Preparation of Cu/ZnO/Al2O3 catalyst by intercalated hydrotalcite precursor and its catalytic performance in methanol synthesis
-
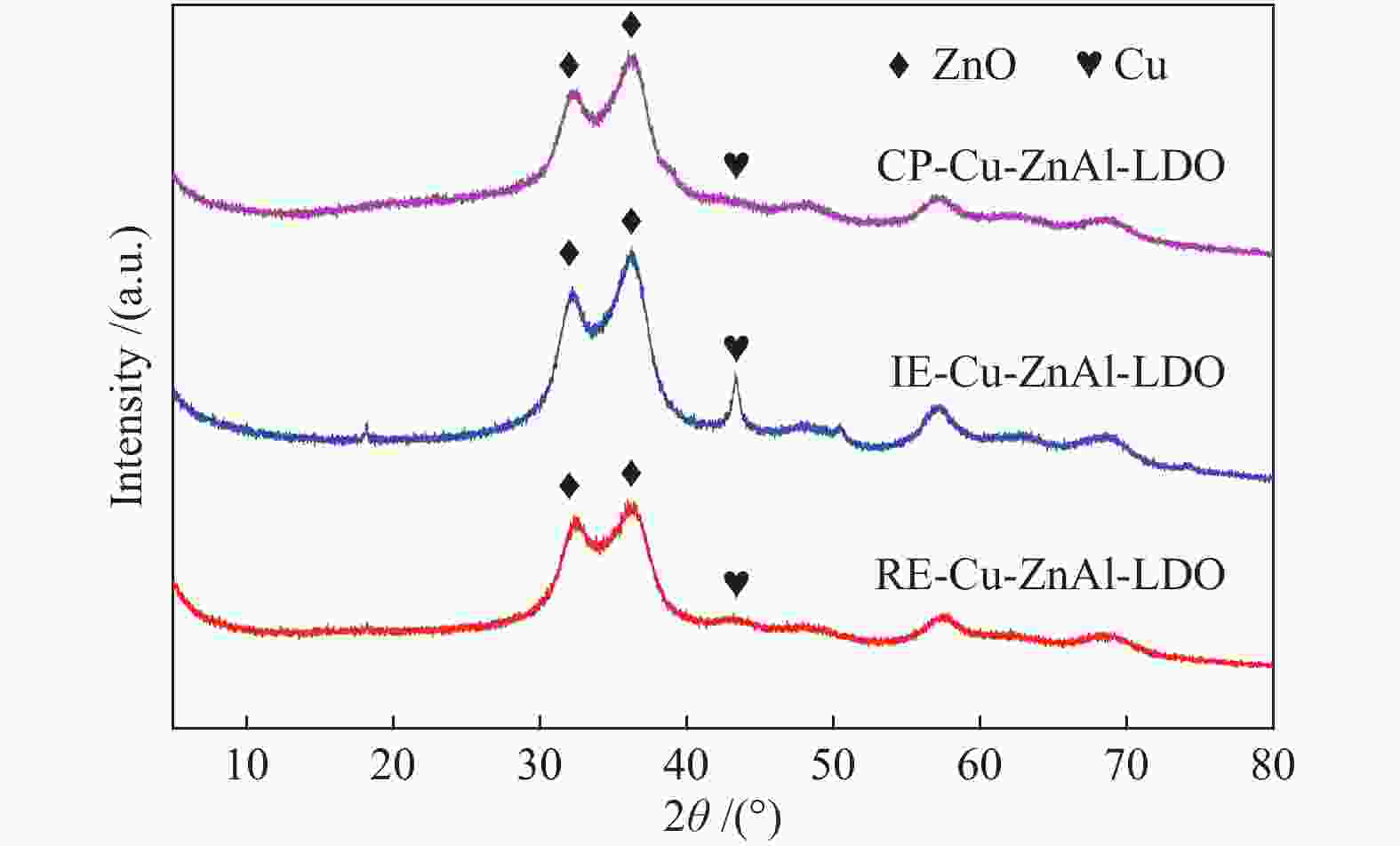
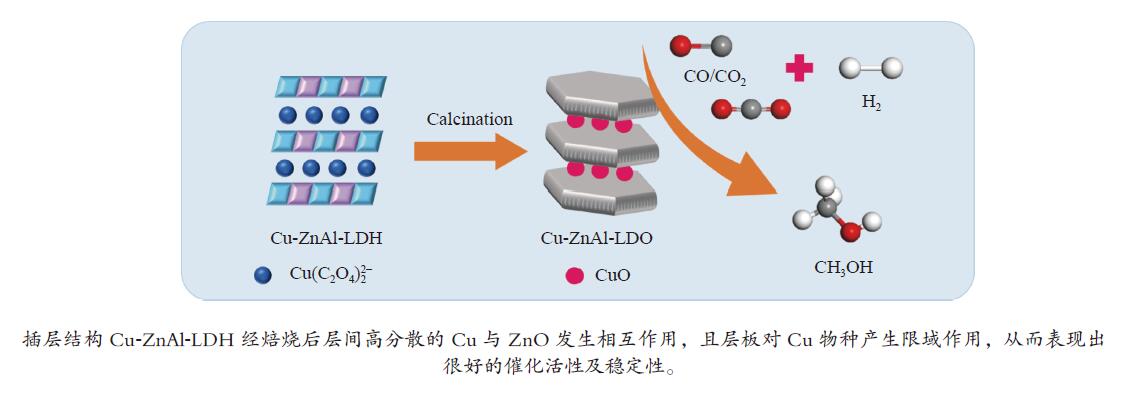
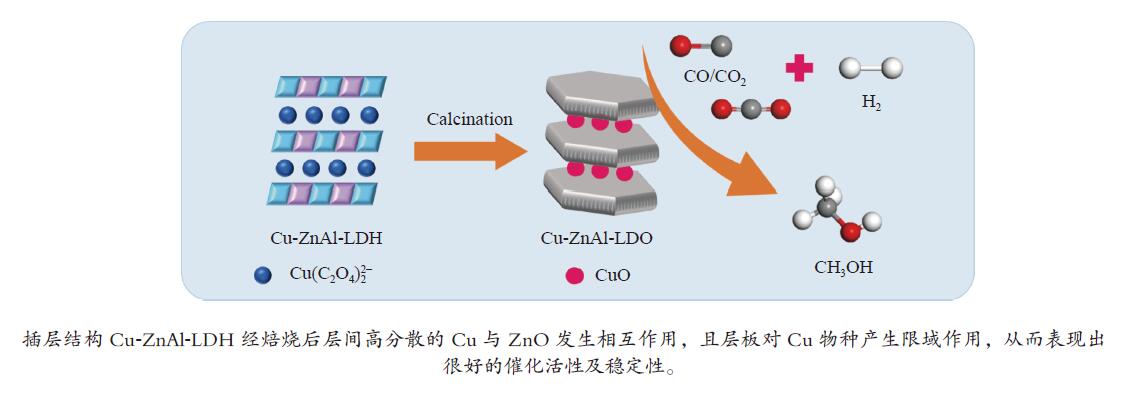
摘要: 分别采用共沉淀法、离子交换法和焙烧复原法制备
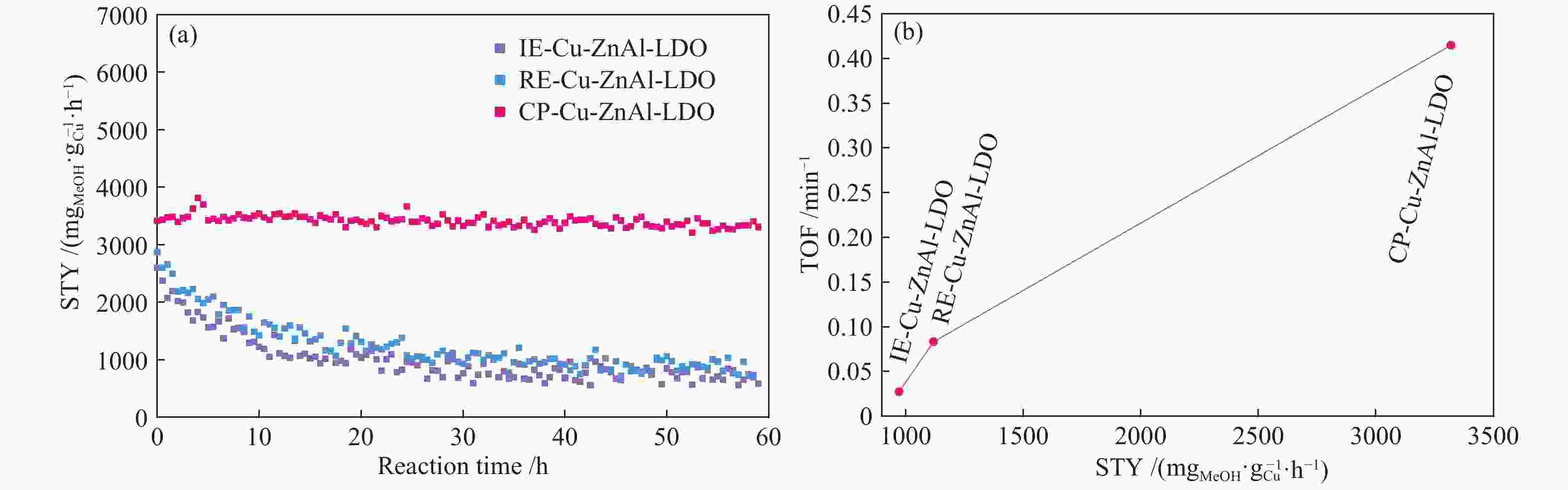
$ {\rm{Cu(}}{{\rm{C}}_{\rm{2}}}{{\rm{O}}_{\rm{4}}}{\rm{)}}_2^{2 - } $ 络合阴离子插层ZnAl-LDH水滑石纯物相前驱体,经焙烧得到片层结构的Cu-ZnAl-LDO催化剂。通过XRD、TEM、ICP、H2-TPR、N2O-H2氧化还原滴定及N2物理吸附-脱附等方式对前驱体和催化剂进行表征分析,考察了不同制备方法对插层结构催化剂结构性质及其合成甲醇催化活性及稳定性的影响。结果表明,相比于离子交换法和焙烧复原法,共沉淀法制备的CP-Cu-ZnAl-LDH前驱体具有较完整的水滑石二维层状结构,且插层Cu组分含量最大,焙烧后层间高分散的Cu物种颗粒尺寸较小,与ZnO发生相互作用,表现出很好的催化活性,单位质量Cu的甲醇收率可达3412 mg/(gCu·h);同时,层板对Cu物种的限域作用有效抑制了Cu颗粒的团聚长大,连续反应60 h过程中催化剂未发现失活。Abstract: The$ {\rm{Cu(}}{{\rm{C}}_{\rm{2}}}{{\rm{O}}_{\rm{4}}}{\rm{)}}_2^{2 - } $ complexed anion intercalated ZnAl-LDH hydrotalcite pure phase precursors were prepared by co-precipitation, ion exchange and calcination-reconstruction methods; after that, the Cu-ZnAl-LDO catalysts with lamellar structure were obtained by calcination of the precursors. The precursors and catalysts were characterized by XRD, TEM, ICP, H2-TPR, N2O-H2 TPD titrations and N2 physisorption; the effect of preparation method on the confinement degree of intercalated active Cu species and the catalytic activity and stability of Cu-ZnAl-LDO in methanol synthesis was investigated. The results indicate that compared with the ion exchange and calcination-reconstruction methods, the CP-Cu-ZnAl-LDO catalyst prepared by co-precipitation method has a higher interlayer Cu content as well as a stronger confinement effect of layer plate on the Cu species. In addition, the unique ordered layered structure of hydrotalcite can also improve the dispersion of active Cu species between the layers and inhibit the sintering of Cu particles. As a result, the CP-Cu-ZnAl-LDO catalyst prepared by co-precipitation method exhibits excellent performance in methanol synthesis; the space-time yield of methanol reaches 3412 mg/(gCu·h) and basically no deactivation is observed after continuous reaction for 60 h.-
Key words:
- hydrotalcite /
- copper-based catalyst /
- confinement effect /
- methanol synthesis
-
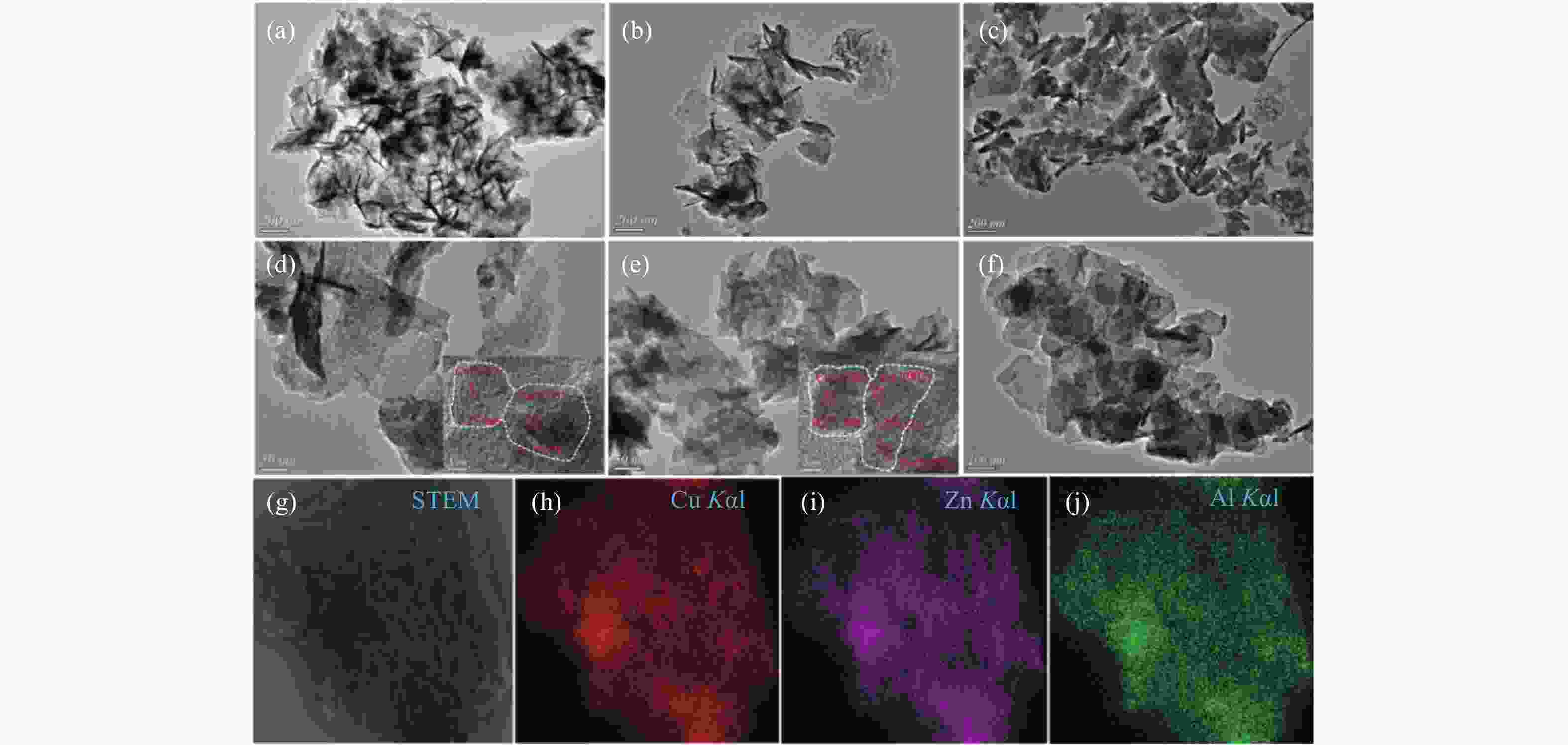
图 2 不同前驱体和催化剂的TEM照片及CP-Cu-ZnAl-LDO催化剂的STEM-Mapping照片
Figure 2 TEM images of different precursors and catalysts and STEM-Mapping diagrams of CP-Cu-ZnAl-LDO catalyst
(a): CP-Cu-ZnAl-LDH; (b): IE-Cu-ZnAl-LDH; (c): RE-Cu-ZnAl-LDH; (d): CP-Cu-ZnAl-LDO; (e): IE-Cu-ZnAl-LDO; (f): RE-Cu-ZnAl-LDO; (g)−(j): Mapping diagrams of Cu, Zn and Al elements in CP-Cu-ZnAl-LDO catalyst
表 1 不同水滑石前驱体结构参数
Table 1 Structural parameters of different hydrotalcite precursors
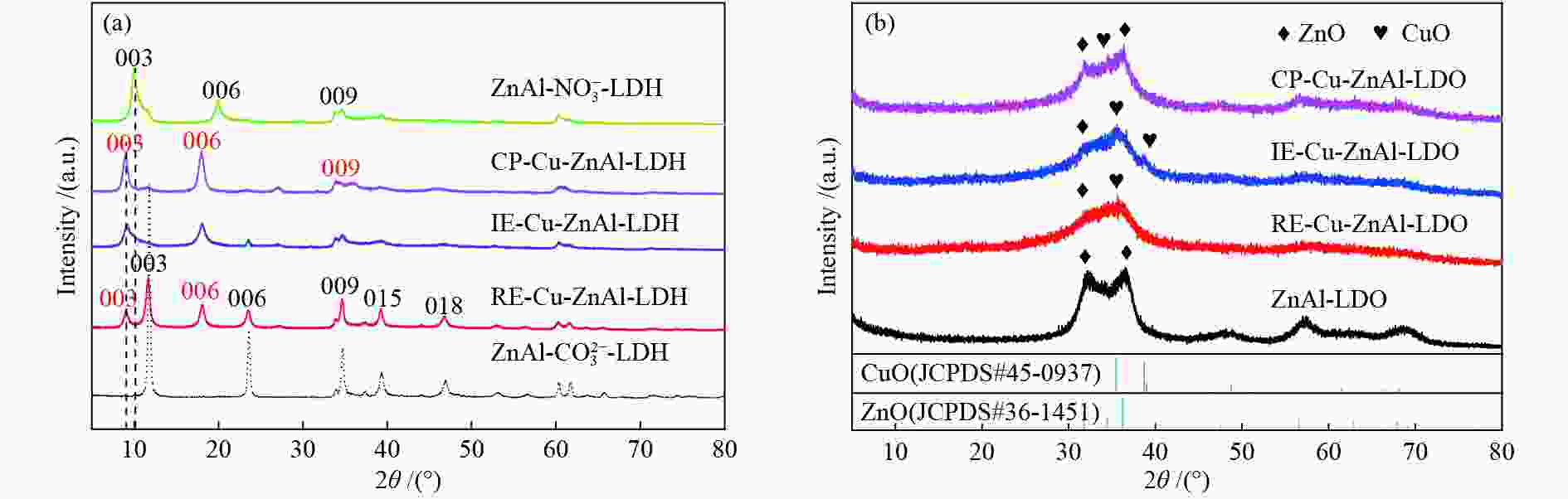
Sample 2 θ of (003) diffraction peak /(°) Basal spacing d003 /nm Interlayer distance①/nm CP-Cu-ZnAl-LDH 9.02 0.98 0.50 IE-Cu-ZnAl-LDH 9.05 0.98 0.50 RE-Cu-ZnAl-LDH 9.06/11.56 0.98/0.77 0.50/0.29 ${\rm{ZnAl } }{\text{-}} {\rm{NO} }_3^{ - } {\text{-}} {\rm{LDH} }$ 9.98 0.89 0.41 $ {\rm{ZnAl {\text{-}} CO}}_3^{2 - } {\text{-}} {\rm{LDH}} $ 11.56 0.77 0.29 ①interlayer distance = d003−thickness of the brucite layer (0.48 nm)[16] 表 2 不同插层结构Cu-ZnAl-LDO催化剂的织构性质
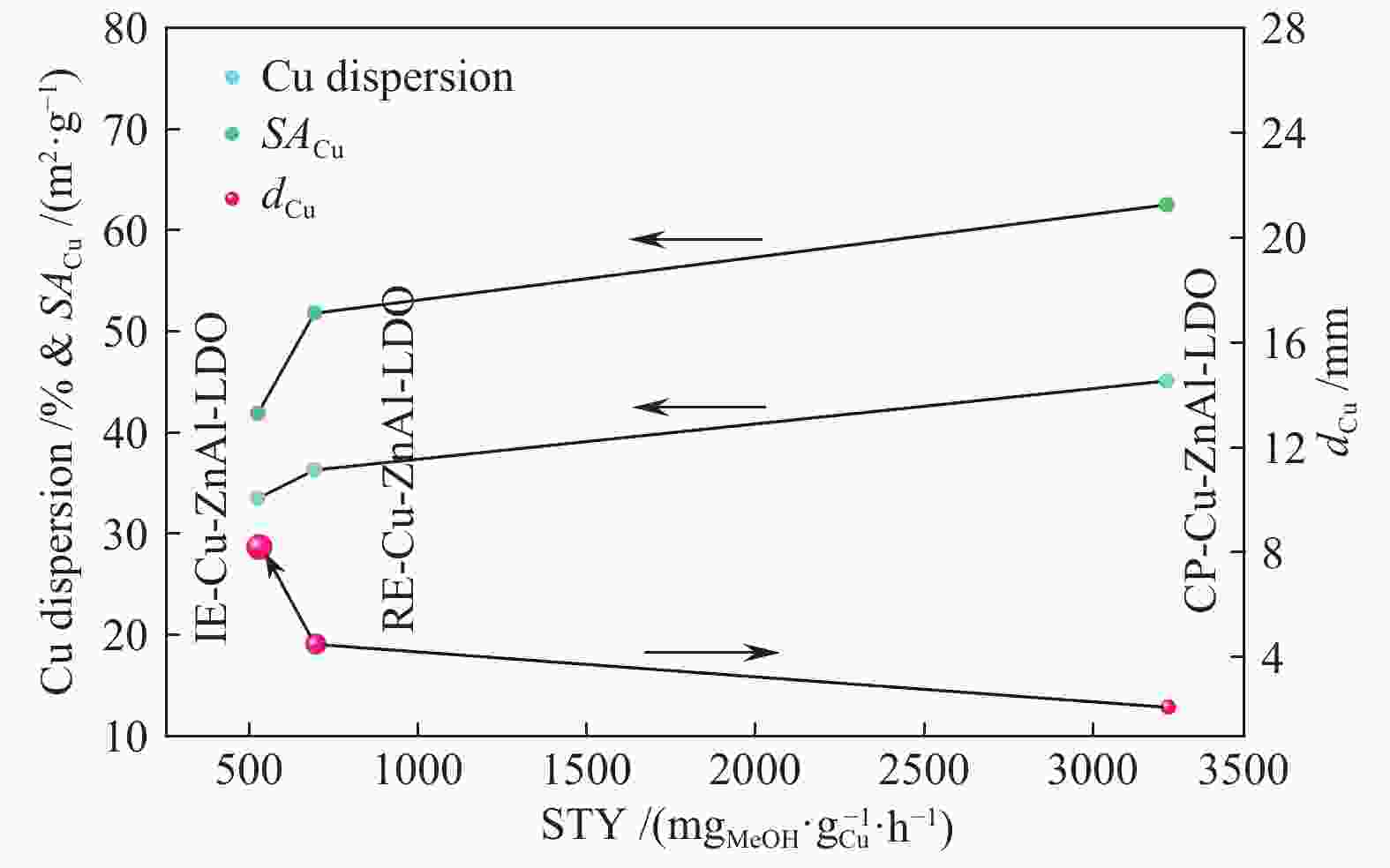
Table 2 Textural properties of Cu-ZnAl-LDO catalysts with different intercalated structures
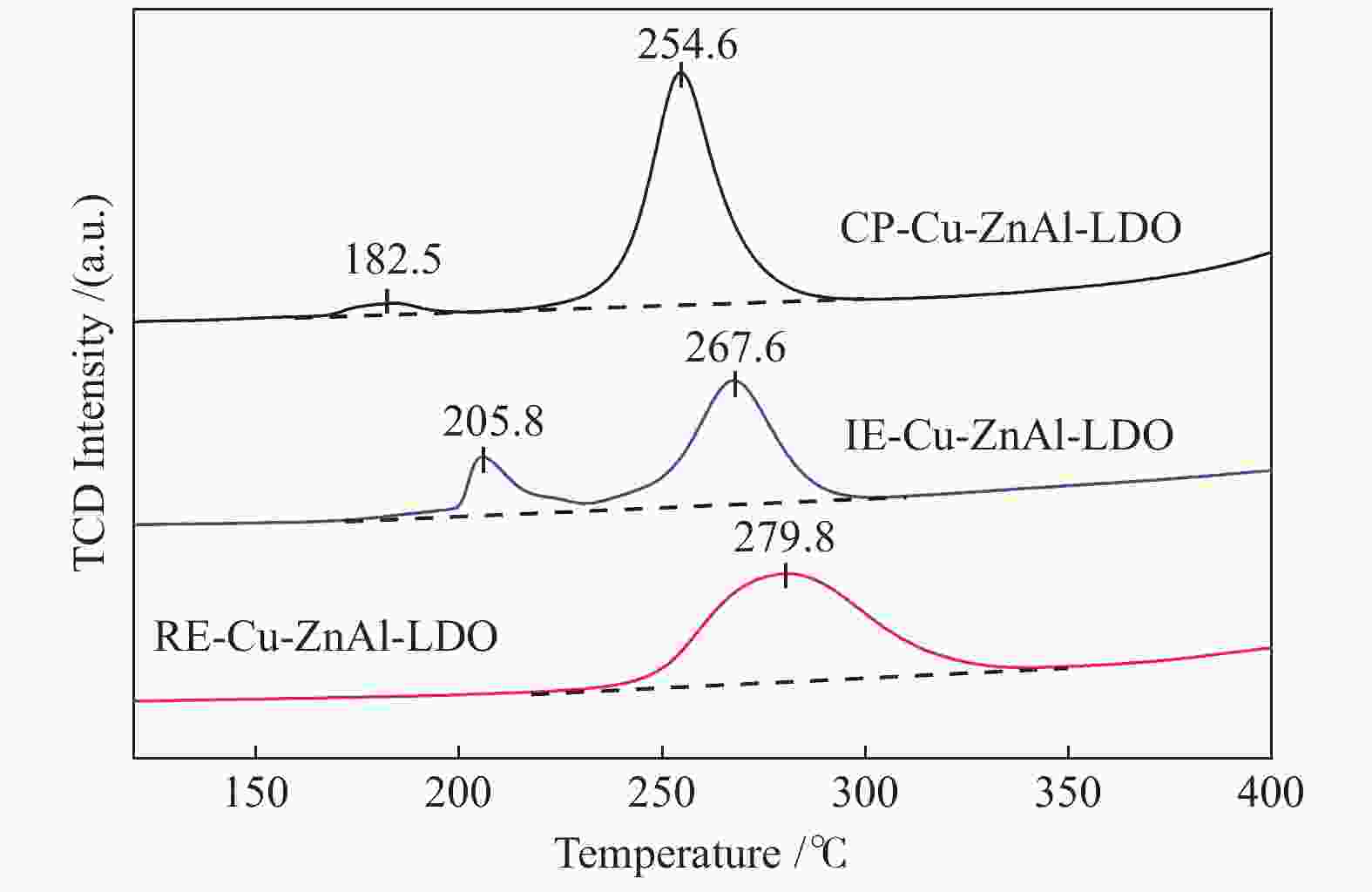
Catalyst Cu content①w/% SA/(m2·g−1) DCu④/% v⑤/(cm3·g−1) dCu⑥/nm catalyst② Cu③ CP-Cu-ZnAl-LDO 10.7 91.2 45.1 62.5 0.52 2.1 IE-Cu-ZnAl-LDO 6.0 59.7 33.5 41.9 0.20 8.2 RE-Cu-ZnAl-LDO 7.9 48.1 36.3 51.8 0.35 4.5 ①: the mass percentage of Cu in the catalyst was calculated by ICP; ②: the specific surface area of the catalyst was calculated by B.E.T method; ③④: the effective specific surface area and Cu dispersion were calculated by N2O-H2 oxidation-reduction titration; ⑤: the mesoporous volume was calculated by t-plot method; ⑥: based on the half peak width of Cu diffraction peak at 2θ = 43.4°, the Cu particle size after catalyst reaction was calculated by Scherrer formula 表 3 不同结构Cu/ZnO/Al2O3催化剂的甲醇时空收率对比
Table 3 Comparison of various Cu/ZnO/Al2O3 catalysts with different structures in the methanol yield
Method Cu content w/% p/t(MPa·℃−1) STYMeOH/($ {\rm{m}}{{\rm{g}}_{{\rm{MeOH}}}} \cdot {\rm{g}}_{{\rm{cat}}}^{ - 1} \cdot {{\rm{h}}^{ - 1}} $) STYMeOH/($ {\rm{m}}{{\rm{g}}_{{\rm{MeOH}}}} \cdot {\rm{g}}_{{\rm{Cu}}}^{ - 1} \cdot {{\rm{h}}^{ - 1}} $) Reference CP 56.9 5/250 642 1128 [21] CP 25.6 5/250 280 1093 [22] CP 67.5 5/250 568 841 [23] CP 10.7 3/250 355 3398 this work IE 6.0 3/250 58 971 this work RE 7.9 3/250 88 1118 this work -
[1] BUKHTIYAROVA M, LUNKENBEIN T, KÄHLER K, SCHLÖGL R. Methanol synthesis from industrial CO2 sources: a contribution to chemical energy conversion[J]. Catal Lett,2017,147(2):416−427. doi: 10.1007/s10562-016-1960-x [2] PRIETO G, ZEČEVIĆ J, FRIEDRICH H, DE JONG K P, DE JONGH P E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles[J]. Nat Mater,2013,12(1):34−39. doi: 10.1038/nmat3471 [3] BEHRENS M, ZANDER S, KURR P, JACOBSEN N, SENKER J R, KOCH G, RESSLER T, FISCHER R W, SCHLÖGL R. Performance improvement of nanocatalysts by promoter-induced defects in the support material: methanol synthesis over Cu/ZnO: Al[J]. J Am Chem Soc,2013,135(16):6061−6068. doi: 10.1021/ja310456f [4] NARKHEDE N, ZHENG H, ZHANG H, ZHANG G, LI Z. Group 13 metal doped Cu/ZnO catalysts from phase pure precursors via an isomorphous substitution route: mechanistic insights into promotional effects for syngas hydrogenation to methanol[J]. Catal Sci Technol, 2020, 10(21): 7386-7398. [5] FENG J, HE Y, LIU Y, DU Y, LI D. Supported catalysts based on layered double hydroxides for catalytic oxidation and hydrogenation: general functionality and promising application prospects[J]. Chem Soc Rev,2015,44(15):5291−5319. doi: 10.1039/C5CS00268K [6] 雒京, 李洪广, 赵宁, 王峰, 肖福魁. 磺化Salen金属配合物插层水滑石选择性催化氧化甘油制备二羟基丙酮的研究[J]. 燃料化学学报,2015,43(6):677−683. doi: 10.1016/S1872-5813(15)30019-0LUO Jing, LI Hong-guang, ZHAO Ning, WANG Feng, XIAO Fu-kui. Selective oxidation of glycerol to dihydroxyacetone over layer double hydroxide intercalated with sulfonato-salen metal complexes[J]. J Fuel Chem Technol,2015,43(6):677−683. doi: 10.1016/S1872-5813(15)30019-0 [7] 吴雁, 雷艳清, 刘新月, 邱月, 王豪. 12-磷钨酸插层MgAl水滑石的合成、表征及催化酯化原油脱酸性能[J]. 燃料化学学报,2017,45(9):1049−1055.WU Yan, LEI Yan-qing, LIU Xin-yue, QIU Yue, WANG Hao. Synthesis and characterization of 12-tungstophosphoric acid intercalated MgAl layered double hydroxides and their application as esterification catalysts for deacidification of crude oil[J]. J Fuel Chem Technol,2017,45(9):1049−1055. [8] MIAO C, HUI T, LIU Y, FENG J, LI D. Pd/MgAl-LDH nanocatalyst with vacancy-rich sandwich structure: Insight into interfacial effect for selective hydrogenation[J]. J Catal,2019,370:107−117. doi: 10.1016/j.jcat.2018.12.006 [9] BARRABÉS N, FRARE A, FÖTTINGER K, URAKAWA A, LLORCA J, RUPPRECHTER G, TICHIT D. Pt-Cu bimetallic catalysts obtained from layered double hydroxides by an anion-exchange route[J]. Appl Clay Sci,2012,69:1−10. doi: 10.1016/j.clay.2012.07.011 [10] SABBAR E M, DE ROY M E, LEROUX F. New Cu and/or Pt complexes intercalated MgAl-hydrotalcite-type anionic clays: synthesis, characterization, thermal behavior and textural properties[J]. Microporous Mesoporous Mater,2007,103(1/3):134−141. doi: 10.1016/j.micromeso.2007.01.044 [11] RĂCIULETE M, LAYRAC G, TICHIT D, MARCU I-C. Comparison of CuxZnAlO mixed oxide catalysts derived from multicationic and hybrid LDH precursors for methane total oxidation[J]. Appl Catal A: Gen,2014,477:195−204. doi: 10.1016/j.apcata.2014.03.018 [12] HUANG Z, WU P, ZHANG X, WANG X, ZHU N, WU J, LI P. Intercalation of Fe (III) complexes into layered double hydroxides: Synthesis and structural preservation[J]. Appl Clay Sci,2012,65:87−94. [13] BEAUDOT P, DE ROY M E, BESSE J P. Preparation and characterization of intercalation compounds of layered double hydroxides with metallic oxalato complexes[J]. Chem Mater,2004,16(5):935−945. doi: 10.1021/cm0311067 [14] STARUKH G, ROZOVIK O, ORANSKA O. Organo/Zn-Al LDH nanocomposites for cationic dye removal from aqueous media[J]. Nanoscale Res Lett,2016,11(1):228. doi: 10.1186/s11671-016-1402-0 [15] FANG X, MEN Y, WU F, ZHAO Q, SINGH R, XIAO P, DU T, WEBLEY P A. Improved methanol yield and selectivity from CO2 hydrogenation using a novel Cu-ZnO-ZrO2 catalyst supported on Mg-Al layered double hydroxide (LDH)[J]. J CO2 Util,2019,29:57−64. doi: 10.1016/j.jcou.2018.11.006 [16] YASAEI M, KHAKBIZ M, GHASEMI E, ZAMANIAN A. Synthesis and characterization of ZnAl-NO3(-CO3) layered double hydroxide: a novel structure for intercalation and release of simvastatin[J]. Appl Surf Sci,2019,467:782−791. [17] CARJA G, DARTU L, OKADA K, FORTUNATO E. Nanoparticles of copper oxide on layered double hydroxides and the derived solid solutions as wide spectrum active nano-photocatalysts[J]. Chem Eng J,2013,222:60−66. doi: 10.1016/j.cej.2013.02.039 [18] KWAK B K, PARK D S, YUN Y S, YI J. Preparation and characterization of nanocrystalline CuAl2O4 spinel catalysts by sol-gel method for the hydrogenolysis of glycerol[J]. Catal Commun,2012,24:90−95. doi: 10.1016/j.catcom.2012.03.029 [19] ABBAS I, KIM H, SHIN C H, YOON S, JUNG K D. Differences in bifunctionality of ZnO and ZrO2 in Cu/ZnO/ZrO2/Al2O3 catalysts in hydrogenation of carbon oxides for methanol synthesis[J]. Appl Catal B: Environ,2019,258:117971. doi: 10.1016/j.apcatb.2019.117971 [20] SIKANDER U, SUFIAN S, SALAM M. A review of hydrotalcite based catalysts for hydrogen production systems[J]. Int J Hydrogen Energy,2017,42(31):19851−19868. doi: 10.1016/j.ijhydene.2017.06.089 [21] SADEGHINIA M, GHAZIANI A N K, REZAEI M. Component ratio dependent Cu/Zn/Al structure sensitive catalyst in CO2/CO hydrogenation to methanol[J]. Mol Catal,2018,456:38−48. doi: 10.1016/j.mcat.2018.06.020 [22] GAO P, XIE R, WANG H, ZHONG L, XIA L, ZHANG Z, WEI W, SUN Y. Cu/Zn/Al/Zr catalysts via phase-pure hydrotalcite-like compounds for methanol synthesis from carbon dioxide[J]. J CO2 Util,2015,11:41−48. doi: 10.1016/j.jcou.2014.12.008 [23] SADEGHINIA M, REZAEI M, KHARAT A N, JORABCHI M N, NEMATOLLAHI B, ZAREIEKORDSHOULI F. Effect of In2O3 on the structural properties and catalytic performance of the CuO/ZnO/Al2O3 catalyst in CO2 and CO hydrogenation to methanol[J]. Mol Catal,2020,484:110776. doi: 10.1016/j.mcat.2020.110776 [24] VAN DEN BERG R, PRIETO G, KORPERSHOEK G, VAN DER WAL L I, VAN BUNNINGEN A J, LAEGSGAARD-JORGENSEN S, DE JONGH P E, DE JONG K P. Structure sensitivity of Cu and CuZn catalysts relevant to industrial methanol synthesis[J]. Nat Commun,2016,7(1):1−7. [25] VAN HELDEN P, CIOBÎCĂ I M, COETZER R L. The size-dependent site composition of FCC cobalt nanocrystals[J]. Catal Today, 2016, 261: 48−59. [26] NARKHEDE N, ZHENG H, ZHANG H, ZHANG G, LI Z. Isomorphous substitution method to fabricating pure phase Al-doped zinc malachite: defects driven promotion improvement and enhanced synergy between Cu-ZnO[J]. ChemCatChem,2020,12(22):5697−5709. doi: 10.1002/cctc.202001030 -






 下载:
下载: