Study on the synthesis of melem catalyst and its application in synthesis of cyclic carbonate
-
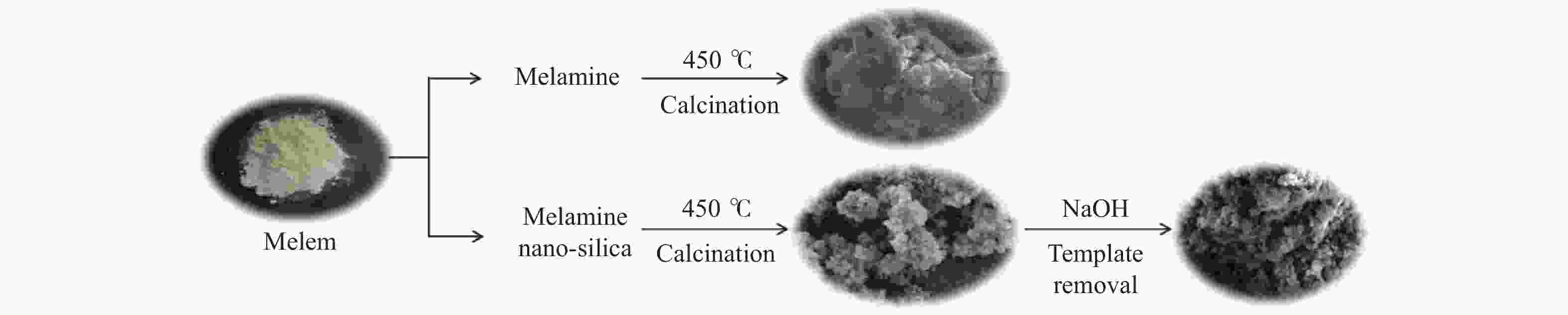
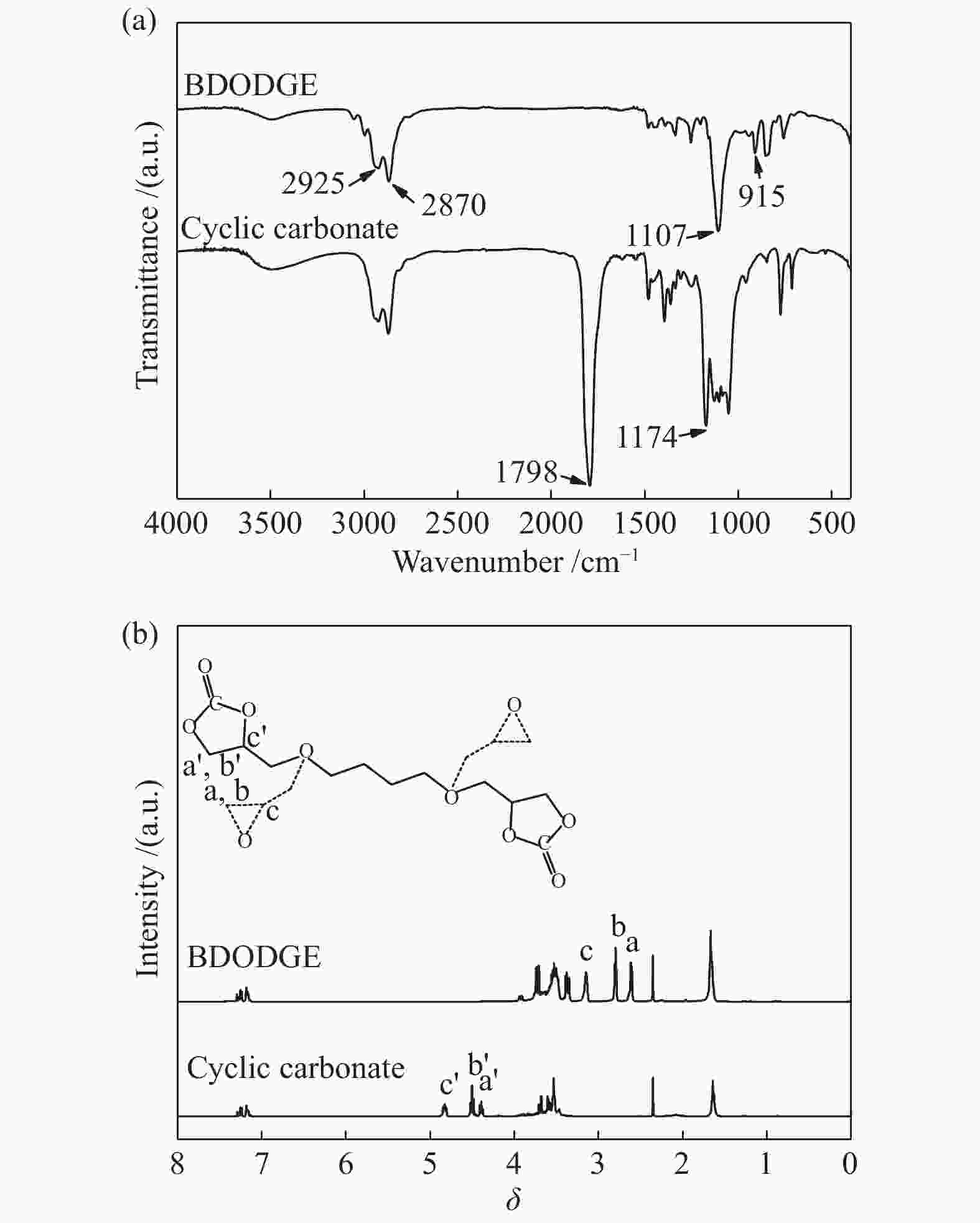
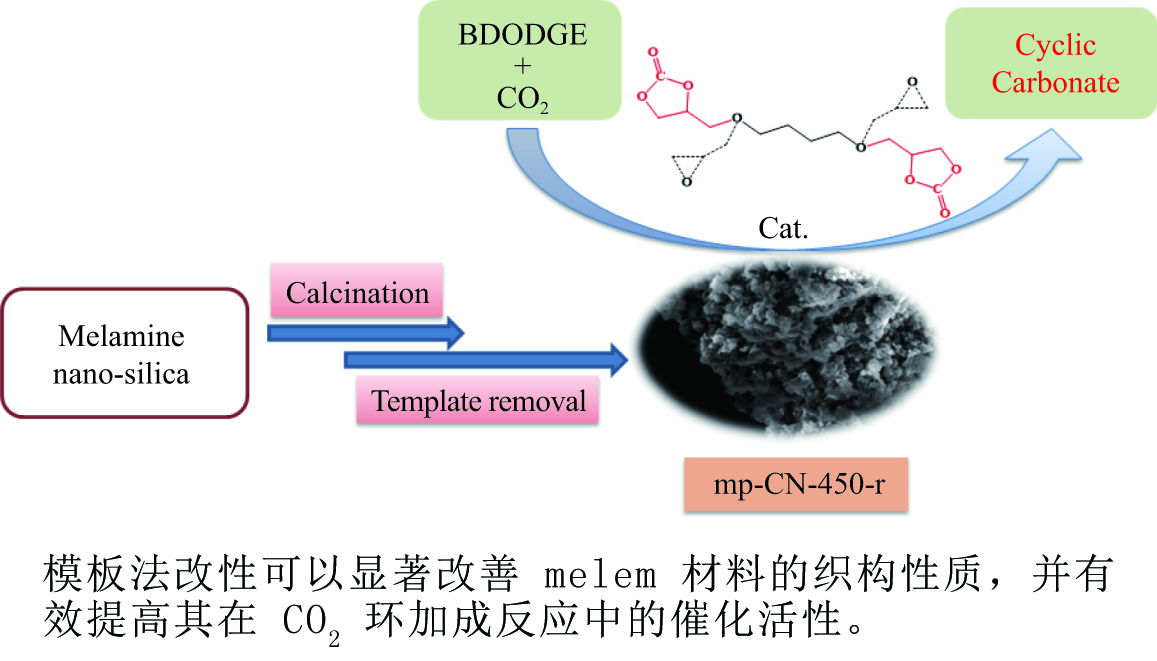
摘要: 以纳米二氧化硅 (SiO2) 为模板,以三聚氰胺为前驱体,采用固相反应法制备一系列介孔蜜勒胺 (melem) 材料。通过调控硬模板剂和前驱体的用量,最终得到一系列高比表面积 (40−92 m2/g) 和孔体积 (0.179−0.407 cm3/g)的介孔 melem材料。将其作为催化剂用于1, 4 -丁二醇二缩水甘油醚 (BDODGE) 与CO2的环加成反应中,结果表明,随着比表面积增大,催化剂活性相比未加入模板剂的melem样品有了显著提高。三聚氰胺与纳米SiO2的质量比为2时制备的催化剂,在130 ℃、20 h、2.0 MPa条件下,BDODGE转化率为99.3%,环碳酸酯选择性为99.5%。Abstract: In this paper, a series of mesoporous melem materials were prepared from melamine by solid-phase reaction using nano-silica (SiO2) as the template. The mesoporous melem materials with high specific surface area (40−92 m2/g) and pore volume (0.179−0.407 cm3/g) were obtained by adjusting the ratio of SiO2 template and melamine. The obtained melem was used as catalyst for the cycloaddition reaction between BDODGE and CO2. The results showed that the catalytic activity was greatly improved with the increase of specific surface area of the catalysts. The catalyst mp-CN-450-2 showed the best performance. After 20 h reaction at 130 ºC, 2.0 MPa, 99.3% of BDODGE conversion and 99.5% of cyclic carbonate selectivity were acquired, respectively.
-
Key words:
- melamine /
- mesoporous melem materials /
- epoxide /
- cyclic carbonate /
- CO2
-
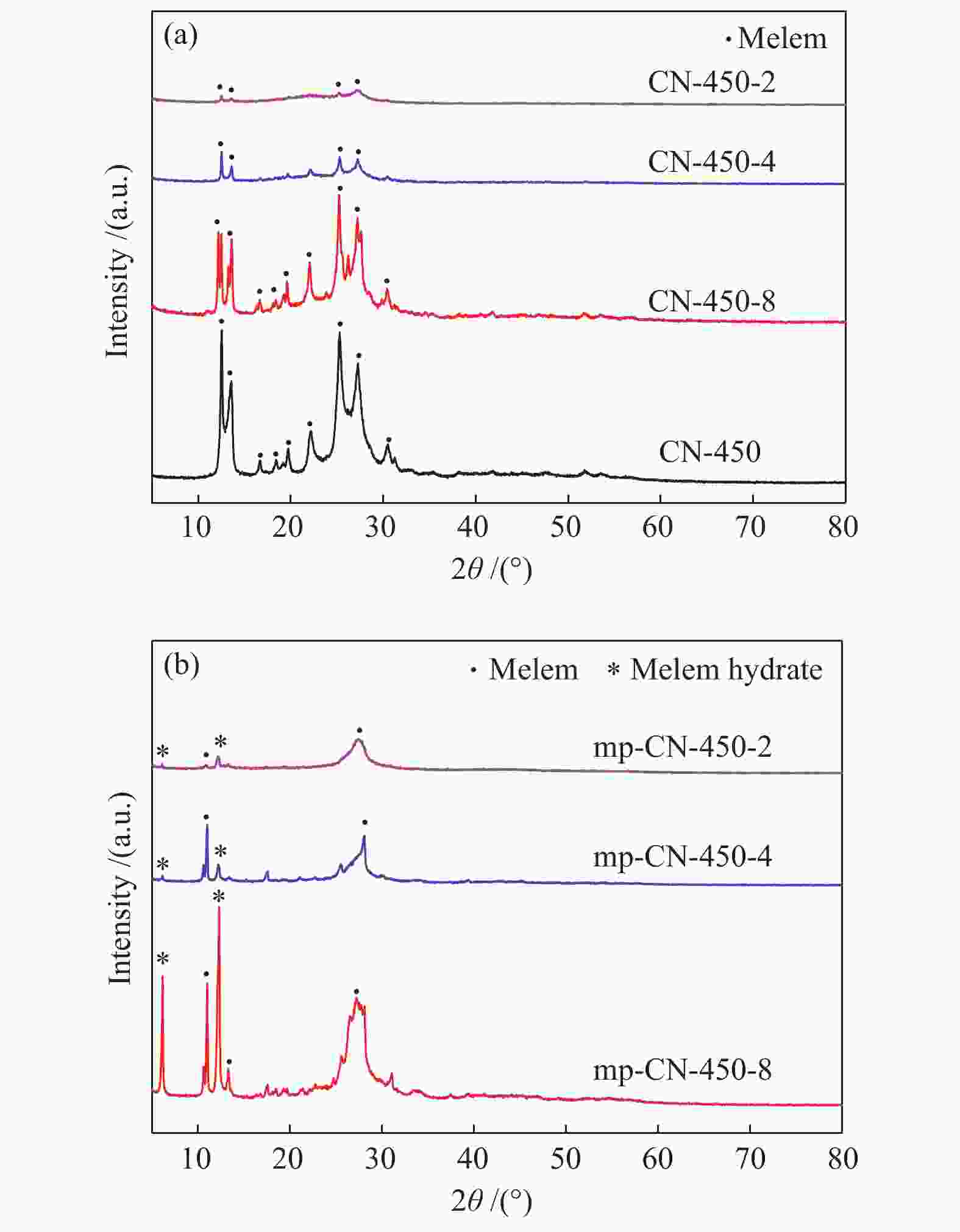
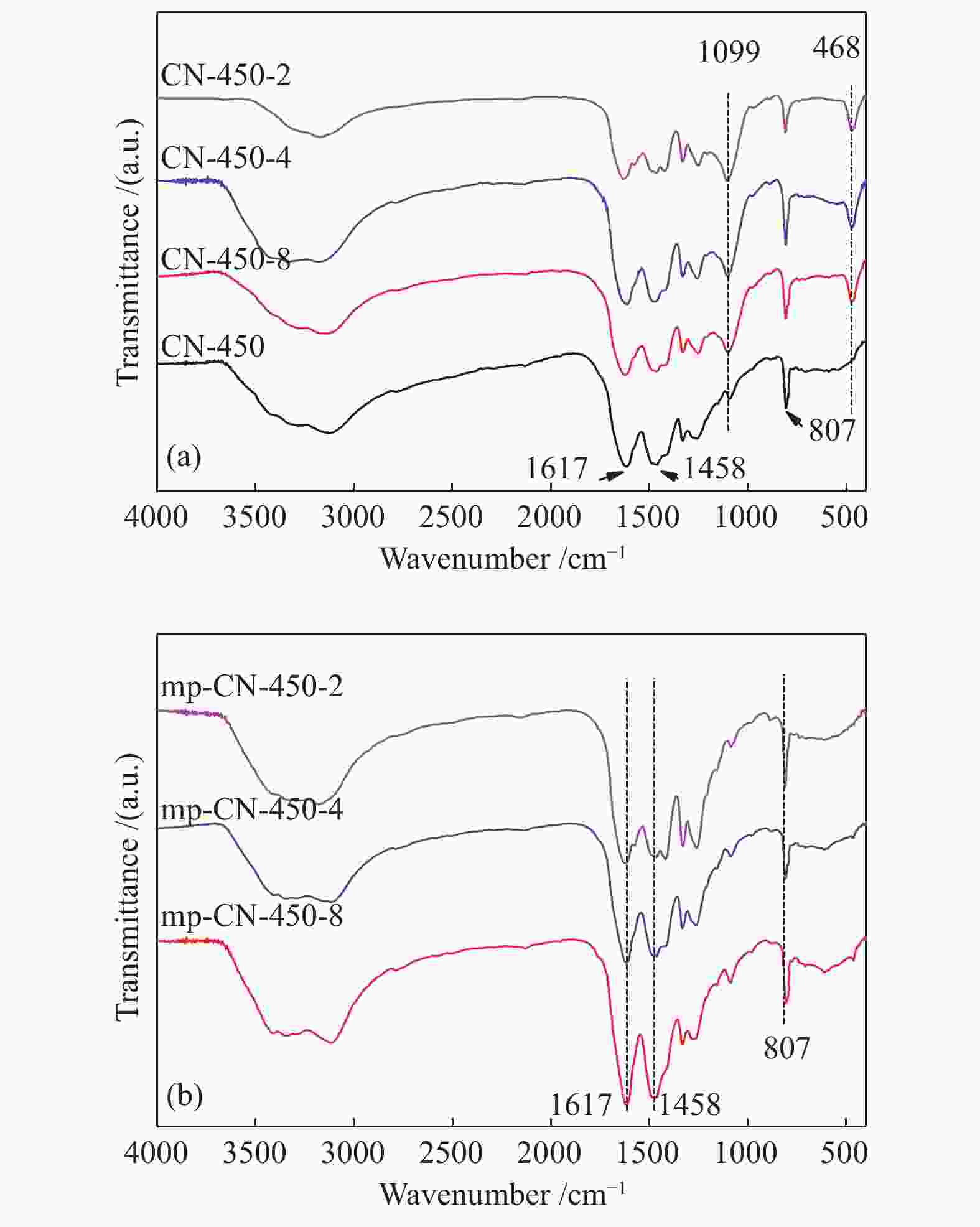
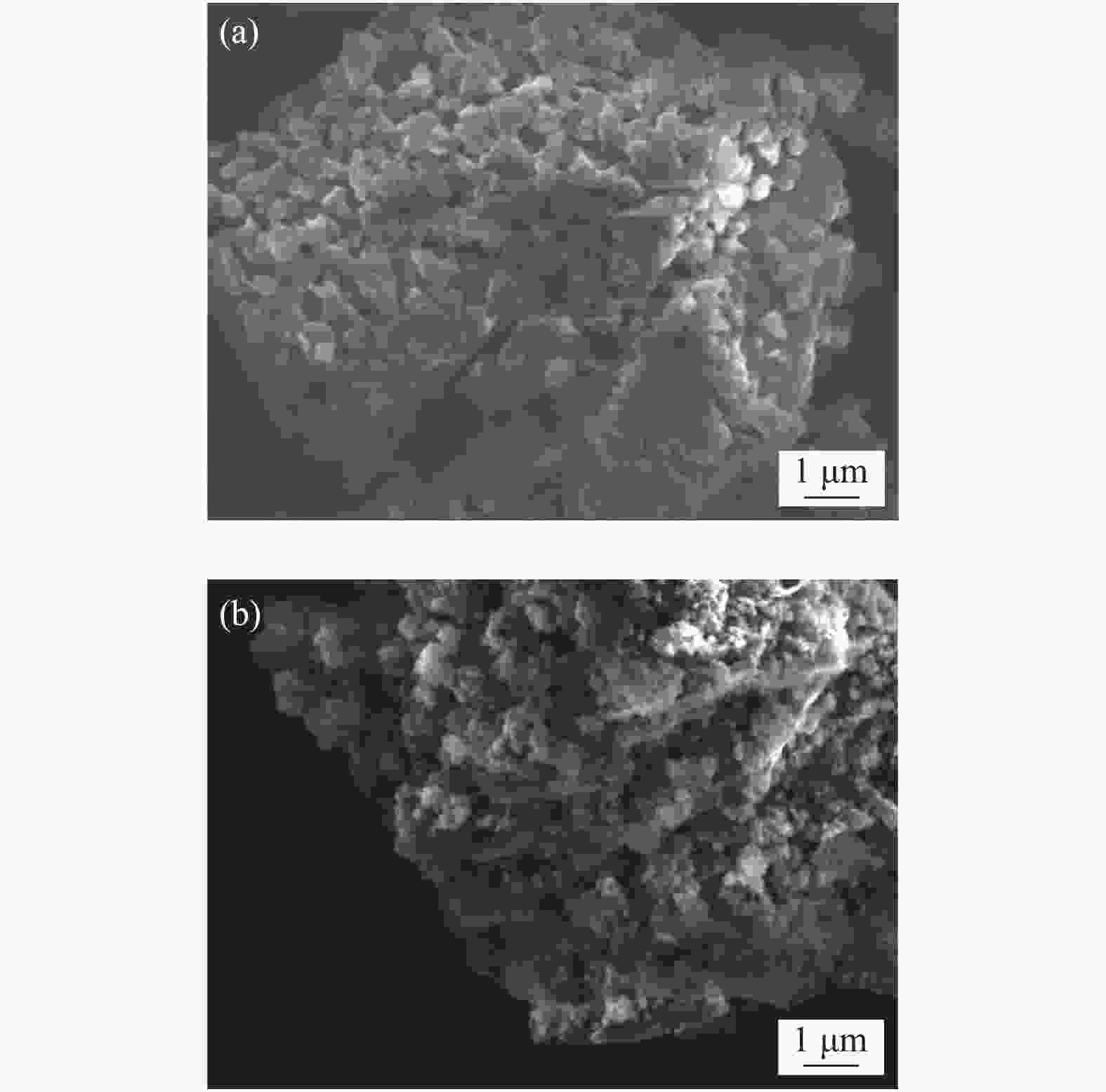
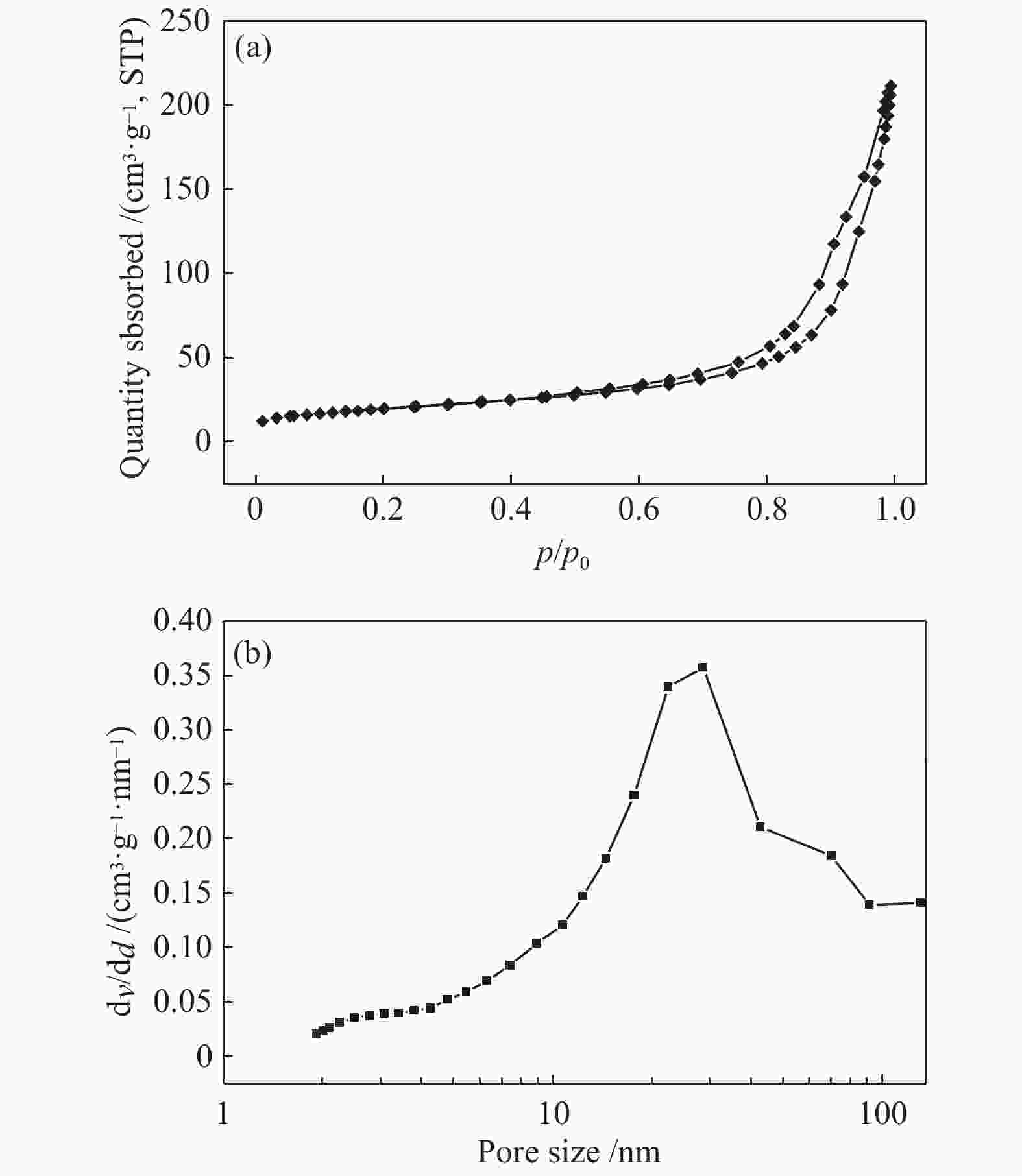
表 1 CN-450-r及mp-CN-450-r材料的织构分析
Table 1 Textual parameters of CN-450-r and mp-CN-450-r materials
Entry Catalyst SBET/ (m2·g−1) Pore volume/ (cm3·g−1) Average pore diameter / nm 1 CN-450 4 0.0157 16.3 2 CN-450-8 7 0.0332 19.1 3 CN-450-4 16 0.0589 14.8 4 CN-450-2 36 0.1394 15.6 5 mp-CN-450-8 40 0.1794 17.7 6 mp-CN-450-4 69 0.3275 18.9 7 mp-CN-450-2 92 0.4072 17.6 8 SiO2 189 0.7826 16.6 表 2 CN-450-r及mp-CN-450-r催化剂对BDODGE与CO2环加成反应的影响
Table 2 Catalytic performance of different CN-450-r and mp-CN-450-r catalysts in the reaction of BDODGE and CO2
Entry Catalyst BDODGE
conversion/%Selectivity/
%1 a CN-450 32.5 91.7 2 a CN-450-8 25.8 99.0 3 a CN-450-4 38.2 99.7 4 a CN-450-2 33.5 94.2 5 b[19] CN-450-W 93.1 99.3 6 c mp-CN-450-8 97.6 98.3 7 c mp-CN-450-4 98.7 98.9 8 c mp-CN-450-2 99.3 99.5 a reaction conditions:W(BDODGE) = 30 g, W(catalyst) = 1.5 g, p(CO2) = 2.0 MPa, t = 140 ℃, t = 20 h; b W(catalyst) = 2.1 g; c t = 130 ℃ 表 3 各种催化剂催化CO2环加成反应对比
Table 3 Comparison of various catalysts for the cycloaddition of CO2
Entry Catalyst Conversion / % Selectivity / % Ref. 1 a m-C3N4 36.1 98.0 [19] 2 a u-C3N4 51.3 98.2 [19] 3 b ZnBr2/u-CN/r-Al2O3 98.9 99.1 [25] 4 c D296 95.8 97.1 [26] 5 d LiBr 94.0 99.1 [27] 6 mp-CN-450-2 99.3 99.5 this work a reaction conditions:W(BDODGE) = 30 g, W(catalyst) = 5.0%, p(CO2) = 1.0 MPa, t = 140 ℃, t = 20 h;b W(catalyst) = 15.1%, t = 30 h;c W(PPGDGE) = 100 g, W(catalyst) = 10%, t = 30 h;d W(E51) = 30 g, W(catalyst) = 1.0%, W(DMF) = 25 g, t = 90 ℃ -
[1] OLAH G A, PRAKASH G K, GOEPPERT A. Anthropogenic chemical carbon cycle for a sustainable future[J]. J Am Chem Soc,2011,133(33):12881−12898. doi: 10.1021/ja202642y [2] LI L, ZHAO N, WEI W, SUN Y H. A review of research progress on CO2 capture, storage, and utilization in Chinese Academy of Sciences[J]. Fuel, 2013, 108 (1): 112−130. [3] COMERFORD J W, INGRAM I D V, NORTH M, WU X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings[J]. Green Chem,2015,17(4):1966−1987. doi: 10.1039/C4GC01719F [4] MARINO T, PONTE F, MAZZONE G, SICILIA E, TOSCANO M, RUSSO N. The ability of a zinc pyrrolidine complex to catalyze the synthesis of cyclic carbonates from carbon dioxide and epoxides: A mechanistic theoretical investigation[J]. Dalton Trans,2017,46(28):9030−9035. doi: 10.1039/C7DT01642E [5] LI J W, REN Y W, QI C R, JIANG H F. A chiral salen-based MOF catalytic material with high thermal, aqueous and chemical stabilities[J]. Dalton Trans,2017,46(24):7821−7832. doi: 10.1039/C7DT01116D [6] DAI W L, YIN S F, GUO R, LUO S L, DU X, AU C T. Synthesis of propylene carbonate from carbon dioxide and propylene oxide using Zn-Mg-Al composite oxide as high-efficiency catalyst[J]. Catal Let,2009,136(1/2):35−44. [7] ADELEYE A I, PATEL D, NIYOGI D, SAHA B. Efficient and greener synthesis of propylene carbonate from carbon dioxide and propylene oxide[J]. Ind Eng Chem Res,2014,53(49):18647−18657. doi: 10.1021/ie500345z [8] DOSKOCIL E J. Effect of water and alkali modifications on ETS-10 for the cycloaddition of CO2 to propylene oxide[J]. J Phys Chem B,2005,109(6):2315−2320. doi: 10.1021/jp048870g [9] SRIVASTAVA R, SRINIVAS D, RATNASAMY P. Synthesis of polycarbonate precursors over titanosilicate molecular sieves[J]. Catal Lett,2003,91(1/2):133−139. [10] FENG D W, CHUNG W C, WEI Z W, GU Z Y, JIANG H L, CHEN Y P, DARENSBOURG D J, ZHOU H C. Construction of ultrastable porphyrin Zr metal-organic frameworks through linker elimination[J]. J Am Chem Soc,2013,135(45):17105−17110. doi: 10.1021/ja408084j [11] BABU R, ROSHAN R, KATHALIKKATTIL A C, KIM D W, PAEK D W. Rapid, microwave-assisted synthesis of cubic, three-dimensional, highly porous MOF-205 for room temperature CO2 fixation via cyclic carbonate synthesis[J]. ACS Appl Mater Interfaces,2016,8(49):33723−33731. doi: 10.1021/acsami.6b12458 [12] SU Q, SUN J, WANG J Q, YANG Z F, CHENG WG, ZHANG S J. Urea-derived graphitic carbon nitride as an efficient heterogeneous catalyst for CO2 conversion into cyclic carbonates[J]. Catal Sci Technol,2014,4(6):1556−1562. doi: 10.1039/c3cy00921a [13] BISWAS T, MAHALINGAM V. g-C3N4 and tetrabutylammonium bromide catalyzed efficient conversion of epoxide to cyclic carbonate under ambient conditions[J]. New J Chem,2017,41(24):14839−14842. doi: 10.1039/C7NJ03720A [14] XU J, LONG K Z, WANG Y, XUE B, LI Y X. Fast and facile preparation of metal-doped g-C3N4 composites for catalytic synthesis of dimethyl carbonate[J]. Appl Catal A: Gen,2015,496:1−8. doi: 10.1016/j.apcata.2015.02.025 [15] ZHU J J, DIAO T T, WANG W Y, XU X L, SUN X Y, CARABINEIRO S, ZHAO Z. Boron doped graphitic carbon nitride with acid-base duality for cycloaddition of carbon dioxide to epoxide under solvent-free condition[J]. Appl Catal B: Environ,2017,219:92−100. doi: 10.1016/j.apcatb.2017.07.041 [16] LIU M S, LAN J W, LIANG L, SUN J M, ARAI M. Heterogeneous catalytic conversion of CO2 and epoxides to cyclic carbonates over multifunctional tri-s-triazine terminal-linked ionic liquids[J]. J Catal,2017,347:138−147. doi: 10.1016/j.jcat.2016.11.038 [17] GOETTMANN F, THOMAS A, ANTONIETTI M. Metal-free activation of CO2 by mesoporous graphitic carbon nitride[J]. Angew Chem Int Ed,2007,46(15):2717−2720. doi: 10.1002/anie.200603478 [18] SONG X H, WU Y F, PAN D H, WEI R P, GAO L J, ZHANG J, XIAO G M. Melem based multifunctional catalyst for chemical fixation of carbon dioxide into cyclic carbonate[J]. J CO2 Util,2018,24:287−297. doi: 10.1016/j.jcou.2018.01.017 [19] 梁宏光. 多官能度五元环状碳酸酯合成中多相催化剂的制备及构效关系研究[D]. 太原: 中国科学院山西煤炭化学研究所, 2019.LIANG hong-guang. The study on the preparation and structure-performance relationship of heterogeneous catalysts for synthesis of polyfunctional five-membered cyclic carbonates[D]. Taiyuan: Institute of Coal Chemistry, Chinese Academy of Sciences, 2019. [20] LI X, KE J, WANG J, LIANG C, KANG M, ZHAO Y, LI Q. A new amino-alcohol originated from carbon dioxide and its application as chain extender in the preparation of polyurethane[J]. J CO2 Util,2018,26:52−9. doi: 10.1016/j.jcou.2018.04.018 [21] JURGENS B, IRRAN E, SENKER J, KROLL P, MULLER H, SCHNICK W. Melem (2,5,8-triamino-tri-s-triazine), an important intermediate during condensation of melamine rings to graphitic carbon nitride: Synthesis, structure determination by X-ray powder diffractometry, solid-state NMR, and theoretical studies[J]. J Am Chem Soc,2003,125(34):10288−10300. doi: 10.1021/ja0357689 [22] MAKOWSKI S J, PIA K S, WOLFGANG S. Formation of a hydrogen-bonded heptazine framework by self-assembly of melem into a hexagonal channel structure[J]. Chem-Eur J,2012,18(11):3248−3257. doi: 10.1002/chem.201103527 [23] 陈圣军, 钮腾飞, 倪邦庆. 介孔氮化碳光催化氧化苄基卤化物的反应研究[J/OL]. 合成化学: 1-12[2020-10-12]. https://doi.org/10.15952/j.cnki.cjsc.1005-1511.20001. [24] CHEN W, ZHONG L X, PENG X W, SUN R C, LU F C. Chemical fixation of carbon dioxide using a green and efficient catalytic system based on sugarcane bagasse-an agricultural waste[J]. ACS Sustainable Chem Eng,2015,3(1):147−152. doi: 10.1021/sc5006445 [25] LIANG H G, WANG J W, LI Q F, LIANG C, FENG Y L, KANG M Q. Supported ZnBr2 and carbon nitride bifunctional complex catalysts for the efficient cycloaddition of CO2 with diglycidyl ethers[J]. New J Chem,2018,42(19):16127−37. doi: 10.1039/C8NJ03499K [26] KE J X, LI X Y, WANG F, JIANG S, KANG M Q, WANG J W, LI Q F, WANG Z J. Non-isocyanate polyurethane/epoxy hybrid materials with different and controlled architectures prepared from a CO2-sourced monomer and epoxy via an environmentally-friendly route[J]. Rsc Adv,2017,7(46):28841−52. doi: 10.1039/C7RA04215A [27] LIANG H G, WANG J W, WANG F, FENG Y L, KANG M Q, WANG Z J. An efficient heterogeneous LiBr/-Al2O3 catalyst for the cycloaddition of CO2 with diglycidyl ethers[J]. J Chem Technol Biot,2018,93(8):2271−80. doi: 10.1002/jctb.5570 -





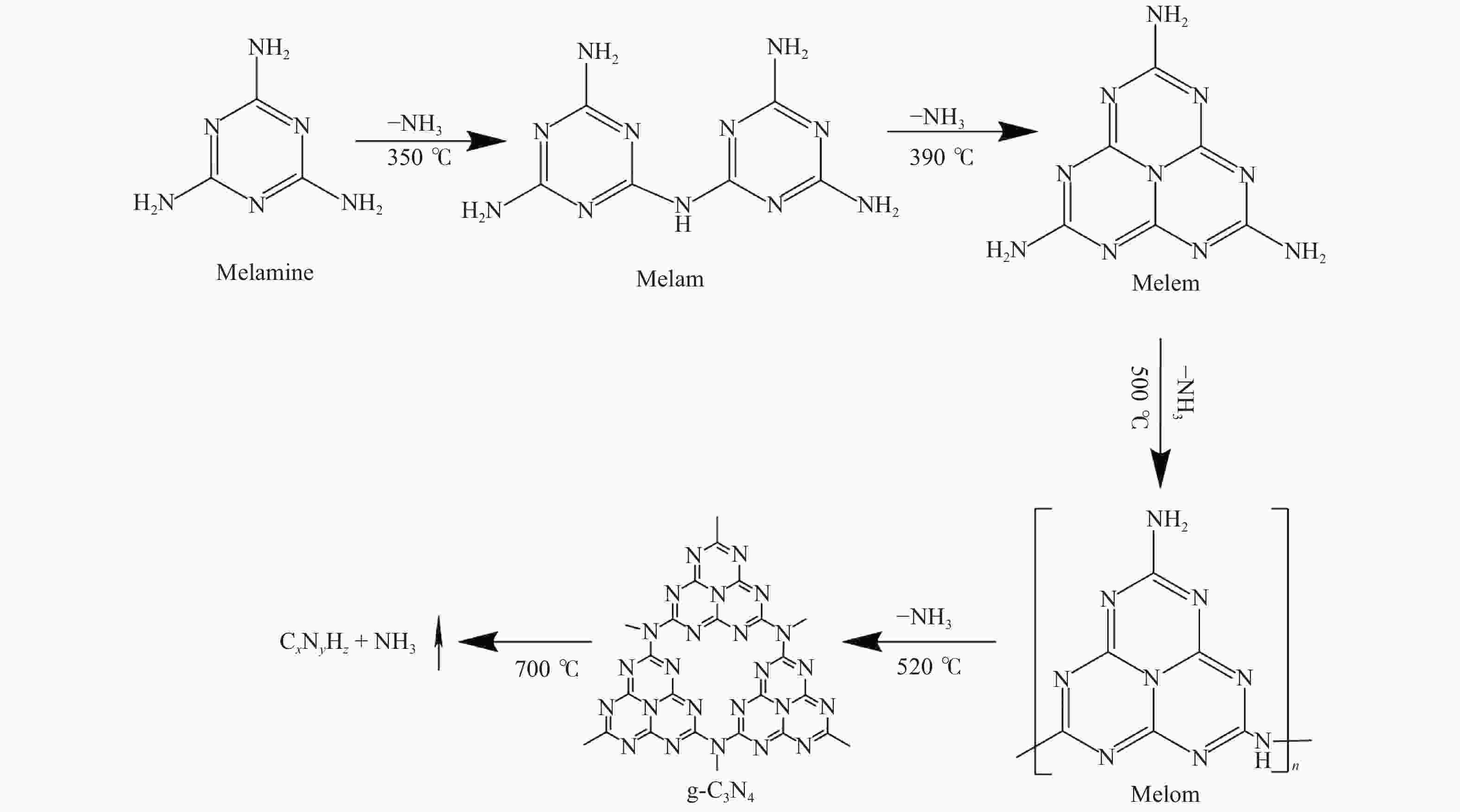
 下载:
下载: