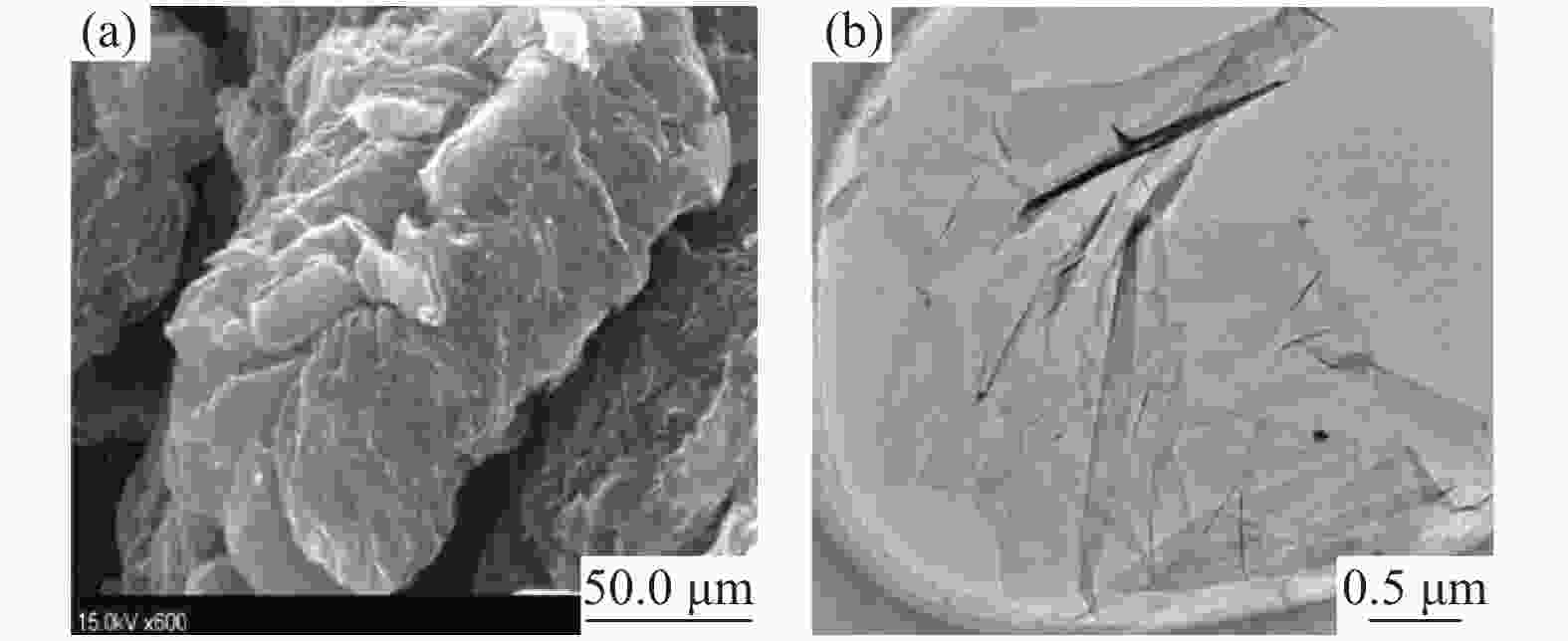
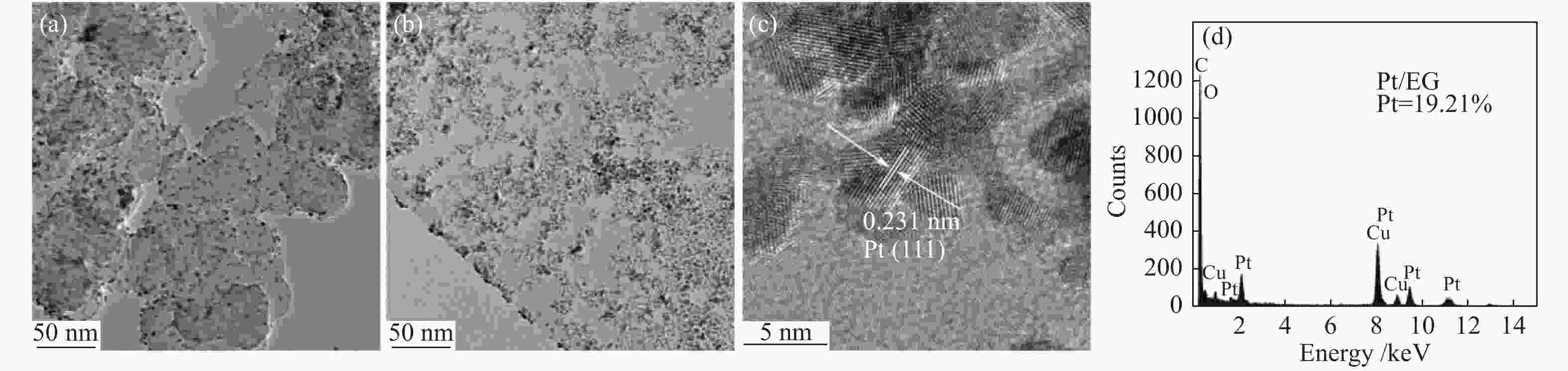
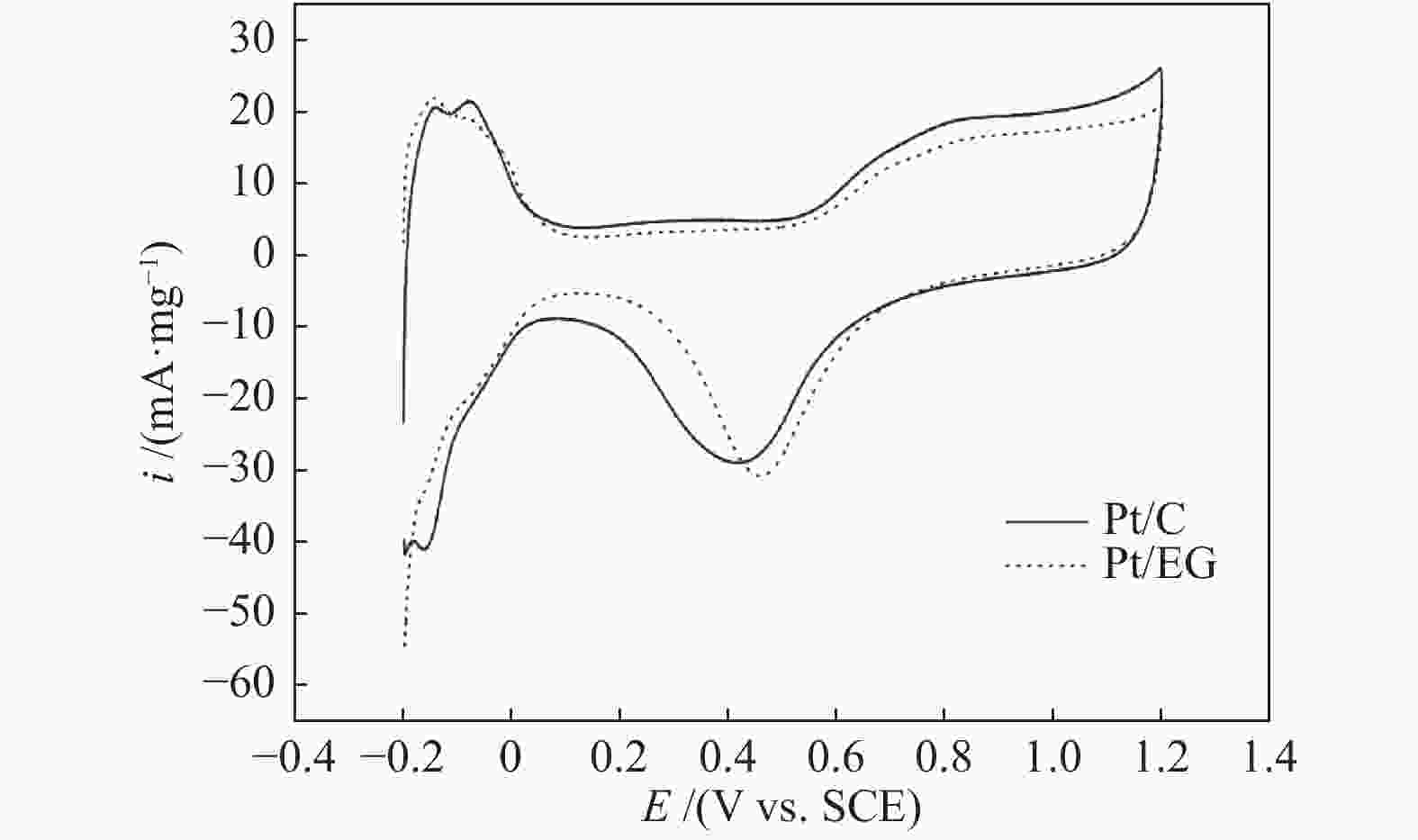
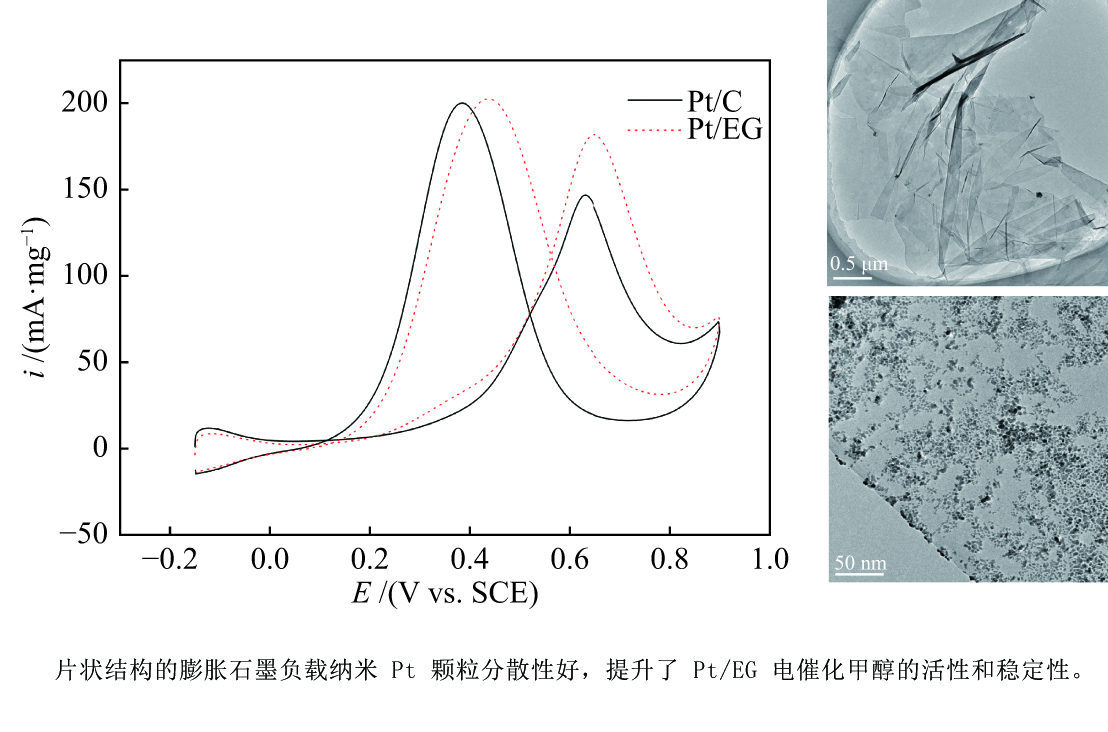
| [1] |
MUNJEWAR S S, THOMBRE S B, MALLICK R K. A comprehensive review on recent material development of passive direct methanol fuel cell[J]. Ionics,2017,23(1):1−18. doi: 10.1007/s11581-016-1864-1
|
| [2] |
KAMARUDIN S K, ACHMAD F, DAUD W R W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices[J]. Int J Hydrogen Energy,2009,34(16):6902−6916. doi: 10.1016/j.ijhydene.2009.06.013
|
| [3] |
LIU H, SONG C, ZHANG L, ZHANG J, WANG H, WILKINSON D P. A review of anode catalysis in the direct methanol fuel cell[J]. J Power Sources,2006,155(2):95−110. doi: 10.1016/j.jpowsour.2006.01.030
|
| [4] |
TOLMACHEV Y V, PETRII O A. Pt-Ru electrocatalysts for fuel cells: Developments in the last decade[J]. J Solid State Electrochem,2017,21(3):613−639. doi: 10.1007/s10008-016-3382-5
|
| [5] |
WEI Y C, LIU C W, CHANG W J, WANG K W. Promotion of Pt-Ru/C catalysts driven by heat treated induced surface segregation for methanol oxidation reaction[J]. J Alloys Compd,2011,509(2):535−541. doi: 10.1016/j.jallcom.2010.09.096
|
| [6] |
HAN F, WANG X, LIAN J, WANG Y. The effect of Sn content on the electrocatalytic properties of Pt-Sn nanoparticles dispersed on graphene nanosheets for the methanol oxidation reaction[J]. Carbon,2012,50(15):5498−5504. doi: 10.1016/j.carbon.2012.07.039
|
| [7] |
PECH-RODRÍGUEZ W J, CALLES-ARRIAGA C, GONZÁLEZ-QUIJANO D, VARGAS-GUTIÉRREZ G, MORAIS C, NAPPORN T W, RODRÍGUEZ-VARELA F J. Electrocatalysis of the Ethylene glycol oxidation reaction and in situ Fourier-transform infared study on PtMo/C electrocatalysts in alkaline and acid media[J]. J Power Sources,2018,375:335−344. doi: 10.1016/j.jpowsour.2017.07.081
|
| [8] |
赵海东, 卢珍, 刘锐, 李作鹏, 郭永. 铂银合金的制备及其对甲醇电氧化反应的催化性能(英文)[J]. 燃料化学学报,2020,48(8):1015−1024. doi: 10.1016/S1872-5813(20)30069-4ZHAO Hai-dong, LU Zhen, LIU Rui, LI Zuo-peng, GUO Yong. Preparation of platinum-silver alloy nanoparticles and their catalytic performance in methanol electro-oxidation[J]. J Fuel Chem Technol,2020,48(8):1015−1024. doi: 10.1016/S1872-5813(20)30069-4
|
| [9] |
CHEN M J, LOU B Y, NI Z J, XU B. PtCo nanoparticles supported on expanded graphite as electrocatalyst for direct methanol fuel cell[J]. Electrochim Acta,2015,165:105−109.
|
| [10] |
OU L. The origin of enhanced electrocatalytic activity of Pt-M (M=Fe, Co, Ni, Cu, and W) alloys in PEM fuel cell cathodes: A DFT computational study[J]. Comput Theor Chem,2014,1048(0):69−76.
|
| [11] |
RETHINASABAPATHY M, KANG S M, HALDORAI Y, JANKIRAMAN M, JONNA N, CHOE S R, HUH Y S, NATESAN B. Ternary PtRuFe nanoparticles supported N-doped graphene as an efficient bifunctional catalyst for methanol oxidation and oxygen reduction reactions[J]. Int J Hydrogen Energy,2017,42(52):30738−30749. doi: 10.1016/j.ijhydene.2017.10.121
|
| [12] |
TELIZ E, DÍAZ V, PÉREZ I, CORENGIA M, ZINOLA C F. Carbon supported Pt, Ru and Mo catalysts for methanol electrooxidation[J]. Int J Hydrogen Energy,2012,37(19):14761−14768. doi: 10.1016/j.ijhydene.2011.12.084
|
| [13] |
LEE E, MURTHY A, MANTHIRAM A. Effect of Mo addition on the electrocatalytic activity of Pt-Sn-Mo/C for direct ethanol fuel cells[J]. Electrochim Acta,2011,56(3):1611−1618. doi: 10.1016/j.electacta.2010.10.086
|
| [14] |
KIM J, POLIQUIT B Z, NAM H S, KIM S H, LEE Y M, KIM K M, KO J M. Electrochemical activities of Pt-Ru-Co and Pt-Ru-Ni electrocatalysts supported by a carbon fiber web[J]. Curr Appl Phys,2012,12(1):254−258. doi: 10.1016/j.cap.2011.06.014
|
| [15] |
THAMER B, EL-NEWEHY M, BARAKAT N, AL-DEYAB S, KIM H. Preparation of zero-valent Co/N-CNFs as an immobilized thin film onto graphite disc for methanol electrooxidation[J]. Fiber Polym,2017,18:696−705. doi: 10.1007/s12221-017-1068-y
|
| [16] |
ZHANG L R, ZHAO J, LI M, NI H T, ZHANG J L, FENG X M, MA Y W, FAN Q L, WANG X Z, HU Z, HUANG W. Preparation of graphene supported nickel nanoparticles and their application to methanol electrooxidation in alkaline medium[J]. New J Chem,2012,36(4):1108−1113. doi: 10.1039/c2nj20690k
|
| [17] |
SHUIHUA T, GONGQUAN S, JING Q, SHIGUO S, JUNSONG G, QIN X, HAARBERG G M. Review of new carbon materials as catalyst supports in direct alcohol fuel cells[J]. Chin J Catal,2010,31(1):12−17. doi: 10.1016/S1872-2067(09)60034-6
|
| [18] |
AHMADI R, AMINI M K. Synthesis and characterization of Pt nanoparticles on sulfur-modified carbon nanotubes for methanol oxidation[J]. Int J Hydrogen Energy,2011,36(12):7275−7283. doi: 10.1016/j.ijhydene.2011.03.013
|
| [19] |
钟盛文, 胡仙超, 俞园, 余长林, 周阳. 核壳结构碳化钨复合微球催化剂对甲醇电催化性能(英文)[J]. 燃料化学学报,2018,46(5):585−591. doi: 10.3969/j.issn.0253-2409.2018.05.011ZHONG Sheng-wen, HU Xian-chao, YU Yuan, YU Chang-lin, ZHOU Yang. Core-shell hierarchical tungsten carbide composite microspheres towards methanol electrooxidation[J]. J Fuel Chem Technol,2018,46(5):585−591. doi: 10.3969/j.issn.0253-2409.2018.05.011
|
| [20] |
CALVILLO L, LÁZARO M, GARCÍA-BORDEJÉ E, MOLINER R, CABOT P, ESPARBÉ I, PASTOR E, QUINTANA J. Platinum supported on functionalized ordered mesoporous carbon as electrocatalyst for direct methanol fuel cells[J]. J Power Sources,2007,169(1):59−64. doi: 10.1016/j.jpowsour.2007.01.042
|
| [21] |
PARK I S, PARK K W, CHOI J H, PARK C R, SUNG Y E. Electrocatalytic enhancement of methanol oxidation by graphite nanofibers with a high loading of PtRu alloy nanoparticles[J]. Carbon,2007,45(1):28−33. doi: 10.1016/j.carbon.2006.08.011
|
| [22] |
BHAVANI K S, ANUSHA T, BRAHMAN P K. Fabrication and characterization of gold nanoparticles and fullerene-C60 nanocomposite film at glassy carbon electrode as potential electro-catalyst towards the methanol oxidation[J]. Int J Hydrogen Energy,2019,44(47):25863−25873. doi: 10.1016/j.ijhydene.2019.08.005
|
| [23] |
ANTOLINI E. Graphene as a new carbon support for low-temperature fuel cell catalysts[J]. Appl Catal B-Environ,2012,123:52−68.
|
| [24] |
魏英祥, 涂伟霞. 膨胀石墨负载钯纳米颗粒催化六价铬还原反应[J]. 高等学校化学学报,2014,35(11):2397−2402.WEI Yingxiang, TUWeixia. Reduction of hexavalent chromium over the expanded graphite supported palladium nanocatalyst[J]. Chem J Chin Univ,2014,35(11):2397−2402.
|
| [25] |
YUAN J, TANG S, ZHU Z, QIN X, QU R, DENG Y, WU L, LI J, HAARBERG G M. Facile synthesis of high-performance Ni(OH)2/expanded graphite electrodes for asymmetric supercapacitors[J]. J Mater Sci-Mater El,2017,28(23):18022−18030. doi: 10.1007/s10854-017-7745-1
|
| [26] |
LI H, ZHANG Y, WAN Q, LI Y, YANG N. Expanded graphite and carbon nanotube supported palladium nanoparticles for electrocatalytic oxidation of liquid fuels[J]. Carbon,2018,131:111−119. doi: 10.1016/j.carbon.2018.01.093
|
| [27] |
DHAKATE S, SHARMA S, BORAH M, MATHUR R, DHAMI T. Expanded graphite-based electrically conductive composites as bipolar plate for PEM fuel cell[J]. Int J Hydrogen Energy,2008,33(23):7146−7152. doi: 10.1016/j.ijhydene.2008.09.004
|
| [28] |
RAMACHANDRAN R, FELIX S, JOSHI G M, RAGHUPATHY B P C, JEONG S K, GRACE A N. Synthesis of graphene platelets by chemical and electrochemical route[J]. Mater Res Bull,2013,48(10):3834−3842. doi: 10.1016/j.materresbull.2013.05.085
|
| [29] |
HYDE T. Final analysis: Crystallite size analysis of supported platinum catalysts by XRD[J]. Platin Met Rev,2008,52(2):129−130. doi: 10.1595/147106708X299547
|
| [30] |
OJANI R, RAOOF J B, GOLI M, VALIOLLAHI R. Pt–Co nanostructures electrodeposited on graphene nanosheets for methanol electrooxidation[J]. J Power Sources,2014,264:76−82. doi: 10.1016/j.jpowsour.2014.03.147
|
| [31] |
VELÁZQUEZ-PALENZUELA A, CENTELLAS F, GARRIDO J A, ARIAS C, RODRÍGUEZ R M, BRILLAS E, CABOT P L. Kinetic analysis of carbon monoxide and methanol oxidation on high performance carbon-supported Pt-Ru electrocatalyst for direct methanol fuel cells[J]. J Power Sources,2011,196(7):3503−3512. doi: 10.1016/j.jpowsour.2010.12.044
|





 下载:
下载: