Effects of HNO3 modification on the mechanism of low temperature NO reduction over activated carbon
-
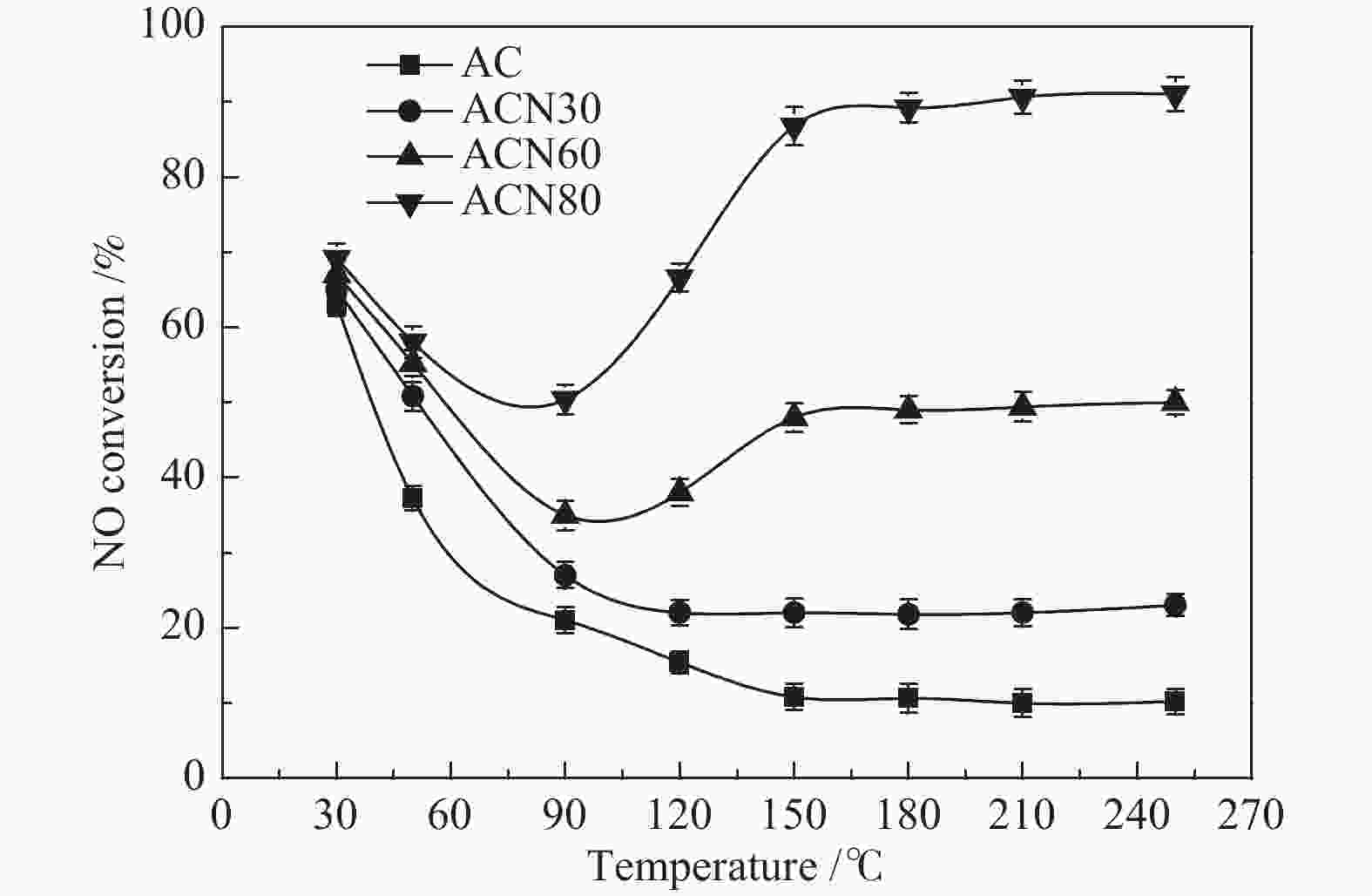
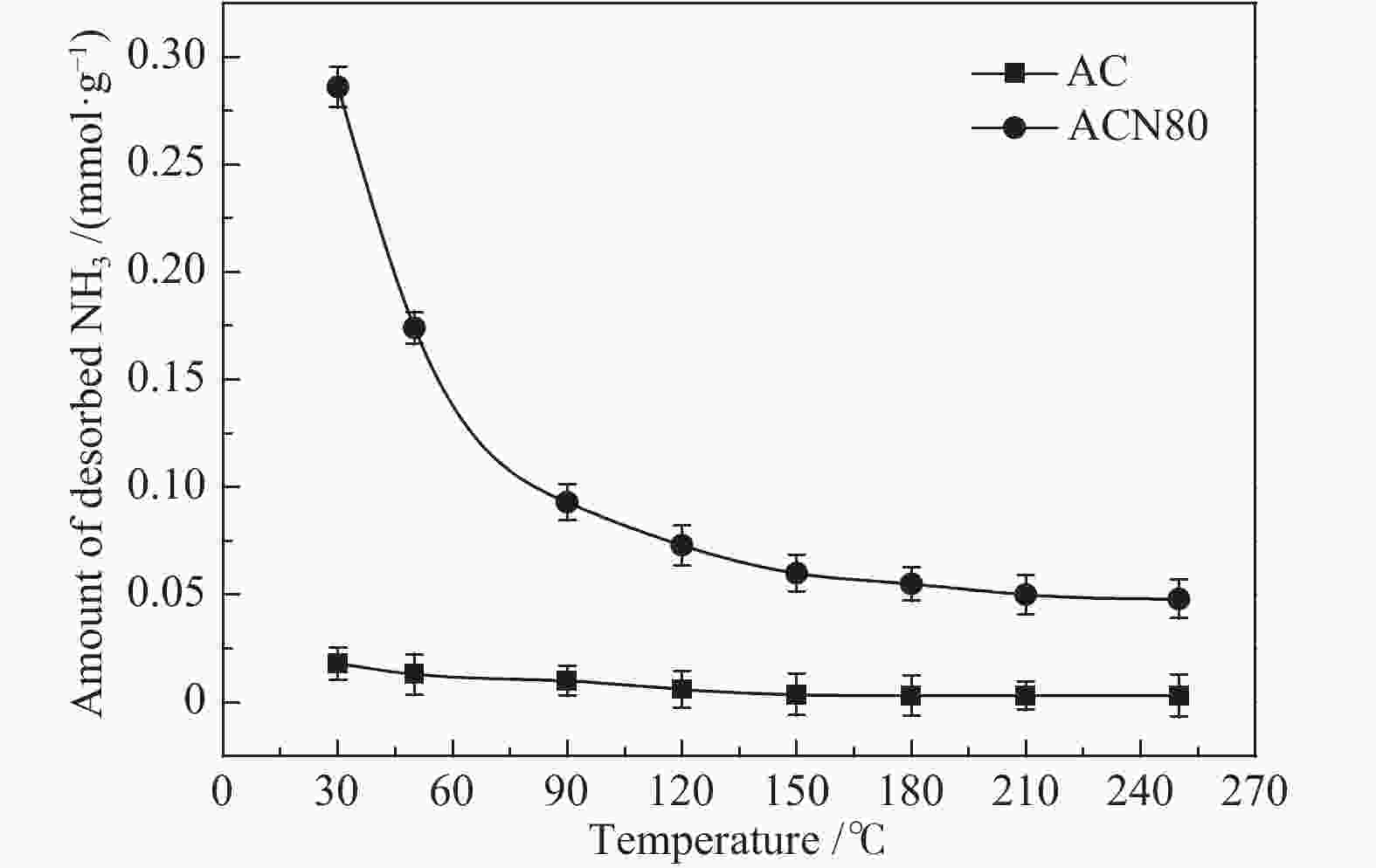
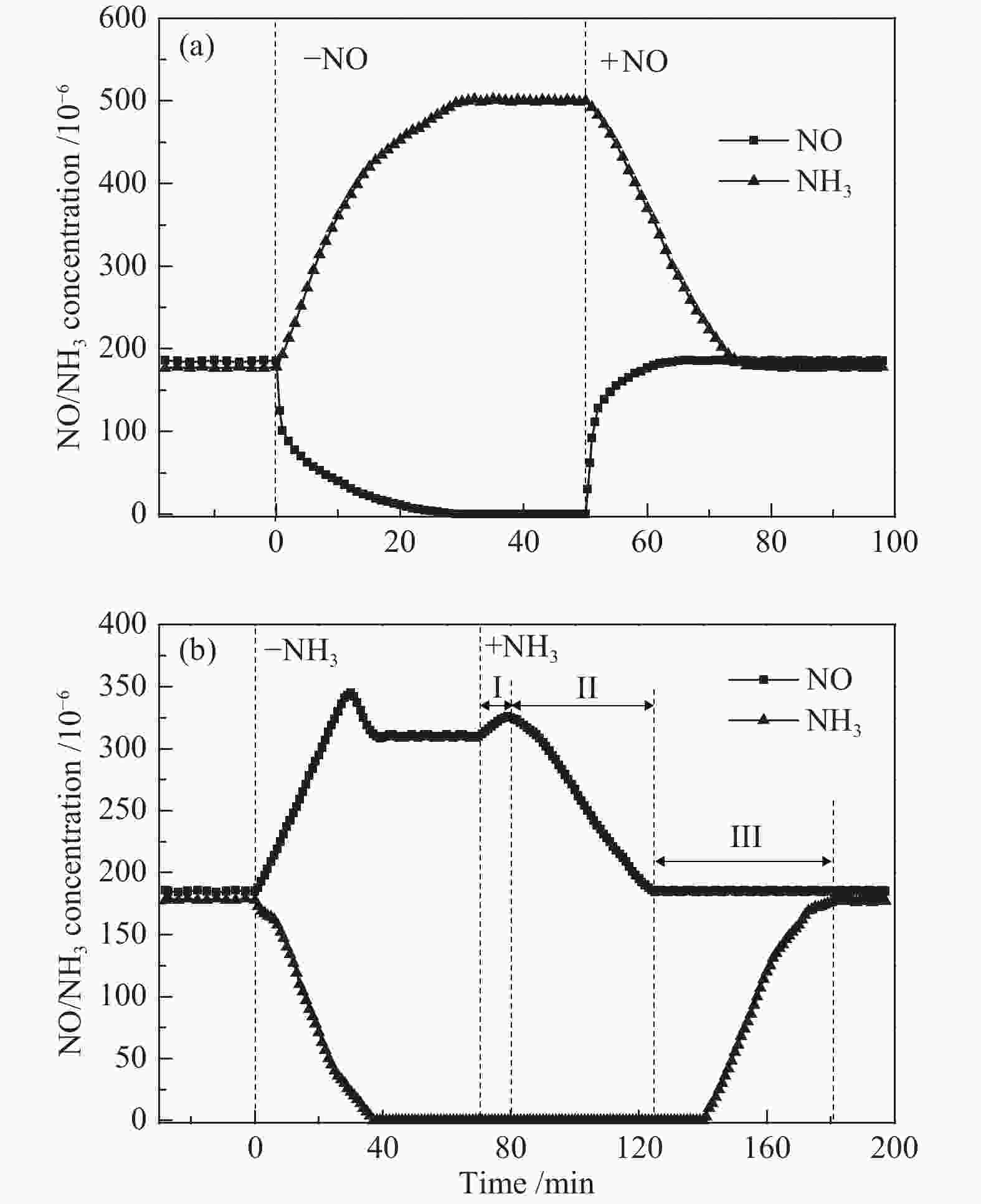
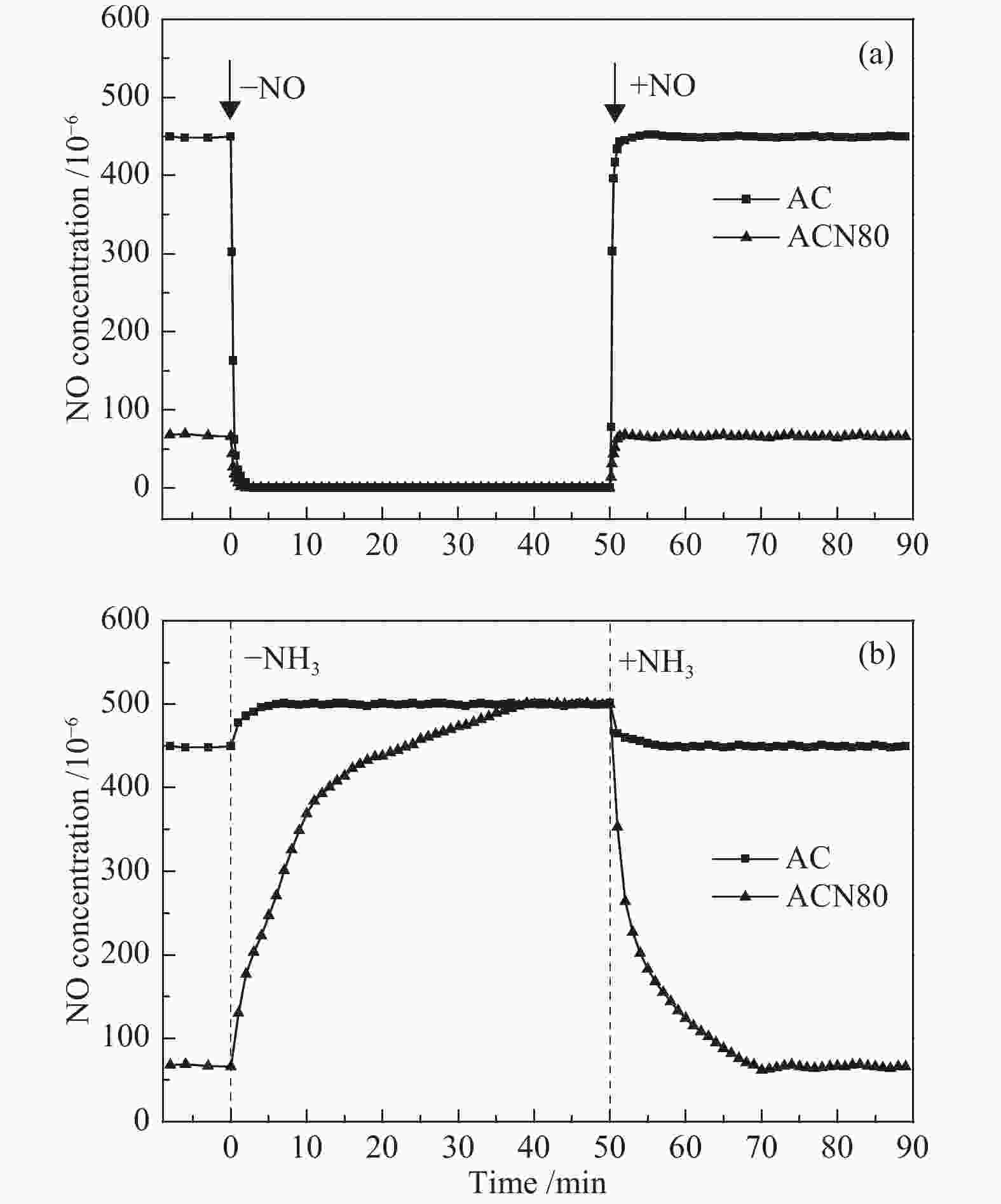
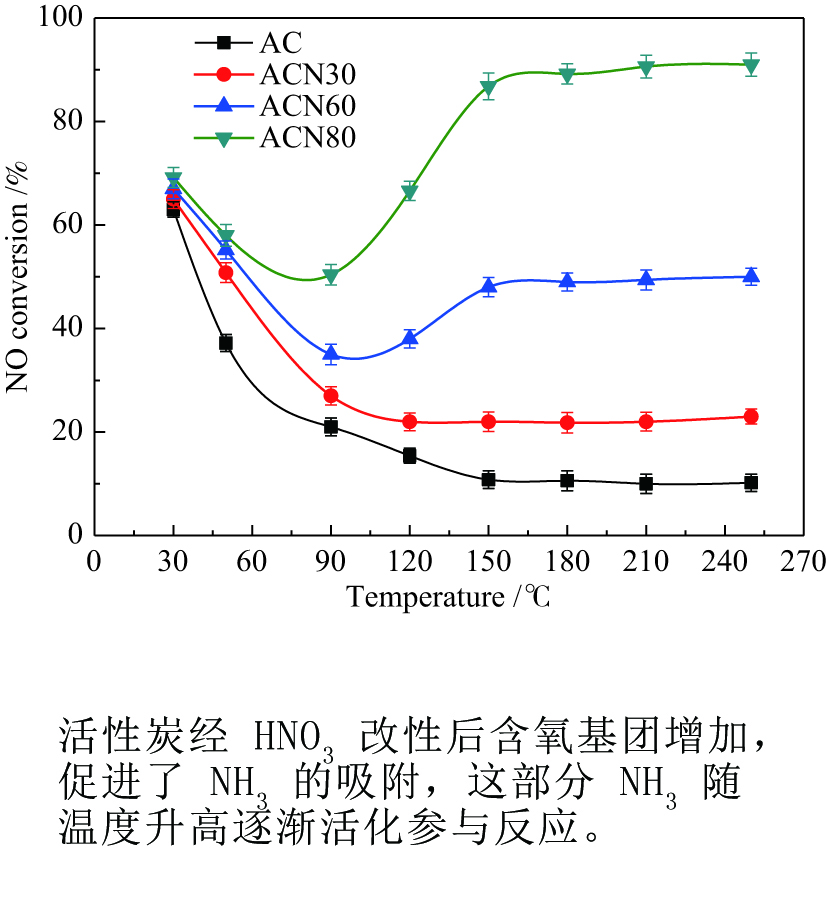
摘要: 为了研究HNO3改性对活性炭(AC)低温脱硝机理的影响,采用HNO3在不同温度下对AC进行改性处理,改性前后样品在30−250 ℃进行脱硝活性测试,通过程序升温脱附(TPD)和瞬态响应实验对脱硝机理进行分析。结果表明,AC上NO转化率随反应温度升高逐渐降低再趋于稳定,反应物的吸附是速率控制步骤。含氧基团增加促进了NH3的吸附,但这部分吸附态NH3在30 ℃时几乎不参与反应,随反应温度升高不同程度活化,在30−90 ℃反应物的吸附是速率控制步骤,90 ℃后吸附态NH3的活化是速率控制步骤。Abstract: To study the effects of HNO3 modification on NO reduction over activated carbon (AC) at low temperatures, the AC was modified using HNO3 at different temperatures. The activity of the resulted activated carbons towards NO reduction with NH3 was investigated in a temperature range of 30−250 ℃. The temperature programmed desorption (TPD) and transient response experiments were used to study the mechanism of NO reduction. The results reveal that the NO conversion decreased with temperature at the initial stage and then kept stable. The adsorption of reactants was the rate-determining step. The increase of oxygen groups on the catalyst surface enhanced NH3 adsorption. The adsorbed NH3 hardly reacted with NO at 30 ℃. With increasing temperature, the adsorbed NH3 was gradually activated. In the temperature range of 30−90 ℃, the adsorption of reactants was rate-determining step of NO reduction. When the temperature was higher than 90 ℃, the reaction was dominated by the activation of adsorbed NH3.
-
Key words:
- NO reduction /
- activated carbon /
- SCR /
- oxygen groups /
- HNO3 modification
-
表 1 活性测试反应条件
Table 1 Reaction conditions for activity test
Unit Range NO 1 5×10−4 NH3 1 5×10−4 O2 1 4% N2 balance Total flow rate mL/min 400 Temperature ℃ 30−250 -
[1] 梁彦正, 王学涛, 张乾蔚, 罗绍峰, 周瑜枫. 双金属Ce-Mn/ZSM-5催化剂的制备及NH3-SCR脱硝性能研究[J]. 燃料化学学报,2020,48(2):205−212. doi: 10.3969/j.issn.0253-2409.2020.02.010LIANG Yan-zheng, WANG Xue-tao, ZHANG Qian-wei, LUO Shao-feng, ZHOU Yu-feng. Study on the preparation and catalytic performance of bimetallic Ce-Mn /ZSM-5 catalyst for selective catalytic reduction of nitric oxide by NH3[J]. J Fuel Chem Technol,2020,48(2):205−212. doi: 10.3969/j.issn.0253-2409.2020.02.010 [2] YULIANG W, KETING G, XIANGXIANG L, HUI L, SHAOCHEN G, DONGDONG R. Performance of Mn-Ce co-doped siderite catalysts in the selective catalytic reduction of NOx by NH3[J]. J Fuel Chem Technol,2019,47(12):1495−1502. doi: 10.1016/S1872-5813(19)30061-1 [3] LI S, WANG X, TAN S, SHI Y, LI W. CrO3 supported on sargassum-based activated carbon as low temperature catalysts for the selective catalytic reduction of NO with NH3[J]. Fuel,2017,191:511−517. doi: 10.1016/j.fuel.2016.11.095 [4] 杨辉, 刘豪, 朱德力, 王泽安, 邱建荣, 曾汉才. 活性炭纤维联合脱硫脱硝的机理分析[J]. 中国电机工程学报,2015,35:2495−2503.YANG Hui, LIU Hao, ZHU De-li, WANG Ze-an, QIU Jian-rong, ZENG Han-cai. Mechanism of Combined Removal of SO2 and NO Over Activated Carbon Fibers[J]. Proc CSEE,2015,35:2495−2503. [5] YAN W, LI S, FAN C, DENG S. Effect of surface carbon-oxygen complexes during NO reduction by coal char[J]. Fuel,2017,204:40−46. doi: 10.1016/j.fuel.2017.05.045 [6] 步学朋, 徐振刚, 李文华, 梁大明, 孙仲超, 李雪飞, 熊银伍, 吴涛, 李兰廷, 张科达. 中国活性焦烟气净化研究分析[J]. 煤质技术,2010,16(2):67−71. doi: 10.3969/j.issn.1007-7677.2010.01.027BU Xue-peng, XU Zhen-gang, LI Wen-hua, LIANG Da-ming, SUN Zhong-chao, LI Xue-fei, XIONG Yin-wu, WU Tao, LI Lan-ting, ZHANG Ke-da. Study and analysis on flue gas purification of active coke in China[J]. Coal Qual Technol,2010,16(2):67−71. doi: 10.3969/j.issn.1007-7677.2010.01.027 [7] YOU F, YU G, XING Z, LI J, XIE S, LI C, WANG G, REN H, WANG Y. Enhancement of NO catalytic oxidation on activated carbon at room temperature by nitric acid hydrothermal treatment[J]. Appl Surf Sci,2019,471:633−644. doi: 10.1016/j.apsusc.2018.12.066 [8] JIANG L, LIU Q, RAN G, KONG M, REN S, YANG J, LI J. V2O5-modified Mn-Ce/AC catalyst with high SO2 tolerance for low-temperature NH3-SCR of NO[J]. Chem Eng J,2019,370:810−821. doi: 10.1016/j.cej.2019.03.225 [9] LIN Y, LI Y, XU Z, XIONG J, ZHU T. Transformation of functional groups in the reduction of NO with NH3 over nitrogen-enriched activated carbons[J]. Fuel,2018,223:312−323. doi: 10.1016/j.fuel.2018.01.092 [10] ZHU L, HUANG B, WANG W, WEI Z, YE D. Low-temperature SCR of NO with NH3 over CeO2 supported on modified activated carbon fibers[J]. Catal Commun,2011,12(6):394−398. doi: 10.1016/j.catcom.2010.10.028 [11] GUO Q, JING W, HOU Y, HUANG Z, MA G, HAN X, SUN D. On the nature of oxygen groups for NH3-SCR of NO over carbon at low temperatures[J]. Chem Eng J,2015,270:41−49. doi: 10.1016/j.cej.2015.01.086 [12] HUANG C C, LI H S, CHEN C H. Effect of surface acidic oxides of activated carbon on adsorption of ammonia[J]. J Hazard Mater,2008,159(2/3):523−527. doi: 10.1016/j.jhazmat.2008.02.051 [13] LE LEUCH L M, BANDOSZ T J. The role of water and surface acidity on the reactive adsorption of ammonia on modified activated carbons[J]. Carbon,2007,45(3):568−578. doi: 10.1016/j.carbon.2006.10.016 [14] ZHANG W J, RABIEI S, BAGREEV A, ZHUANG M S, RASOULI F. Study of NO adsorption on activated carbons[J]. Appl Catal B: Environ,2008,83(1/2):63−71. doi: 10.1016/j.apcatb.2008.02.003 [15] ATKINSON J D, ZHANG Z, YAN Z, ROOD M J. Evolution and impact of acidic oxygen functional groups on activated carbon fiber cloth during NO oxidation[J]. Carbon,2013,54:444−453. doi: 10.1016/j.carbon.2012.11.060 [16] LAZARO M, GALVEZ M, RUIZ C, JUAN R, MOLINER R. Vanadium loaded carbon-based catalysts for the reduction of nitric oxide[J]. Appl Catal B: Environ,2006,68(3/4):130−138. doi: 10.1016/j.apcatb.2006.07.025 [17] ZHU Z, LIU Z, LIU S, NIU H, HU T, LIU T, XIE Y. NO reduction with NH3 over an activated carbon-supported copper oxide catalysts at low temperatures[J]. Appl Catal B: Environ,2000,26:25−35. doi: 10.1016/S0926-3373(99)00144-7 [18] ZHU Z, LIU Z, LIU S, NIU H. Adsorption and reduction of NO over activated coke at low temperatures[J]. Fuel,2000,79:651−658. doi: 10.1016/S0016-2361(99)00192-1 [19] GÁLVEZ M E, LÁZARO M J, MOLINER R. Novel activated carbon-based catalyst for the selective catalytic reduction of nitrogen oxide[J]. Catal Today,2005,102−103:142−147. doi: 10.1016/j.cattod.2005.02.020 [20] MOCHIDAA I, SHIRAHAMAA N, KAWANOA S, KORAIA Y, YASUTAKEB A, TANOURAC M, FUJIIC S, YOSHIKAWAD M. NO oxidation over activated carbon fiber (ACF). Part 1. Extended kinetics over a pitch based ACF of very large surface area[J]. Fuel,2000,79:1713−1723. doi: 10.1016/S0016-2361(00)00034-X [21] BUSCAA G, LIETTIB L, RAMISA G, BERTIC F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review[J]. Appl Catal B: Environ,1998,18:1−36. doi: 10.1016/S0926-3373(98)00040-X [22] 吴孝敏, 倪凯文, 宇小龙, 赵宁. 暴露CeO2不同晶面的VOx-MnOx/CeO2催化剂低温NH3-SCR脱硝的原位红外研究[J]. 燃料化学学报,2020,48:179−188. doi: 10.3969/j.issn.0253-2409.2020.02.007WU Xiao-min, NI Kai-wen, YU Xiao-long, ZHAO Ning. In-situ DRIFTs study on different exposed facets of VOx-MnOx/CeO2 catalysts for low-temperature NH3-SCR[J]. J Fuel Chem Technol,2020,48:179−188. doi: 10.3969/j.issn.0253-2409.2020.02.007 [23] LEI Z, HAN B, YANG K, CHEN B. Influence of H2O on the low-temperature NH3-SCR of NO over V2O5/AC catalyst: An experimental and modeling study[J]. Chem Eng J,2013,215−216:651−657. doi: 10.1016/j.cej.2012.11.011 [24] FU M, LI C, LU P, QU L, ZHANG M, ZHOU Y, YU M, FANG Y. A review on selective catalytic reduction of NOx by supported catalysts at 100-300 °C-catalysts, mechanism, kinetics[J]. Catal Sci Technol,2014,4(1):14−25. doi: 10.1039/C3CY00414G [25] CAO F, XIANG J, SU S, WANG P, SUN L, HU S, LEI S. The activity and characterization of MnOx-CeO2-ZrO2/γ-Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3[J]. Chem Eng J,2014,243:347−354. doi: 10.1016/j.cej.2014.01.034 [26] TENG H, TU Y T, LAI Y C, LIN C C. Reduction of NO with NH3 over carbon catalysts: The effects of treating carbon with H2SO4 and HNO3[J]. Carbon,2001,39:575−582. doi: 10.1016/S0008-6223(00)00171-8 [27] SUN D, LIU Q, LIU Z, GUI G, HUANG Z. Adsorption and oxidation of NH3 over V2O5/AC surface[J]. Appl Catal B: Environ,2009,92:462−467. doi: 10.1016/j.apcatb.2009.09.005 [28] 张强, 刘璐, 于梦云, 周洲. 氧化铝载体硫酸化对锰铈催化剂SCR脱硝性能的影响[J]. 燃料化学学报,2019,47(9):1137−1145. doi: 10.3969/j.issn.0253-2409.2019.09.014ZHANG Qiang, LIU Lu, YU Meng-yun, ZHOU Zhou. Effect of sulfuric acid modification of Al2O3 support on the SCR performance of MnCe/Al2O3 catalysts[J]. J Fuel Chem Technol,2019,47(9):1137−1145. doi: 10.3969/j.issn.0253-2409.2019.09.014 -





 下载:
下载: