Catalytic performance of CuO/La1−xCexCrO3 in the steam reforming of methanol
-
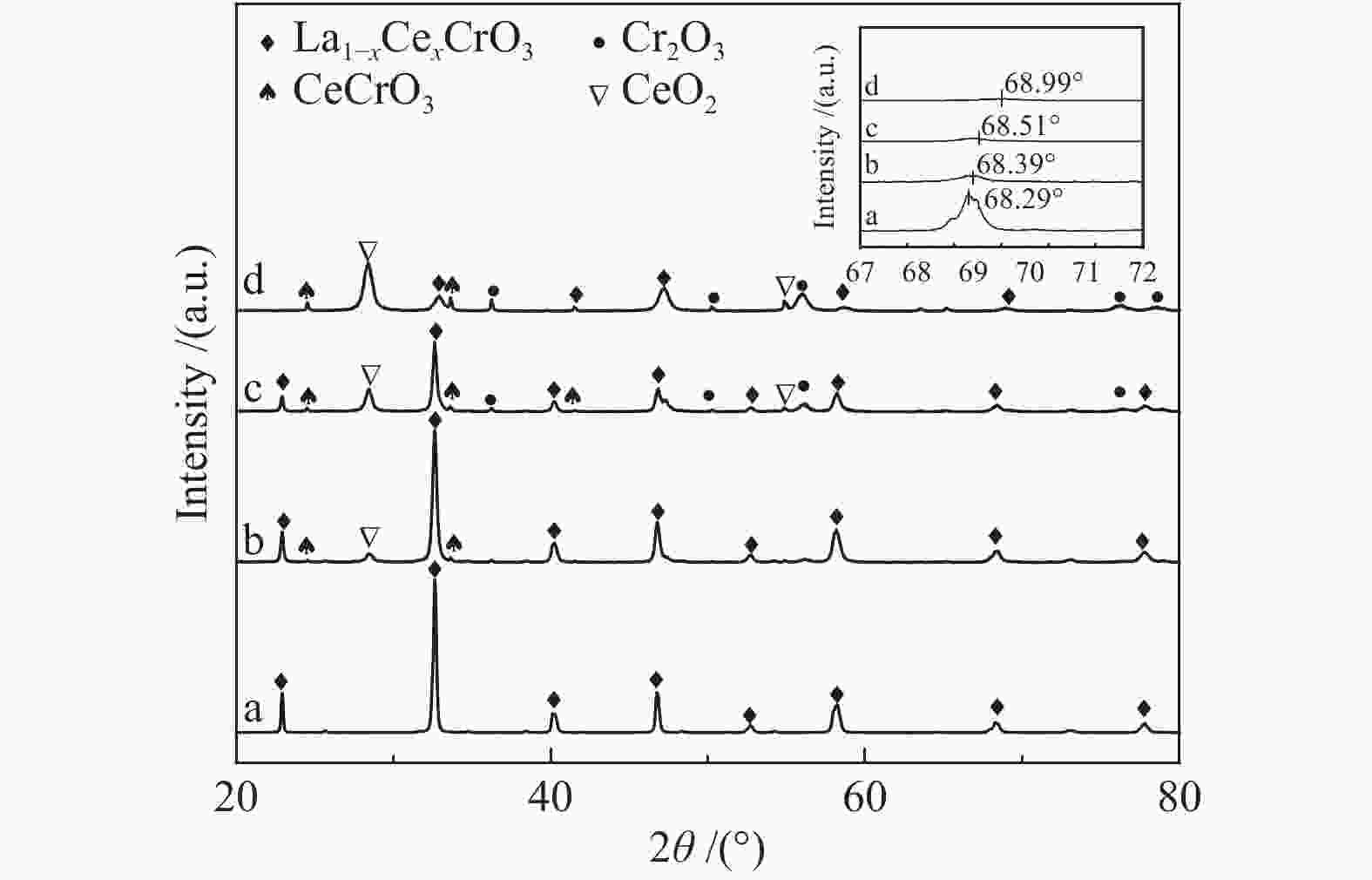
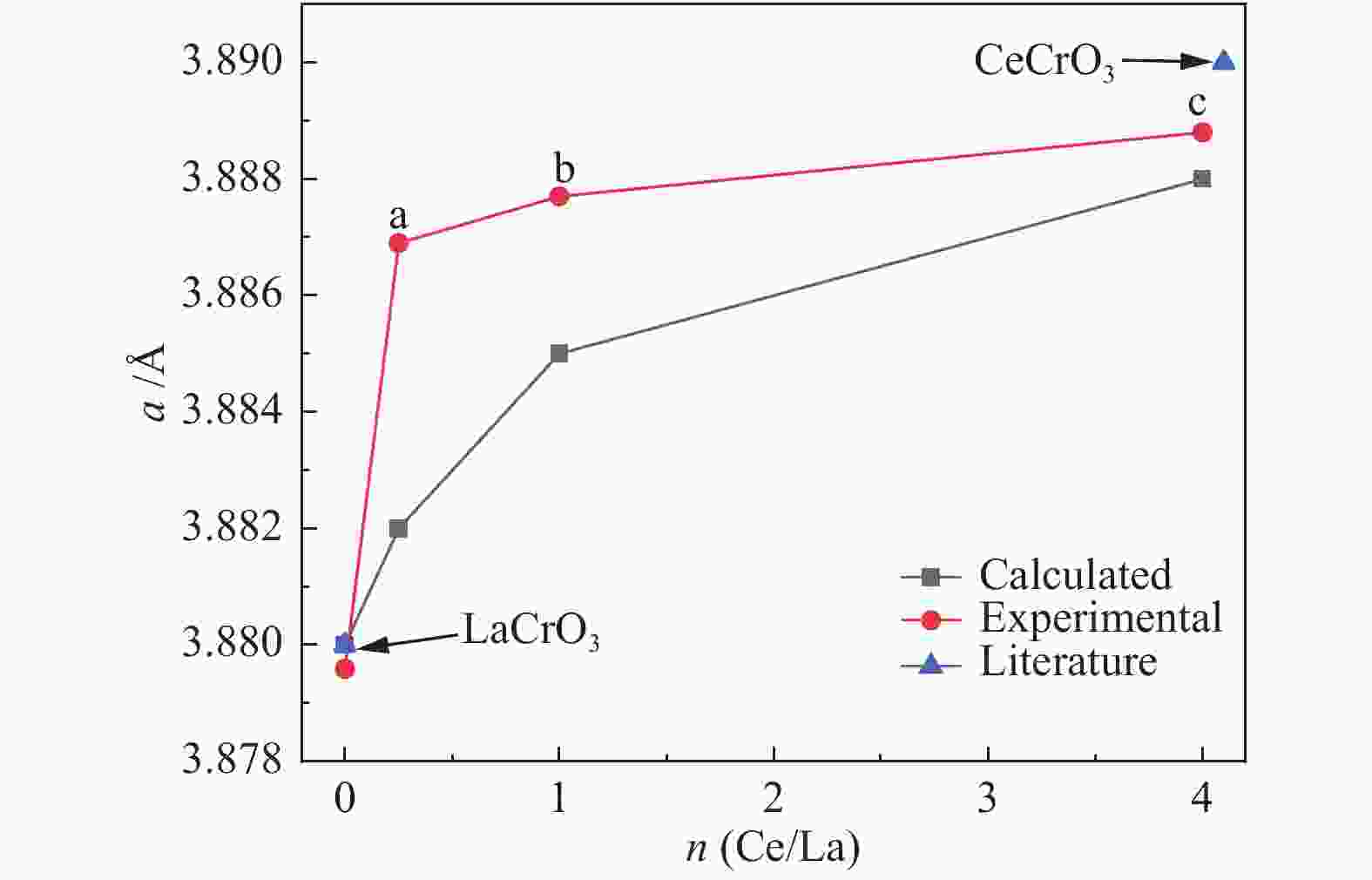
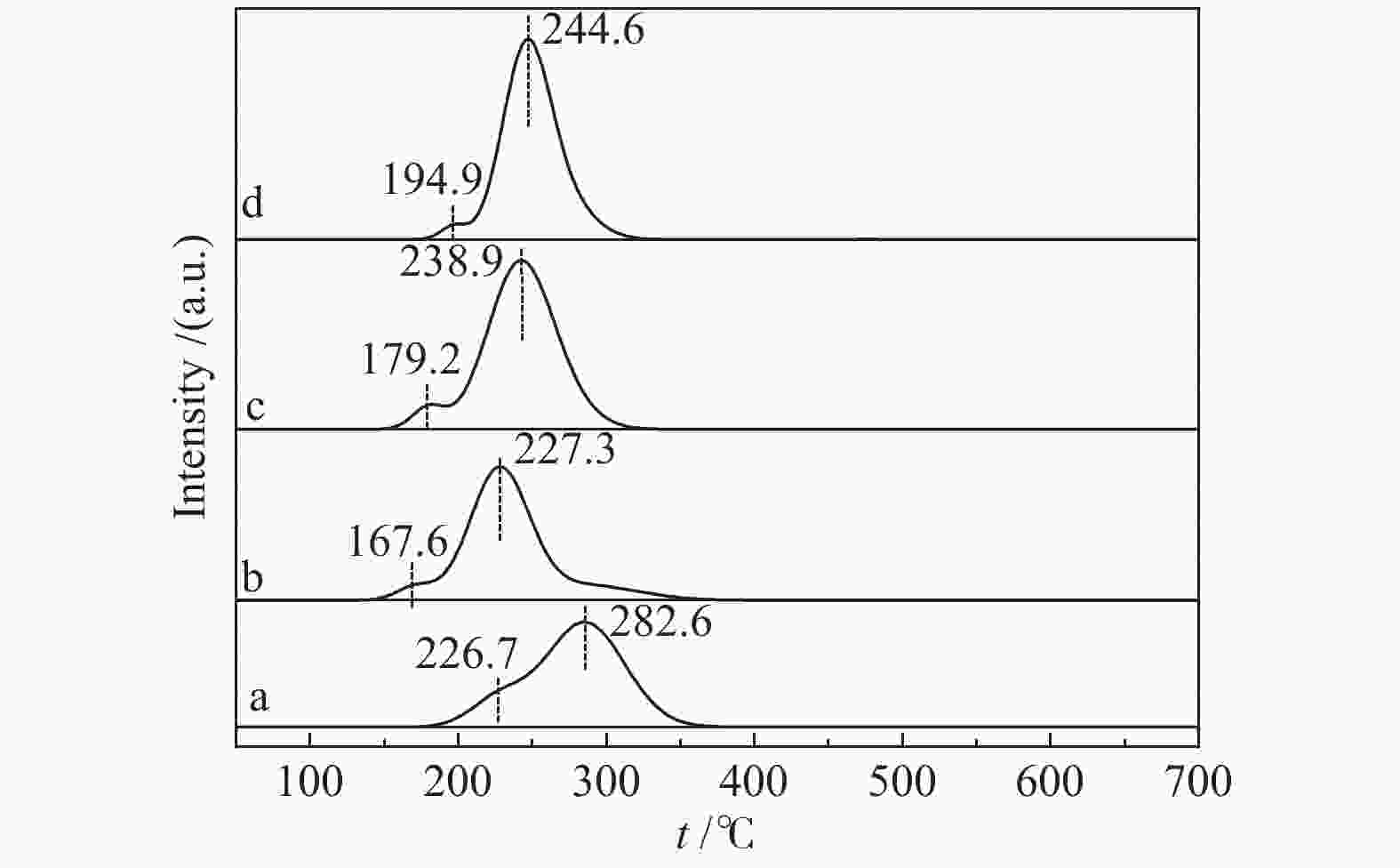
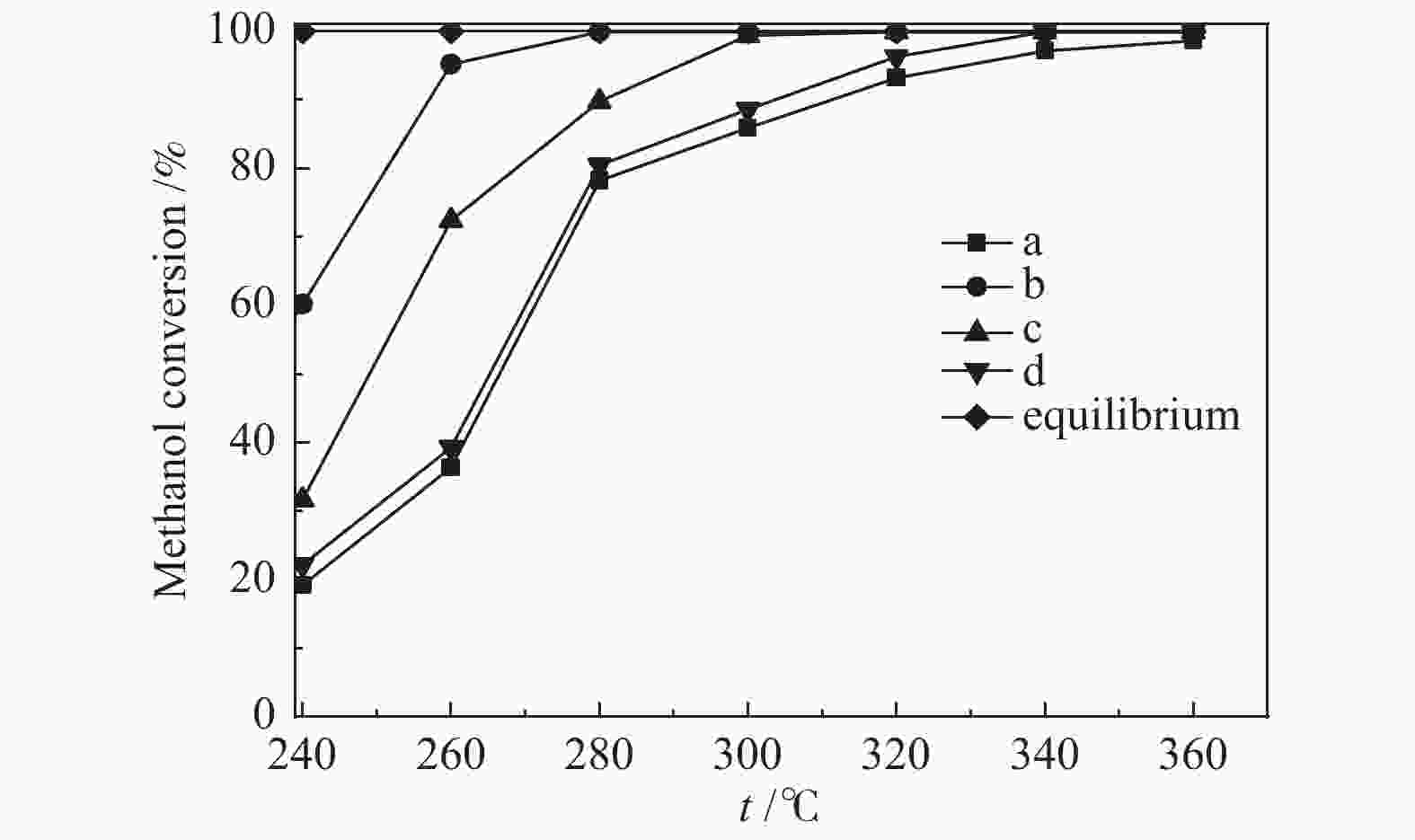
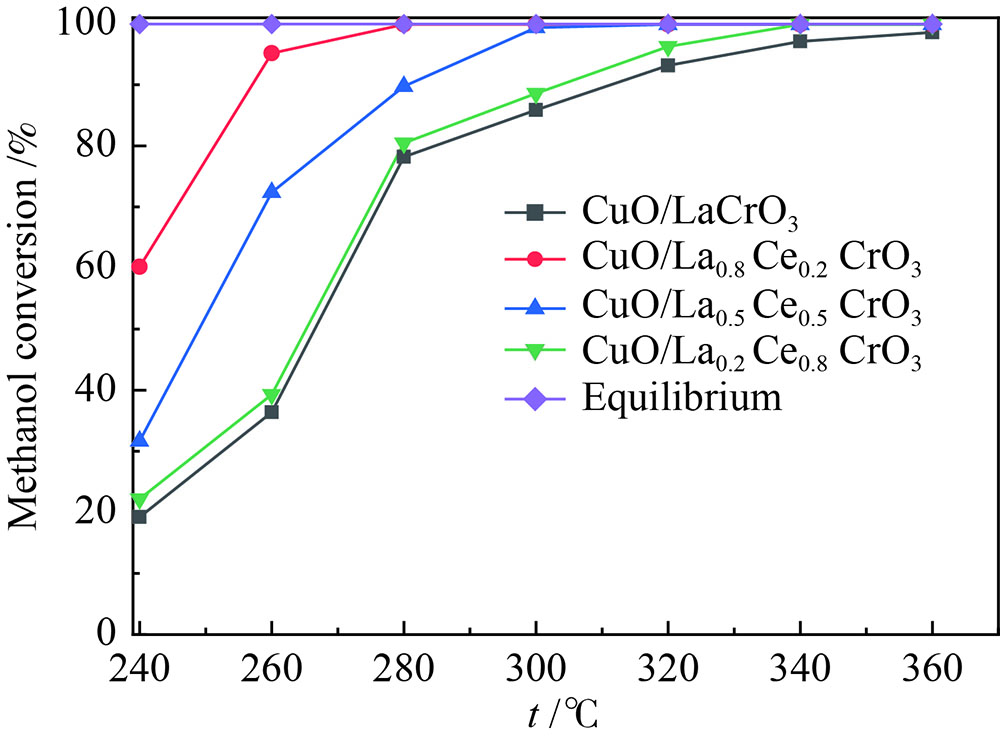
摘要: 采用溶胶凝胶法制备了负载型CuO/La1−xCexCrO3催化剂,研究了A位中Ce元素掺杂量对CuO/La1−xCexCrO3催化剂结构、性质及其催化甲醇水蒸气重整制氢性能的影响。结果表明,掺杂Ce元素影响了CuO的还原性能和钙钛矿载体与CuO之间的作用力,进而影响了CuO/La1−xCexCrO3催化剂对甲醇水蒸气重整制氢反应的催化性能。其中,CuO/La0.8Ce0.2CrO3具有较好的催化性能,在280 °C、水醇物质的量比为1.2、甲醇气体空速为800 h−1的条件下,甲醇转化率达到100%。Abstract: A series of supported CuO/La1−xCexCrO3 catalysts were prepared by the sol-gel method and the effect of Ce doping in the A site on their structure, properties and catalytic performance in the steam reforming of methanol were investigated. The results indicate that the Ce doping impacts mainly on the reduction performance of CuO and the interaction between the perovskite support and CuO, which in turn influences the catalytic performance of CuO/La1−xCexCrO3 in methanol steam reforming. In particular, the CuO/La0.8Ce0.2CrO3 catalyst demonstrates adequate performance in methanol steam reforming; over it, the methanol conversion reaches 100% under 280 °C, water/methanol molar ratio of 1.2 and methanol gas hourly space velocity of 800 h−1.
-
Key words:
- perovskite /
- methanol steam reforming /
- hydrogen /
- sol-gel method
-
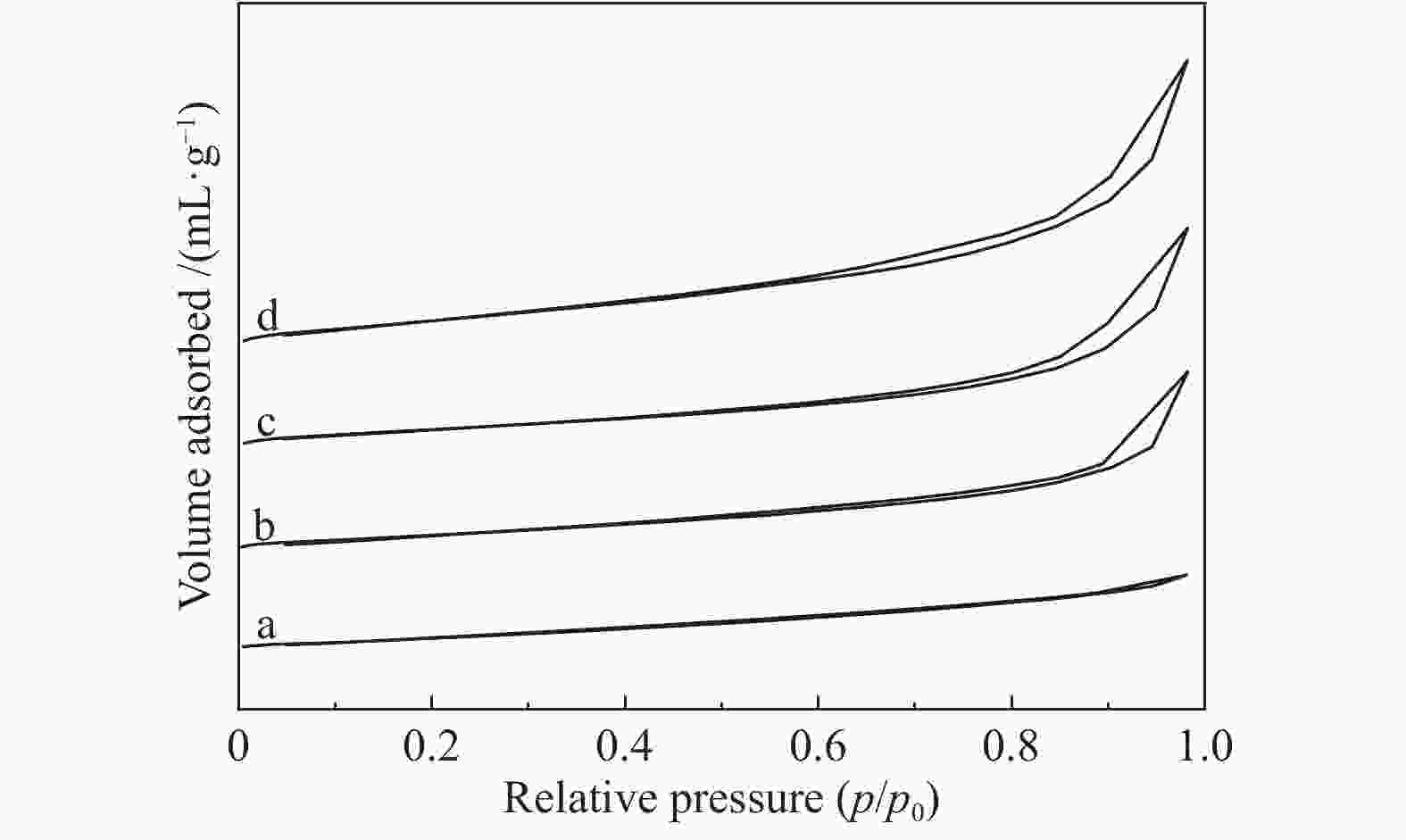
表 1 La1-xCexCrO3载体和CuO/La1-xCexCrO3催化剂的比表面积及孔结构参数
Table 1 Surface area and pore structure parameters of La1−xCe x CrO3 support and CuO/La1−xCe x CrO3 catalysts
Catalyst ABET/
(m2·g−1)Pore volume v/
(cm3·g−1)Bore diameter/nm Cu surface area A/
(m2·gcat−1)H2 production rate/
(mL·kgcat−1·s−1)LaCrO3 10.6 0.02 3.06 − − La0.8Ce0.2CrO3 15.3 0.06 3.44 − − La0.5Ce0.5CrO3 22.6 0.12 3.66 − − La0.2Ce0.8CrO3 24.1 0.15 3.91 − − CuO/LaCrO3 12.1 0.03 3.09 2.1 651 CuO/La0.8Ce0.2CrO3 16.6 0.06 3.54 3.1 1056 CuO/La0.5Ce0.5CrO3 25.5 0.13 3.73 2.8 746 CuO/La0.2Ce0.8CrO3 27.1 0.15 3.92 2.8 669 表 2 催化剂的产氢速率对比
Table 2 A comparison of various catalysts in the hydrogen production rate
-
[1] ZAICENKO V M, SHPILRAIN E E, SHTERENBERG V Y. Hydrogen energy: Present state and lines of future development[J]. Teploenergetika,2003,1(5):61−67. [2] HERDEM M S, SINAKI M Y, FARHAD S, HAMDULLAHPUR F. An overview of the methanol reforming process: comparison of fuels, catalysts, reformers, and systems[J]. Int J Hydrogen Energy,2019,43:5076−5085. [3] CLAUDE L. From hydrogen production by water electrolysis to its utilization in a PEM fuel cell or in a SO fuel cell: Some considerations on the energy efficiencies[J]. Int J Hydrogen Energy,2016,41(34):15415−15425. doi: 10.1016/j.ijhydene.2016.04.173 [4] HOSSAIN M A, JEWARATNAM J, GANESAN P. Prospect of hydrogen production from oil palm biomass by thermochemical process-A review[J]. Int J Hydrogen Energy,2016,41(38):16637−16655. doi: 10.1016/j.ijhydene.2016.07.104 [5] SA S, SILVA H, BRANDAO L, SOUSA J M, MENDES A. Catalysts for methanol steam reforming—A review[J]. Appl Catal B: Environ,2010,99(1-2):43−57. doi: 10.1016/j.apcatb.2010.06.015 [6] LYTKINA A A, ZHILYAEVA N A, ERMILOVA M M, OREKHOVA N V, YAROSLAVTSEV A B. Influence of the support structure and composition of Ni-Cu-based catalysts on hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy,2015,40(31):9677−9684. doi: 10.1016/j.ijhydene.2015.05.094 [7] HE J P, YANG Z X, ZHANG L, LI Y, PAN L W. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming[J]. Int J Hydrogen Energy,2017,42(15):9930−9937. doi: 10.1016/j.ijhydene.2017.01.229 [8] WANG Z J, WANG C X, CHEN S Q, LIU Y. Co-Ni bimetal catalyst supported on perovskite-type oxide for steam reforming of ethanol to produce hydrogen[J]. Int J Hydrogen Energy,2014,39(11):5644−5652. doi: 10.1016/j.ijhydene.2014.01.151 [9] GLISENTI A, GALENDA A, NATILE M M. Steam reforming and oxidative steam reforming of methanol and ethanol: The behaviour of LaCo0.7Cu0.3O3[J]. Appl Catal A: Gen,2013,453:102−112. doi: 10.1016/j.apcata.2012.11.031 [10] 肖国鹏, 乔韦军, 王丽宝, 张磊, 张健, 王宏浩. LaNiO3的焙烧温度对甲醇水蒸气重整制氢CuO/LaNiO3催化剂的影响[J]. 燃料化学学报,2020,48(2):213−240. doi: 10.3969/j.issn.0253-2409.2020.02.011XIAO Guo-peng, QIAO Wei-jun, WANG Li-bao, ZHANG Lei, ZHANG Jian, WANG Hong-hao. The effect of LaNiO3 roasting temperature on CuO/LaNiO3 catalysts for hydrogen production by methanol steam reforming[J]. J Fuel Chem Technol,2020,48(2):213−240. doi: 10.3969/j.issn.0253-2409.2020.02.011 [11] KHALESI A, ARANDIYAN H R, PARVARI M. Effects of Lanthanum Substitution by Strontium and Calcium in La-Ni-Al Perovskite Oxides in Dry Reforming of Methane[J]. J Catal,2008,29(10):18−26. [12] YANG S Q, ZHOU F, LIU Y J, ZHANG L, YU C, WANG H H, TIAN Y, ZHANG C S, LIU D S. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy,2019,44(14):7252−7261. doi: 10.1016/j.ijhydene.2019.01.254 [13] XI H J, HOU X N, LIU Y J, QING S J, GAO Z X. Cu–Al spinel oxide as an efficient catalyst for methanol steam reforming[J]. Angew Chem Int Ed,2014,126:12080−12083. [14] 王丽宝, 王东哲, 张磊, 庆绍军, 韩蛟, 张财顺, 高志贤, 张海娟, 冯旭浩. 铈源对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J]. 燃料化学学报,2020,48(7):852−859.WANG Li-bao, WANG Dong-zhe, ZHANG Lei, QING Shao-jun, HAN Jiao, ZHANG Cai-shun, GAO Zhi-xian, ZHANG Hai-juan, FENG Xu-hao. Effect of cerium source on CuO/CeO2 catalysts for hydrogen production by methanol steam reforming[J]. J Fuel Chem Technol,2020,48(7):852−859. [15] 苏石龙, 张磊, 张艳, 雷俊腾, 桂建舟, 刘丹, 刘道胜, 潘立卫. 千瓦级PEMFC甲醇水蒸气重整制氢过程热力学模拟[J]. 石油化工高等学校学报,2015,28(2):21−25.SU Shi-long, ZHANG Lei, ZHANG Yan, LEI Jun-teng, GUI Jian-zhou, LIU Dan, LIU Dao-sheng, PAN Li-wei. Thermodynamic simulation of hydrogen production process from kilowatt PEMFC methanol steam reforming[J]. J Petrochem College,2015,28(2):21−25. [16] 杨淑倩, 贺建平, 张娜, 隋晓伟, 张磊, 杨占旭. 稀土掺杂改性对Cu/ZnAl水滑石衍生催化剂甲醇水蒸气重整制氢性能的影响[J]. 燃料化学学报,2018,46(2):179−188. doi: 10.3969/j.issn.0253-2409.2018.02.007YANG Shu-qian, HE Jian-ping, ZHANG Na, SUI Xiao-wei, ZHANG Lei, YANG Zhan-xu. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol,2018,46(2):179−188. doi: 10.3969/j.issn.0253-2409.2018.02.007 [17] 刘玉娟, 王东哲, 张磊, 王宏浩, 陈琳, 刘道胜, 韩蛟, 张财顺. 载体焙烧气氛对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J]. 燃料化学学报,2018,46(8):992−999. doi: 10.3969/j.issn.0253-2409.2018.08.011LIU Yu-juan, WANG Dong-zhe, ZHANG Lei, WANG Hong-hao, CHEN Lin, LIU Dao-sheng, HAN Jiao, ZHANG Cai-shun. Effect of carrier roasting atmosphere on CuO/CeO2 catalyst for methanol steam reforming[J]. J Fuel Chem Technol,2018,46(8):992−999. doi: 10.3969/j.issn.0253-2409.2018.08.011 [18] ZHANG L, PAN L W, NI C J, SUN T J, ZHAO S S, WANG S D, WANG A J, HU Y K. CeO2-ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming[J]. Int J Hydrogen Energy,2013,38(11):4397−4406. doi: 10.1016/j.ijhydene.2013.01.053 [19] SONG Q L, MEN Y, WANG J G, LIU S, CHAI S S, AN W, WANG K, LI Y Y, TANG Y H. Methanol steam reforming for hydrogen production over ternary composite ZnyCe1Zr9Ox catalysts[J]. Int J Hydrogen Energy,2020,45(16):9592−9602. doi: 10.1016/j.ijhydene.2020.01.175 -





 下载:
下载: