-FTIR等表征方法探究了晶化条件对Silicalite-1分子筛结晶度、孔结构和酸性质的影响。实验结果表明,在晶种投入量15%、PVP添加量3.75%时产品相对结晶度达到最高(103%),粒径约为200 nm。改性后的Silicalite-1具有更丰富的酸位点,醛氨缩合反应的初始活性由66%增加至85%,在反应进行15 h后,原料转化率和吡啶碱收率分别保持在66%和40%以上。研究提出的原位改性Silicalite-1分子筛策略极大扩宽了纯硅沸石在酸催化领域的应用,具有显著的科研价值和工业化潜力。
-
关键词:
- Silicalite-1分子筛 /
- 原位改性 /
- 水热法 /
- 催化剂 /
- 醛氨缩合反应
Abstract: Pyridine and its derivatives, collectively referred to as pyridine bases, find extensive applications in fields such as pesticides and pharmaceuticals, serving as crucial intermediates in the chemical industry. In recent years, with the development of the pesticide and pharmaceutical industries, the demand for pyridine bases has rapidly increased. The Chichibabin condensation reaction is the most widely used route for industrial production of pyridine bases. Currently, the most commonly used ZSM-5 zeolite catalyst is limited by the instability of its silicon-aluminum framework structure, resulting in a short active reaction cycle (5 hours). In response to this issue, this study selected the thermally stable and hydrothermally stable Silicalite-1 zeolite. Polyvinylpyrrolidone (PVP) was employed as a colloidal dispersant using a hydrothermal synthesis method. In situ modification was utilized to introduce Fe into the MFI framework during zeolite synthesis. The influence of PVP dosage, template agent dosage, and other crystallization conditions on the crystallinity, pore structure, and acidity of Silicalite-1 zeolite products was investigated using X-ray diffraction (XRD), scanning electron microscopy (SEM), thermogravimetric analysis (TG), and BET surface area analysis. The acidity of Fe-modified Silicalite-1 zeolites was characterized using NH3-temperature programmed desorption (NH3-TPD), pyridine infrared (Py-FTIR) spectroscopy, Fourier-transform infrared (FT-IR) spectroscopy, and X-ray photoelectron spectroscopy (XPS). Experimental results indicated that the addition of seed crystals effectively reduced the particle size of the molecular sieve to approximately 200 nm. Fe-modified Silicalite-1 exhibited a disk-like morphology with good crystal dispersion. The highest relative crystallinity of the product reached 103% with a seed crystal input of 15% and PVP addition of 3.75%. Fe-modified Silicalite-1 possessed a greater abundance of both Lewis (L) and Brønsted (B) acid sites. The modified Silicalite-1 exhibits a higher abundance of B and L acid sites, resulting in an increase in the initial activity for the pyridine bases synthesis with Chichibabin condensation from 66% to 85%. Compared to ZSM-5, Fe-modified Silicalite-1 exhibited superior catalytic stability, maintaining the conversion rates and yields of pyridine bases above 66% and 40%, respectively, over a 15 hour reaction period. Finally, the strategy proposed in this study, which utilizes polyvinylpyrrolidone as a colloidal stabilizer to modify Silicalite-1 zeolite, significantly broadened the application prospects of weakly acidic pure silica zeolites in the field of acid catalysis. This approach has demonstrated significant scientific value and industrial potential. -
图 11 TS-S-1和T-S-1催化剂的总碳转化率(a),吡啶碱收率(b)和吡啶碱选择性(c)Fe-S-1、TS-S-1与HZSM-5催化剂的总碳转化率(d),吡啶碱收率(e)和吡啶碱选择性(f)
Figure 11 Total carbon conversion rate (a), pyridine bases yield (b), and pyridine bases selectivity (c) of TS-S-1 and T-S-1 catalysts,Total carbon conversion rate (d), pyridine bases yield (e), and pyridine bases selectivity(f) of Fe-S-1, TS-S-1, and HZSM-5 catalysts
表 1 Silicalite-1分子筛的孔结构
Table 1 Pore structure properties of Silicalite-1 zeolites
Sample PVP/% SBET/(m2·g−1) SMicro/(m2·g−1) vTotal/(cm3·g−1) vMicro/(cm3·g−1) vMeso/(cm3·g−1) Fe-S-1 3.75 348 215 0.18 0.10 0.08 TS-S-1 3.75 341 211 0.18 0.11 0.07 TS-S-1 — 357 231 0.18 0.11 0.07 T-S-1 3.75 302 235 0.19 0.11 0.08 T-S-1 — 311 247 0.18 0.11 0.07 表 2 Fe-S-1分子筛样品元素分析与酸性
Table 2 Elemental composition analysis and acidity of Fe-S-1 zeolite sample
Sample PVP addition
amount/%Fe/% Si/% Fe/Si Acidityc/(μmol·g−1) balka surfaceb balka surfaceb balka surfaceb 200 ℃ 300 ℃ BASd LASe BASd LASe Fe-S-1 3.75 2.39 2.21 23.24 22.12 0.10 0.10 7.2 19.9 5.8 7.9 Fe-S-1 2.5 1.91 1.59 20.31 19.41 0.09 0.08 6.3 16.3 4.9 8.1 TS-S-1 0 — — 24.12 22.60 — — 1.3 6.7 0.8 3.0 a: ICP test result; b: XPS test result; c: Py-FTIR test result; d: Brønsted acid site; e: Lewis acid site. 表 3 反应前后催化剂的孔结构和酸性质对比
Table 3 Comparison of the pore structure and acidity properties of the catalyst before and after the reaction
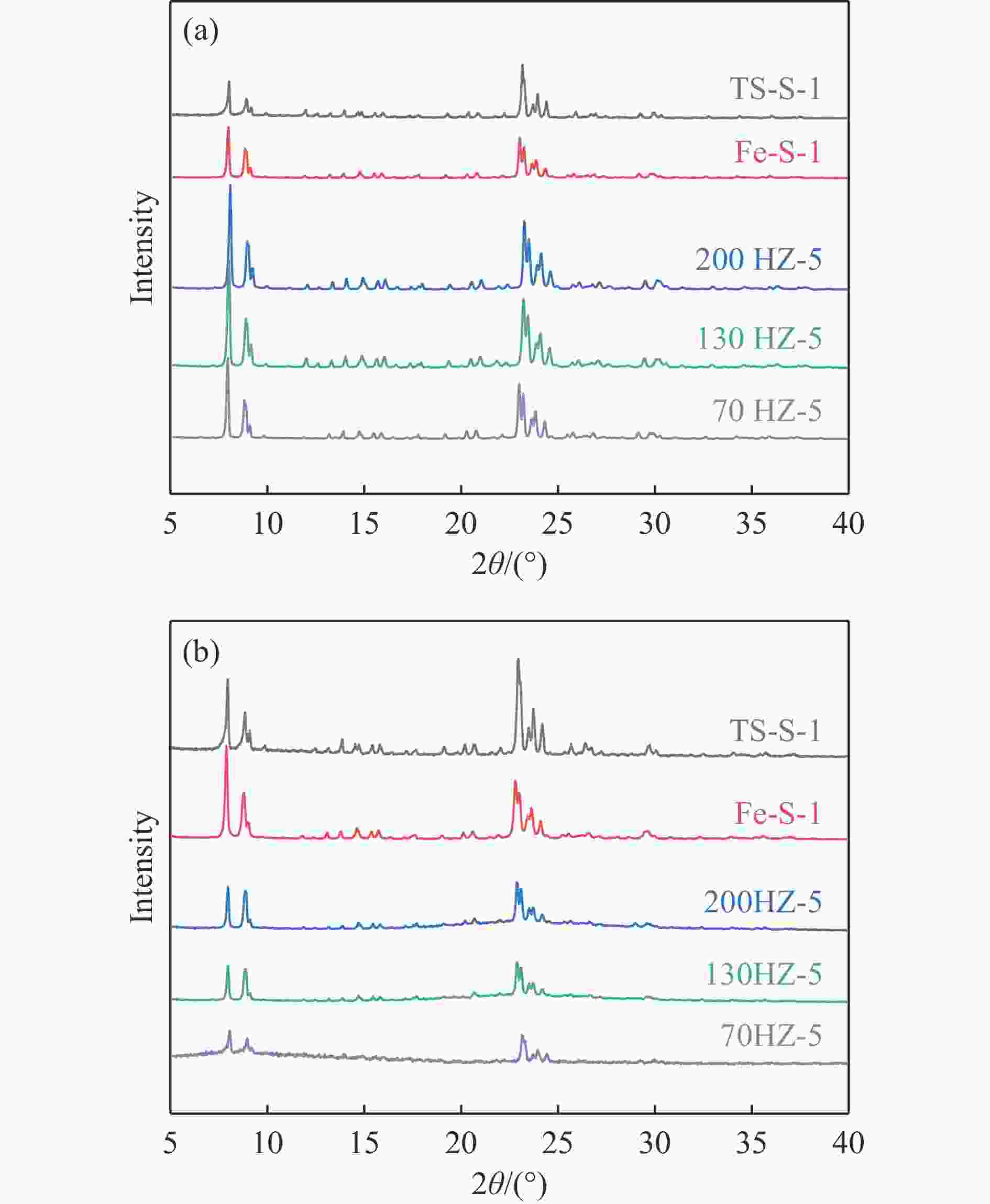
Sample Before reaction After reaction Acid loss/% SBET loss/% R.C.% lossa Total acid/(μmol·g−1) SBET/(m2·g−1) Total acid/(μmol·g−1) SBET/(m2·g−1) 200HZ-5 594 351 210 195 64.5 44.4 67 130HZ-5 1791 345 415 206 77.6 40.2 71 70HZ-5 2166 383 598 163 72.4 57.4 77 TS-S-1 47 357 16 233 65.9 34.7 29 Fe-S-1 227 348 127 261 44.8 26.8 33 Note: (a) the difference in relative crystallinity (R.C.%) between fresh catalyst and waste catalyst before and after the reaction -
[1] JIN F, CUI Y, LI Y. Effect of alkaline and atom-planting treatment on the catalytic performance of ZSM-5 catalyst in pyridine and picolines synthesis[J/OL]. Appl Catal A: gen, 2008, 350(1): 71-78. [2] JIN F, TIAN Y, LI Y. Effect of alkaline treatment on the catalytic performance of ZSM-5 catalyst in pyridine and picolines synthesis[J/OL]. Ind Eng Chem res, 2009, 48(4): 1873-1879. [3] LUO C W, FENG X Y, LIU W, et al. Deactivation and regeneration on the ZSM-5-based catalyst for the synthesis of pyridine and 3-picoline[J/OL]. Micropor Mesopor Mat, 2016, 235: 261-269. [4] PALA-ROSAS I, CONTRERAS J L, SALMONES J, et al. Effects of the acidic and textural properties of Y-Type zeolites on the synthesis of pyridine and 3-picoline from acrolein and ammonia[J/OL]. Catalysts, 2023, 13(4): 652. [5] BOOSA V, VARIMALLA S, DUMPALAPALLY M, et al. Influence of brønsted acid sites on chemoselective synthesis of pyrrolidones over H -ZSM-5 supported copper catalyst[J/OL]. Appl Catal B: environ, 2021, 292: 120177. [6] CALVIN J R, DAVIS R D, MCATEER C H. Mechanistic investigation of the catalyzed vapor-phase formation of pyridine and quinoline bases using 13CH2O, 13CH3OH, and deuterium-labeled aldehydes[J/OL]. Appl Catal A-gen, 2005, 285(1): 1-23. [7] LUO C W, CHAO Z S, LEI B, et al. The mild liquid-phase synthesis of 3-picoline from acrolein diethyl acetal and ammonia over heterogeneous catalysts[J/OL]. Ees, 2017, 94(1): 012031. [8] KULKARNI S J, RAO R R, SUBRAHMANYAM M, et al. Synthesis of pyridine and picolines from ethanol over modified ZSM-5 catalysts[J/OL]. Appl Catal A-gen, 1994, 113(1): 1-7. [9] ZHANG X, LI Y, HUO Y, et al. Synthesis of pyridine bases from ethanol, methanol and ammonia over micro-mesoporous Zn–OH/HZSM-5 catalyst[J/OL]. Micropor Mesopor Mat, 2020, 306: 110442. [10] JIANG F, HUANG J J, XIAO W Y, et al. Synthesis of picolines over metal modified HZSM-5 catalyst[J/OL]. Asian J Chem, 2015, 27(7): 2415-2419. [11] SINGH, BALDEV, Role of acidity of pillared inter-layered clay (PILC) for the synthesis of pyridine bases[J/OL]. J Chem technol Biot, 1998, 71(3): 246-252. [12] YAMAMURA M, CHAKI K, WAKATSUKI T, et al. Synthesis of ZSM -5 zeolite with small crystal size and its catalytic performance for ethylene oligomerization[J/OL]. Zeolites, 1994, 14(8): 643-649. [13] XIAO J, LI H, ZHU G bin. Hierarchically porous MFI zeolite synthesized by zeolite seeding and alkaline steaming-mediated crystallization[J/OL]. Adv Powder Technol, 2016, 27(4): 1396-1403. [14] YANG X, DIB E, LANG Q, et al. Silicalite-1 formation in acidic medium: synthesis conditions and physicochemical properties[J/OL]. Micropor Mesopor Mat, 2022, 329: 111537. [15] NAWAB M, BAROT S, BANDYOPADHYAY R. Nano-sized Silicalite-1: novel route of synthesis, metal impregnation and its application in selective oxidation of toluene[J/OL]. J Chem Sci, 2018, 131(1): 2. [16] SUÁREZ S, POSTOLACHE R, GARCÍA-GARCÍA F J, et al. Silicalite-1 synthesized with geothermal and ludox colloidal silica and corresponding TiO2/Silicalite-1 hybrid photocatalysts for VOC oxidation[J/OL]. Micropor Mesopor Mat, 2020, 302: 110202. [17] CRECI S, WANG X, CARLSSON P A, et al. Tuned acidity for catalytic reactions: synthesis and characterization of Fe-and Al-MFI zeotypes[J/OL]. Top Catal, 2019, 62(7): 689-698. [18] LIU H, WANG H, XING A H, et al. Effect of Al distribution in MFI framework channels on the catalytic performance of ethane and ethylene aromatization[J/OL]. J Phys Chem C, 2019, 123(25): 15637-15647. [19] THONGKAM M, WORAMONGKOLCHAI S, SAOWSUPA S, et al. A facile method to synthesize b -oriented Silicalite-1 thin film[J/OL]. Membranes, 2022, 12(5): 520. [20] KUZ’MICHEVA G, CHERNYSHEV V, KRAVCHENKO G, et al. Impact of composition and structural parameters on the catalytic activity of MFI type titanosilicalites[J/OL]. Dalton T, 2022, 51(9): 3439-3451. [21] GAO M, GONG Z, WENG X, et al. Methane combustion over palladium catalyst within the confined space of MFI zeolite[J/OL]. Chinese J Catal, 2021, 42(10): 1689-1699. [22] PERATHONER S, PINO F, CENTI G, et al. Benzene selective oxidation with N2O on Fe/MFI catalysts: Role of zeolite and iron sites on the deactivation mechanism[J/OL]. Top Catal, 2003, 23(1): 125-136. [23] NAUMANN D’ALNONCOURT R, FRIEDRICH M, KUNKES E, et al. Strong metal–support interactions between palladium and iron oxide and their effect on CO oxidation[J/OL]. J Catal, 2014, 317: 220-228. -




 下载:
下载: