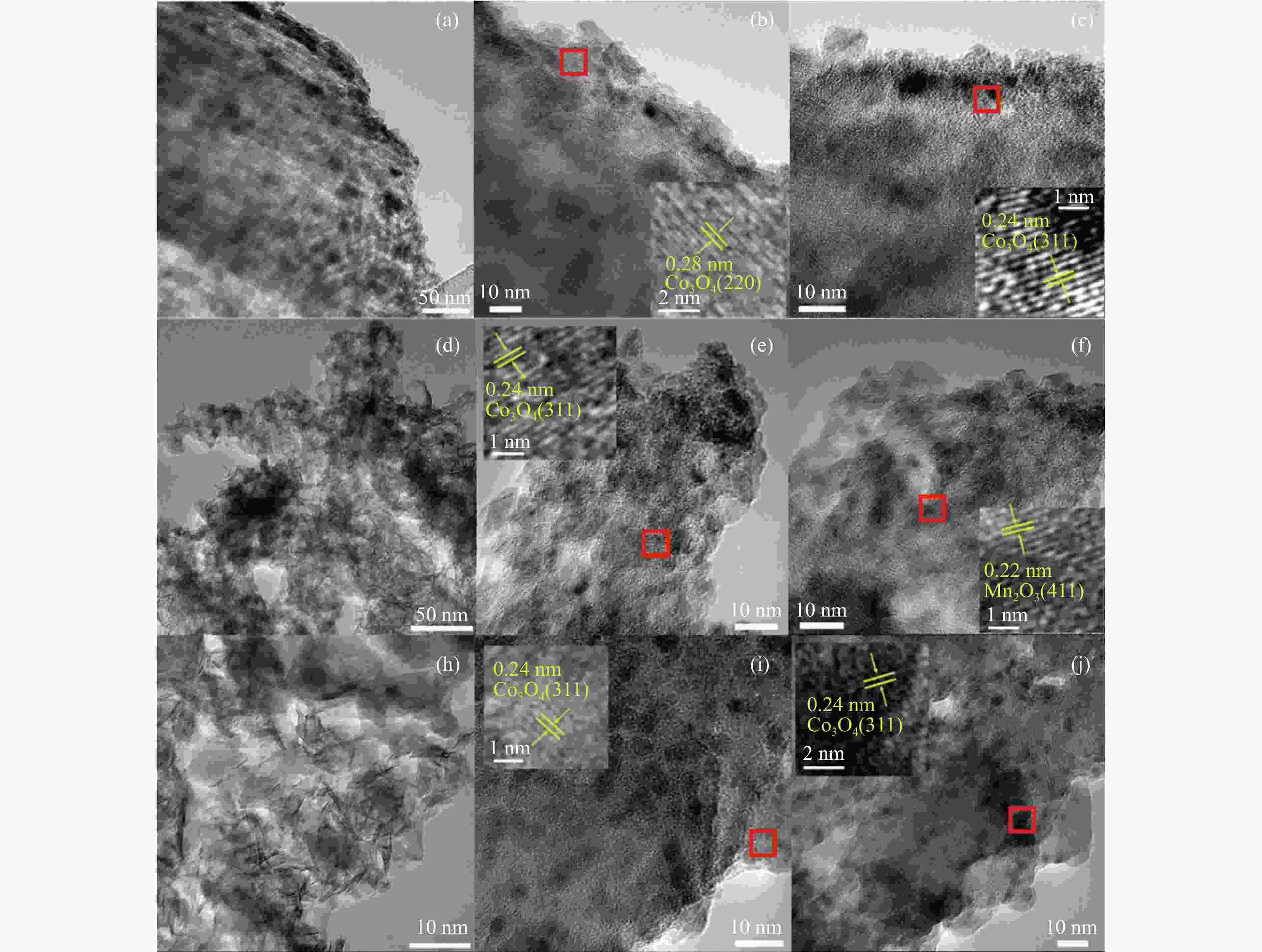
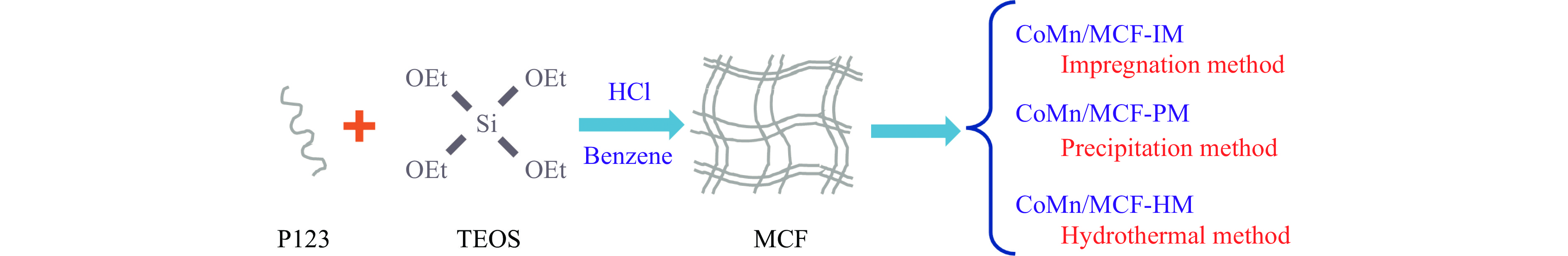
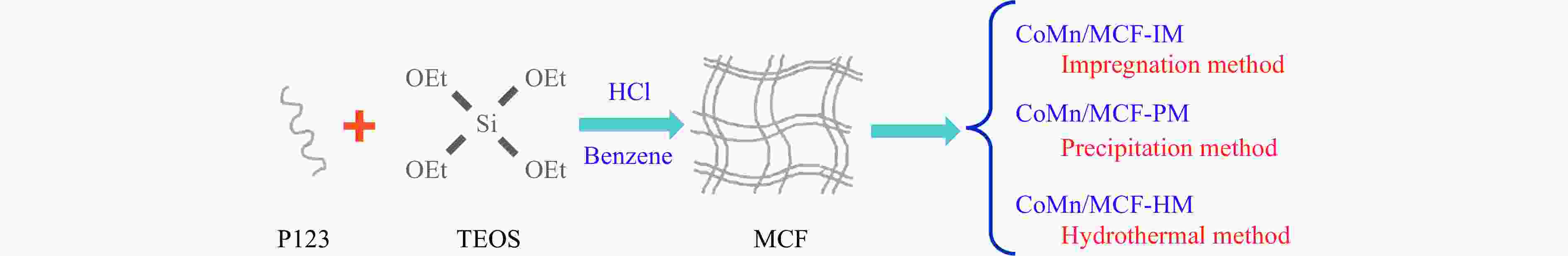
Preparation of silicon foam supported CoMn catalysts and their catalytic performances in higher alcohol synthesis via syngas
-
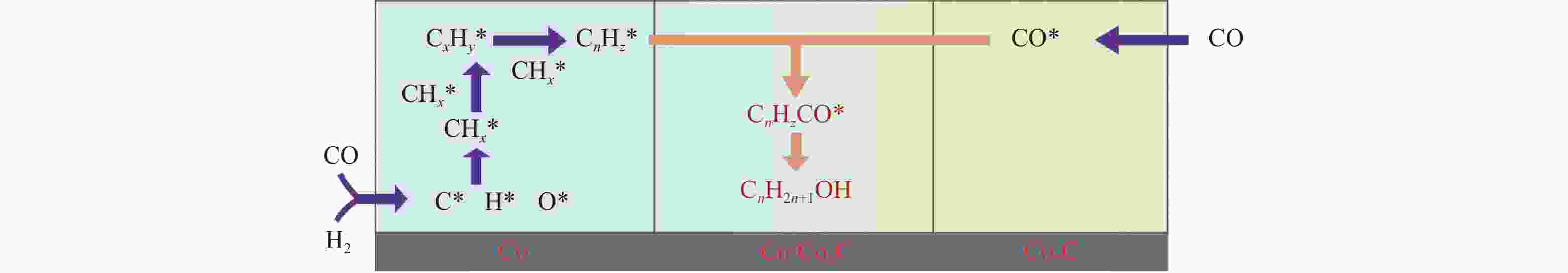
摘要: 本研究采用浸渍法、沉淀法和水热合成法制备了一系列泡沫硅负载CoMn基催化剂,并结合XRD、H2-TPR、N2物理吸附、TEM和XPS等表征技术考察了制备方法对催化剂在合成气制低碳醇反应中的性能影响。研究表明,催化剂表面存在Co2+(Co2C)、Co0物种,水热合成法制备的催化剂表面Co2C-Co0活性位点存在良好的协同作用有利于醇的生成,较高比例的Co2C也促进了CO的非解离吸附和插入,从而呈现最高的醇选择性。在t=260 ℃,p=5.0 MPa,GHSV=4500 h−1,H2/CO(体积比)=2∶1的反应条件下,该泡沫催化剂可实现CO转化率11.1%,总醇选择性34.7%,C2+OH选择性34.5%的反应性能。Abstract: A series of silicon foam supported CoMn catalysts were prepared using impregnation, precipitation, and hydrothermal methods. Combining the characterization techniques such as XRD, H2-TPR, N2 physical adsorption, TEM, and XPS, the effect of different catalyst preparation methods on the catalytic performance in the synthesis of higher alcohols from syngas was investigated. Research has shown that there are Co2+(Co2C) and Co0 species on the surface of the catalyst. The active sites of Co2C-Co0 on the surface of the catalyst prepared by hydrothermal synthesis have a good synergistic effect, which is conducive to the generation of alcohols. A higher proportion of Co2C also promotes the associative adsorption and insertion of CO, resulting in the highest alcohol selectivity. Under the reaction conditions: t=260 ℃, p=5.0 MPa, GHSV=4500 h−1, H2/CO(volume ratio)=2∶1, the catalyst exhibited the best reaction performance to achieve CO conversion of 11.1%, total alcohol selectivity of 34.7%, and C2+OH selectivity of 34.5%.
-
Key words:
- synthesis gas /
- higher alcohols /
- silicon foam /
- CoMn catalyst
-
表 1 实验用化学试剂
Table 1 Experimental chemical reagents
Chemical reagent Reagent grade Manufacturer Co(NO3)2·6H2O AR Sinopharm Chemical Reagent Co Ltd 50% Mn(NO3)2 solution AR Sinopharm Chemical Reagent Co Ltd PEG-PPG-PEG, Pluronic®P-123, Average Mn ~5800 AR Sinopharm Chemical Reagent Co Ltd C8H20O4Si AR Sinopharm Chemical Reagent Co Ltd C6H6 AR Sinopharm Chemical Reagent Co Ltd HCl AR Sinopharm Chemical Reagent Co Ltd K2CO3 AR Sinopharm Chemical Reagent Co Ltd CH4N2O AR Sinopharm Chemical Reagent Co Ltd NH4F AR Aladdin Experimental water deionized water Laboratory homemade 表 2 样品的织构性质
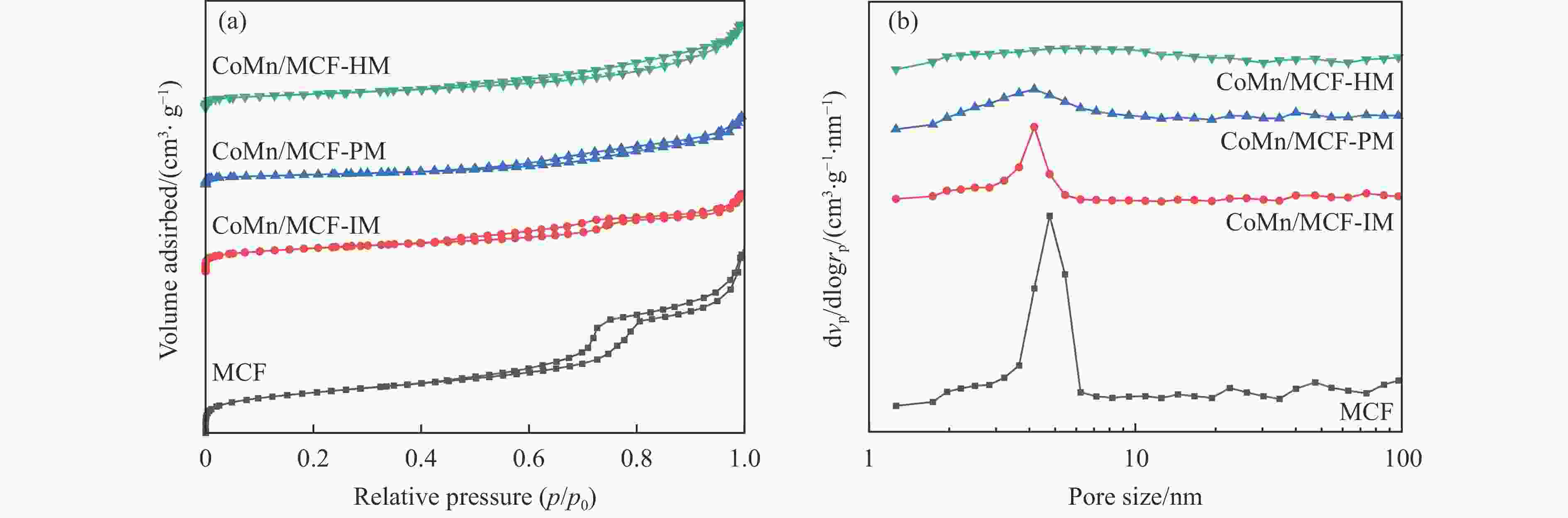
Table 2 Texture properties of the samples
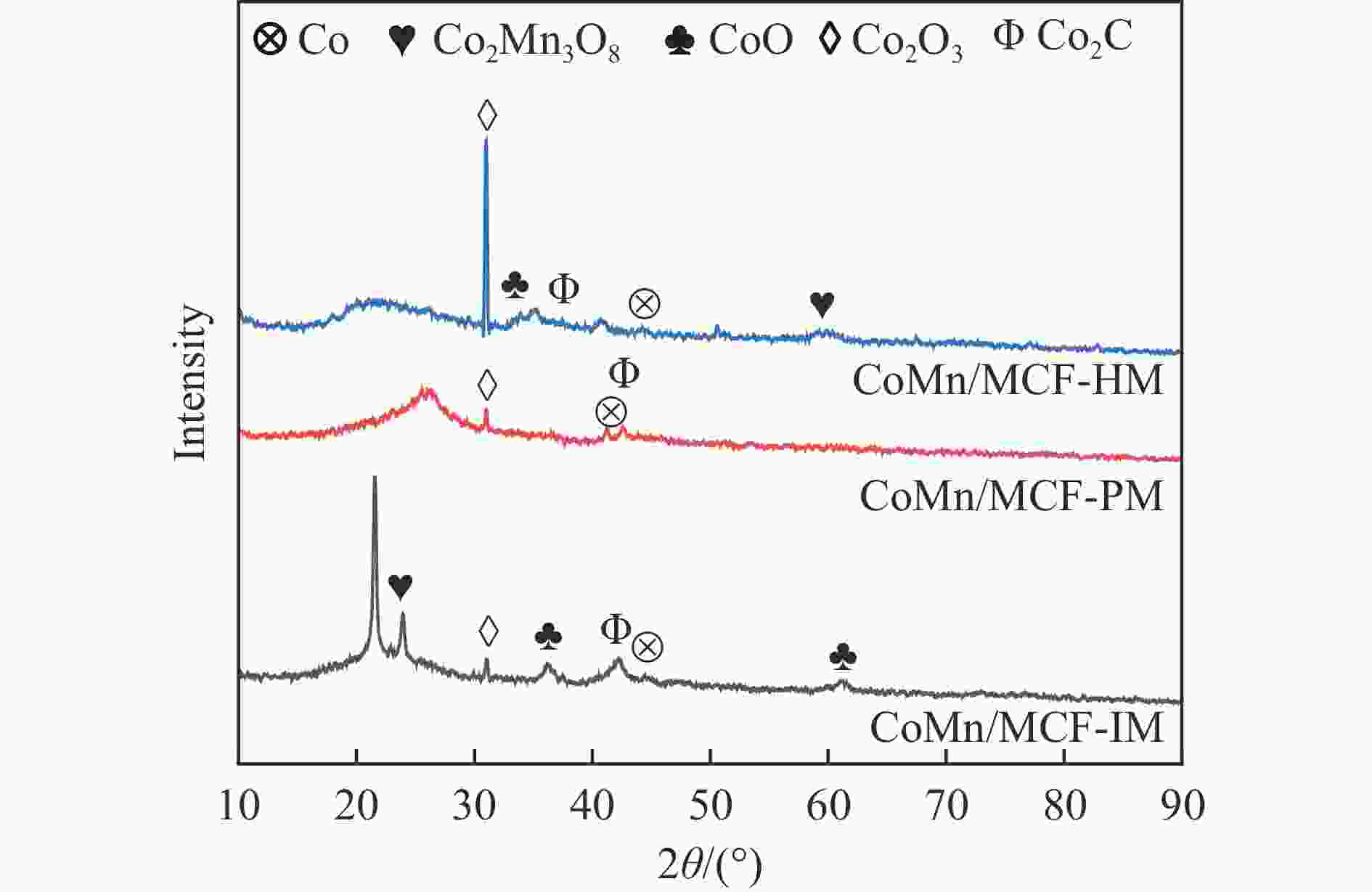
Sample SBET/(m2·g−1) Pore volume/(cm3·g−1) Average pore sizea/nm MCF 668 1.3 6.0 CoMn/MCF-IM 383 0.5 4.5 CoMn/MCF-PM 159 0.5 10.9 CoMn/MCF-HM 229 0.6 8.4 a: Average pore size = 4(pore volume/SBET) 表 3 催化剂的元素含量
Table 3 The element contents of the catalysts
Sample Co/% Mn/% Si/% CoMn/MCF-IM 12.91 4.65 29.90 CoMn/MCF-PM 13.36 4.70 30.89 CoMn/MCF-HM 13.48 4.02 30.82 表 4 催化剂的催化性能
Table 4 The catalytic performance of catalystsa
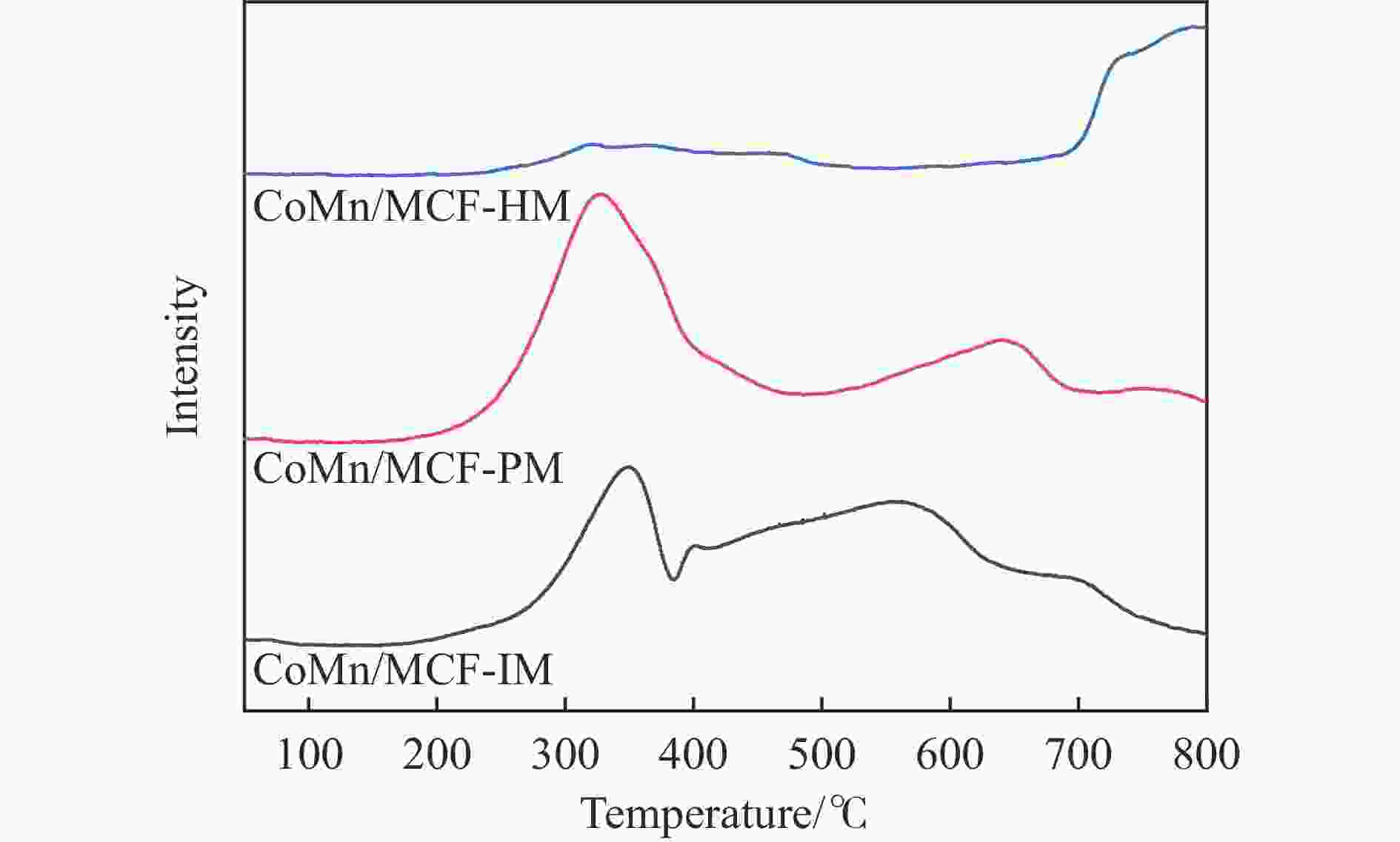
Sample xCO/% Selectivity/% Alcohol distribution STYROH/(g·g·h−1) ROH CHn CO2 MeOH C2+OH CoMn/MCF-IM 36.7 6.9 89.6 3.6 29.1 71.0 0.06 CoMn/MCF-PM 6.3 26.8 73.3 0.0 54.6 45.4 0.02 CoMn/MCF-HM 11.1 34.7 65.4 0.0 65.5 34.5 0.04 a: Reaction conditions: t=260 ℃, p=5.0 MPa, GHSV=4500 h−1, H2/CO=2.0。 表 5 反应后催化剂表面钴元素相对含量
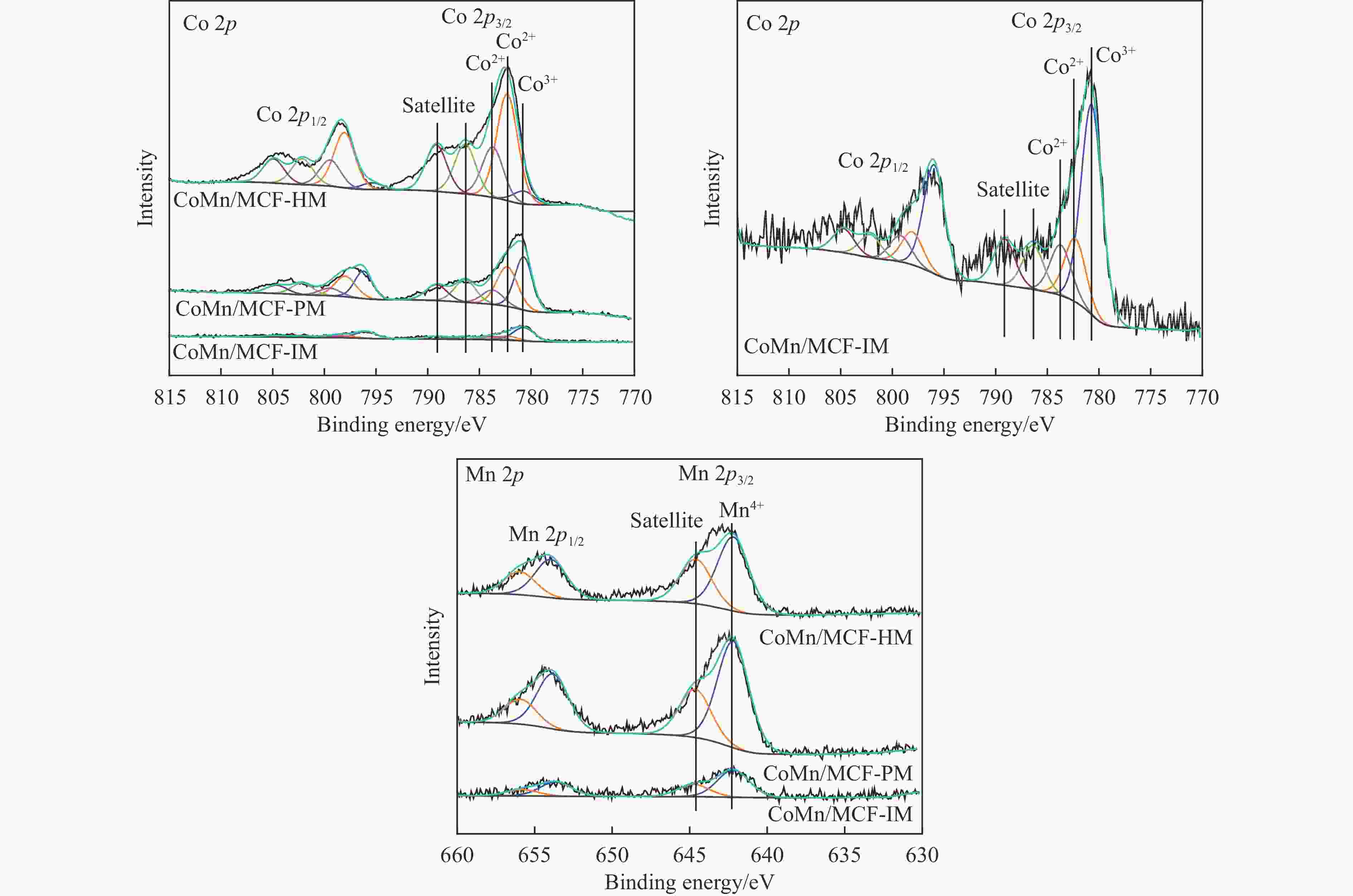
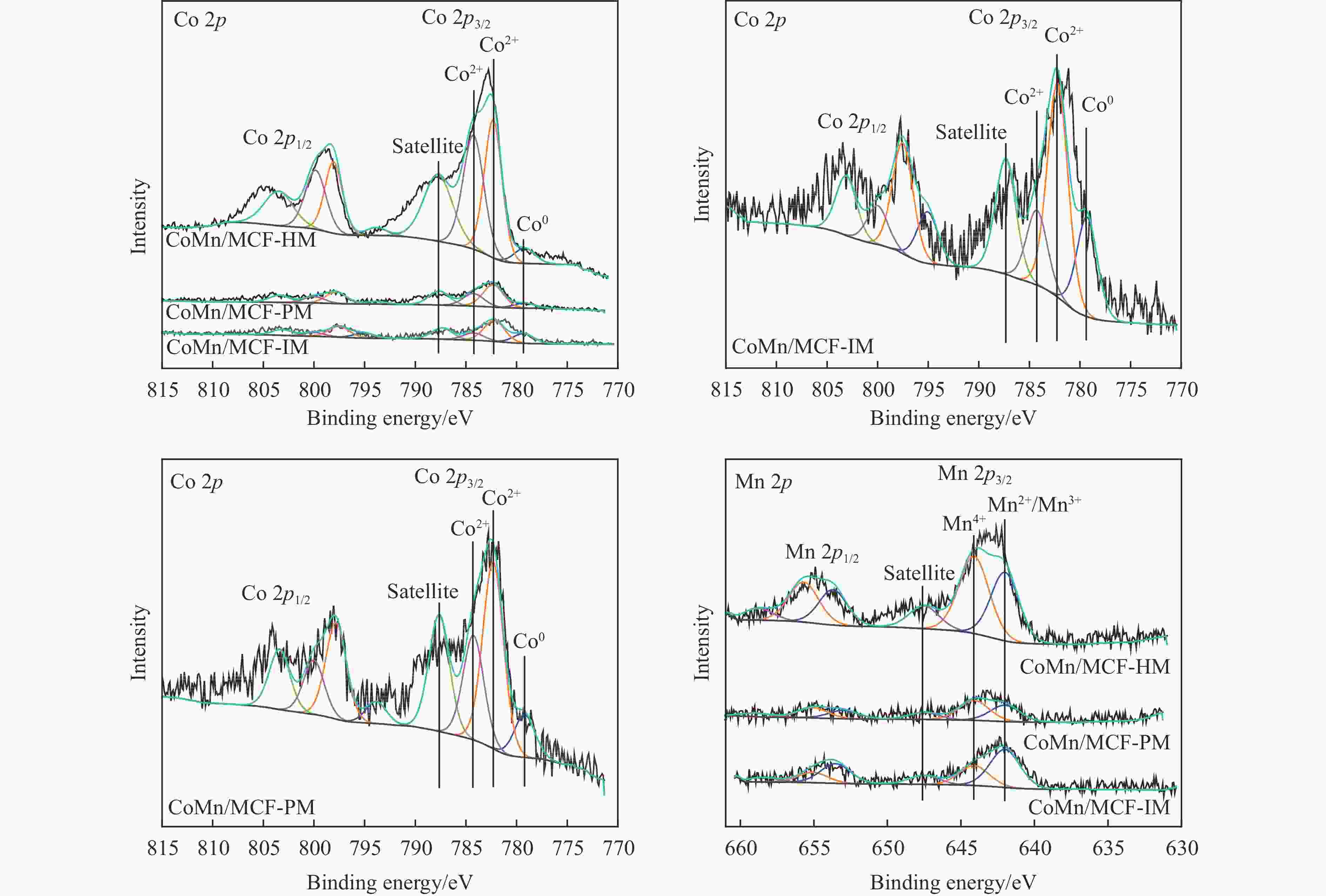
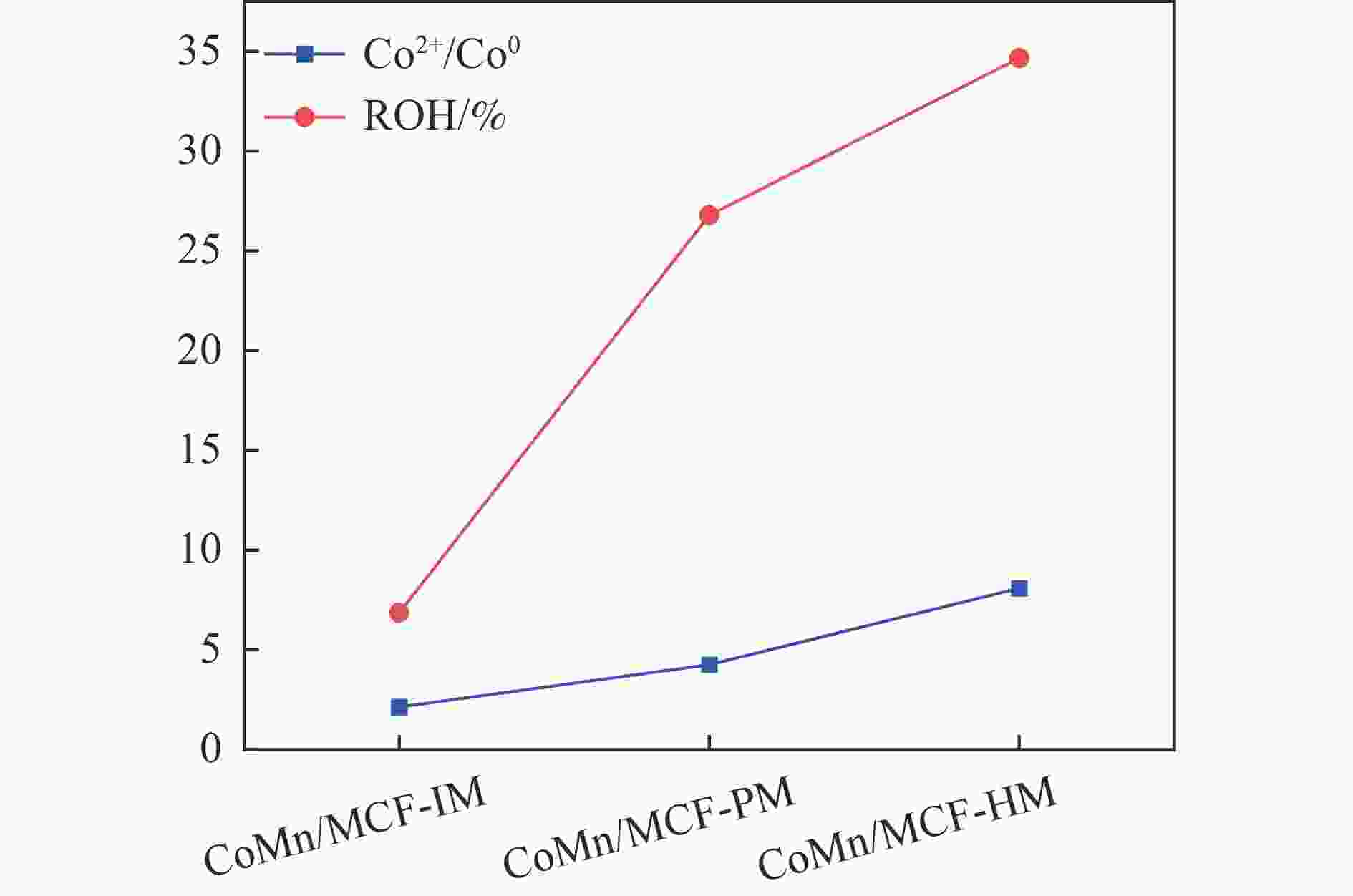
Table 5 Relative contents of cobalt element on the surface of the catalyst after reactiona
Catalyst Co2+(area%) Co0(area%) Co2+/Co0 CoMn/MCF-IM 0.68 0.32 2.13 CoMn/MCF-PM 0.81 0.19 4.26 CoMn/MCF-HM 0.89 0.11 8.09 a: Integrated area based on Co 2p3/2 main peak。 -
[1] AN Y, LIN T, YU F, et al. Advances in direct production of value-added chemicals via syngas conversion[J]. Sci China: Chem,2017,60:887−903. doi: 10.1007/s11426-016-0464-1 [2] LUK H T, MONDELLI C, FERRÉ D C, et al. Status and prospects in higher alcohols synthesis from syngas[J]. Chem Soc Rev,2017,46(5):1358−1426. doi: 10.1039/C6CS00324A [3] XUE X, WENG Y, YANG S, et al. Research progress of catalysts for synthesis of low-carbon alcohols from synthesis gas[J]. RSC Adv,2021,11(11):6163−6172. doi: 10.1039/D0RA08329A [4] 宗弘元, 马宇春, 刘仲能. 合成气制混合燃料醇的研究进展[J]. 化工进展,2015,34(5):1269−1276.ZONG Hongyuan, MA Yuchun, LIU Zhongneng. Research progress of higher alcohols synthesis from syngas[J]. Chem Ind Eng Prog,2015,34(5):1269−1276. [5] LI L, LIN T, LI X, et al. Control of Co0/Co2C dual active sites for higher alcohols synthesis from syngas[J]. Appl Catal, A,2020,602:117704. doi: 10.1016/j.apcata.2020.117704 [6] Luan X, Ren Z, Dai X, et al. Selective conversion of syngas into higher alcohols via a reaction-coupling strategy on multifunctional relay catalysts[J]. ACS Catal,2020,10(4):2419−2430. doi: 10.1021/acscatal.9b04111 [7] Ao M, Pham G H, Sage V, et al. Perovskite-derived trimetallic Co-Ni-Cu catalyst for higher alcohol synthesis from syngas[J]. Fuel Process Technol,2019,193:141−148. doi: 10.1016/j.fuproc.2019.05.002 [8] ZHONG L, YU F, AN Y, et al. Cobalt carbide nano prisms for direct production of lower olefins from syngas[J]. Nature,2016,538(7623):84−87. doi: 10.1038/nature19786 [9] JIAO F, LI J, PAN X, et al. Selective conversion of syngas to light olefins[J]. Science,2016,351(6277):1065−1068. doi: 10.1126/science.aaf1835 [10] YANG X, SU X, CHEN D, et al. Direct conversion of syngas to aromatics: A review of recent studies[J]. Chin J Catal,2020,41(4):561−573. doi: 10.1016/S1872-2067(19)63346-2 [11] AO M, PHAM G H, SUNARSO J, et al. Active centers of catalysts for higher alcohol synthesis from syngas: A review[J]. ACS Catal,2018,8(8):7025−7050. doi: 10.1021/acscatal.8b01391 [12] Kang B, Qi S, Wang X, et al. Ultra-small and highly-dispersed MoP particles for remarkable enhanced catalytic performance in higher alcohols synthesis[J]. Catal Commun,2020,137:105945. doi: 10.1016/j.catcom.2020.105945 [13] Anton J, Nebel J, Song H, et al. The effect of sodium on the structure-activity relationships of cobalt-modified Cu/ZnO/Al2O3 catalysts applied in the hydrogenation of carbon monoxide to higher alcohols[J]. J Catal,2016,335:175−186. doi: 10.1016/j.jcat.2015.12.016 [14] Yang N, Yoo J S, Schumann J, et al. Rh-MnO interface sites formed by atomic layer deposition promote syngas conversion to higher oxygenates[J]. ACS Catal,2017,7(9):5746−5757. doi: 10.1021/acscatal.7b01851 [15] WANG Z, SPIVEY J J. Effect of ZrO2, Al2O3 and La2O3 on cobalt-copper catalysts for higher alcohols synthesis[J]. Appl Catal, A,2015,507:75−81. doi: 10.1016/j.apcata.2015.09.032 [16] GAO W, ZHAO Y, LIU J, et al. Catalytic conversion of syngas to mixed alcohols over CuFe-based catalysts derived from layered double hydroxides[J]. Catal Sci Technol,2013,3(5):1324−1332. doi: 10.1039/c3cy00025g [17] ZHAO Z, LU W, YANG R, et al. Insight into the formation of Co@Co2C catalysts for direct synthesis of higher alcohols and olefins from syngas[J]. ACS Catal,2018,8(1):228−241. doi: 10.1021/acscatal.7b02403 [18] PEI Y, DING Y, ZHU H, et al. One-step production of C1-C18 alcohols via Fischer-Tropsch reaction over activated carbon-supported cobalt catalysts: Promotional effect of modification by SiO2[J]. Chin J Catal,2015,36(3):355−361. doi: 10.1016/S1872-2067(14)60252-7 [19] ZHENG Y, SU Y, PANG C, et al. Interface-enhanced oxygen vacancies of CoCuOx catalysts In situ grown on monolithic Cu foam for VOC catalytic oxidation[J]. Environ Sci Technol Lett,2021,56(3):1905−1916. [20] TANG W, WANG S, AO W, et al. Pre-surface leached cordierite honeycombs for MnxCo3-xO4 nano-sheet array integration with enhanced hydrocarbons combustion[J]. Catal Today,2019,320:196−203. doi: 10.1016/j.cattod.2017.10.045 [21] 韦良. 介孔泡沫硅负载钴催化剂的费-托合成反应性能研究[D]. 苏州大学, 2016.WEI Liang. Study on the performance of mesoporous foam silicon supported cobalt catalyst for fischer tropsch synthesis[D]. Soochow University, 2016.) [22] LIU Z, LI A, QIU Y, et al. MgCo2O4@NiMn layered double hydroxide core-shell nanocomposites on nickel foam as superior electrode for all-solid-state asymmetric supercapacitors[J]. J Colloid Interface Sci,2021,592:455−467. doi: 10.1016/j.jcis.2021.02.011 [23] 韦良, 赵燕熹, 向勇, 等. 大孔径高比表面积介孔硅泡沫的制备和表征[J]. 武汉理工大学学报,2013,35(6):34−38. doi: 10.3963/j.issn.1671-4431.2013.06.007WEI Liang, ZHAO Yan-xi, XIANG Yong, et al. Preparation and characterization of mesoporous silicon foam with large pore size and high specific surface area[J]. J Wuhan Univ Technol,2013,35(6):34−38. doi: 10.3963/j.issn.1671-4431.2013.06.007 [24] BULAVCHENKO O A, GERASIMOV E Y, AFONASENKO T N. Reduction of double manganese-cobalt oxides: in situ XRD and TPR study[J]. Dalton Trans,2018,47(47):17153−17159. doi: 10.1039/C8DT04137G [25] LIAO P, ZHANG C, ZHANG L, et al. Higher alcohol synthesis via syngas over CoMn catalysts derived from hydrotalcite-like precursors[J]. Catal Today,2018,311:56−64. doi: 10.1016/j.cattod.2017.09.022 [26] MARTÍNEZ A, LÓPEZ C, MÁRQUEZ F, et al. Fischer-Tropsch synthesis of hydrocarbons over mesoporous Co/SBA-15 catalysts: the influence of metal loading, cobalt precursor, and promoters[J]. J Catal,2003,220(2):486−499. doi: 10.1016/S0021-9517(03)00289-6 [27] WANG P, CHEN S, BAI Y, et al. Effect of the promoter and support on cobalt-based catalysts for higher alcohols synthesis through CO hydrogenation[J]. Fuel,2017,195:69−81. doi: 10.1016/j.fuel.2017.01.050 [28] QIN T, LIN T, QI X, et al. Tuning chemical environment and synergistic relay reaction to promote higher alcohols synthesis via syngas conversion[J]. Appl Catal, B,2021,285:119840. doi: 10.1016/j.apcatb.2020.119840 [29] ZHAO Z, LU W, ZHU H, et al. Tuning the Fischer-Tropsch reaction over CoxMnyLa/AC catalysts toward alcohols: Effects of La promotion[J]. J Catal,2018,361:156−167. doi: 10.1016/j.jcat.2018.02.008 [30] Chen Y, Wei J, Duyar M S, et al. Carbon-based catalysts for Fischer-Tropsch synthesis[J]. Chem Soc Rev,2021,50(4):2337−2366. doi: 10.1039/D0CS00905A -




 下载:
下载: