Effect of Ce introduced Rh-UiO-66-Zr catalyst in syngas converting to ethanol
-
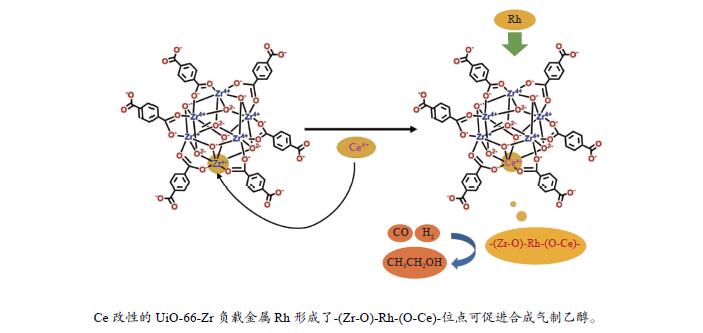
摘要: 在合成气直接合成乙醇过程中,Rh基催化剂对乙醇等C2含氧化合物具有良好的选择性而持续成为研究热点。本工作利用UiO-66作为载体,将金属Ce4 +引入UiO-66-Zr的[Zr6O4(OH)4]金属节点替换部分金属Zr4 +形成[Zr6−xCexO4(OH)4]金属节点,从而较为精确地对合成气制备乙醇活性位点问题进行研究。XRD、TG、Raman、BET、H2-TPR、XPS和in-situ DRIFTS等表征结果显示,随着UiO-66[Zr6O4(OH)4]节点上金属Ce4 +的引入,催化剂上形成了-(Zr-O)-Rh-(O-Ce-位点,而在Rh/UiO-66-Zr催化剂上形成的-(Zr-O)-Rh-(O-Zr)-位点,结合催化反应评价结果发现,乙醇活性位点数量显著提升。由于Rh物种与-(O-Zr)-物种的相互作用较强,Rh与-(O-Ce)-位点的相互作用较弱,这种相互作用的势差有利于电子的高效率传输。另一方面,-(Zr-O)-Rh-(O-Ce)-可以稳定合成气制备乙醇的关键中间体,从而促进乙醇的形成。Abstract: In the process of direct synthesis of ethanol from syngas, rhodium-based catalysts continue becoming a hot topic because of their high selectivity for C2 oxygen-containing compounds such as ethanol. In this work, UiO-66 was used as a support to introduce the metal Ce4 + species into the [Zr6O4(OH)4] metal node of UiO-66-Zr to replace some of the metal Zr4 + species formation [Zr6−xCexO4(OH)4] metal nodes, so as to accurately study the problem of ethanol activity sites for the preparation of syngas. The characterization results of XRD, TG, Raman, BET, H2-TPR, XPS, and in-situ DRIFTS showed that with the introduction of metal Ce4 + species at the UiO-66 [Zr6O4(OH)4] node, -(Zr-O)-Rh-(O-Ce)-sites were formed on the catalyst, and -(Zr-O)-Rh-(O-Zr)-sites were formed on the Rh/UiO-66-Zr catalyst. Combined with the evaluation results of the catalytic reaction, the number of ethanol active sites was significantly increased. Because of the interaction between Rh species and -(O-Zr)-species is stronger than Rh species with -(O-Ce)- sites, the potential difference of this interaction favors the efficient transport of electrons. On the other hand, -(Zr-O)-Rh-(O-Ce)- can stabilize the key intermediates for the preparation of ethanol from syngas, which can promote the formation of ethanol.
-
表 1 不同催化剂的结构参数
Table 1 Properties of different catalysts
Sample SBET/
(m2·g−1)Pore volume/
(cm3·g−1)Average pore
diameter /nmRh/UiO-66-Ce 45.9 0.043 5.59 Rh/UiO-66-Zr 274 0.183 7.14 Rh/UiO-66-ZrCe 253 0.160 6.90 表 2 催化剂Rh/UiO-66-ZrCe中的相对元素含量
Table 2 Relative element content in the catalyst Rh/UiO-66-ZrCe
Element Atomic
fraction / %Atomic
error / %Mass
fraction / %Mass
error / %C 46.41 6.65 22.45 1.92 O 39.59 9.70 25.51 5.52 Zr 12.92 2.45 47.47 7.14 Rh 1.04 0.19 4.31 0.63 Ce 0.05 0.01 0.26 0.04 表 5 Rh基催化剂的XPS定量
Table 5 XPS quantitative results of Rh-based catalysts
Sample Atomic /% Rh Ce Zr O C Rh/UiO-66-Ce 0.46 9.23 0.12 35.40 54.79 Rh/UiO-66-Zr 0.40 0.12 8.44 39.07 51.98 Rh/UiO-66-ZrCe 0.32 0.16 8.44 39.30 51.79 表 3 催化剂Rh/UiO-66-Zr和Rh/UiO-66-ZrCe上Rh 3d的XPS谱图拟合
Table 3 XPS spectrogram fitting results of Rh 3d on Rh/UiO-66-Zr and Rh/UiO-66-ZrCe
Sample Position /eV Chemical specie Relative content /% Rh/UiO-66-Zr 307.00/311.70 Rh0 42.71 308.60/313.30 RhV + 57.29 Rh/UiO-66-ZrCe 307.00/311.70 Rh0 66.60 308.60/313.30 RhV + 33.40 表 4 催化剂上O 1s的XPS谱图拟合
Table 4 XPS spectrogram fitting results of O 1s on Rh/UiO-66-Zr and Rh/UiO-66-ZrCe
Sample Oα Oβ EB /eV area /% EB /eV area /% Rh/UiO-66-Zr 530.16 24.75 531.79 75.25 Rh/UiO-66-ZrCe 530.14 25.90 531.81 74.10 -
[1] ANGUELOV N. Promoting growth in renewables[J]. Nat Energy,2018,3(9):712−713. doi: 10.1038/s41560-018-0223-z [2] LIU B, LU S, LIU E, HU X, FAN J. Methanol aromatization over CrZn-modified HZSM-5 catalysts[J]. Korean J Chem Eng,2018,35(4):867−874. doi: 10.1007/s11814-017-0345-1 [3] LIU W, WANG S, SUN T, WANG S. The promoting effect of Fe doping on Rh/CeO2 for the ethanol synthesis[J]. Catal Lett,2015,145(9):1741−1749. doi: 10.1007/s10562-015-1577-5 [4] CHUANG S S C, STEVENS R W, KHATRI R. Mechanism of C2 + oxygenate synthesis on Rh catalysts[J]. Top Catal,2005,32(3/4):225−232. doi: 10.1007/s11244-005-2897-2 [5] CAVKA J H, JAKOBSEN S, OLSBYE U, GUILLOU N, LAMBERTI C, BORDIGA S, LILLERUD K P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability[J]. J Am Chem Soc,2008,130(42):13850−13851. doi: 10.1021/ja8057953 [6] BAI Y, DOU Y, XIE L H, RUTLEDGE W, LI J R, ZHOU H C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications[J]. Chem Soc Rev,2016,45(8):2327−2367. doi: 10.1039/C5CS00837A [7] WU H, CHUA Y S, KRUNGLEVICIUTE V, TYAGI M, CHEN P, YILDIRIM T, ZHOU W. Unusual and highly tunable missing-linker defects in zirconium metal-organic framework UiO-66 and their important effects on gas adsorption[J]. J Am Chem Soc,2013,135(28):10525−10532. doi: 10.1021/ja404514r [8] CIRUJANO F G, CORMA A, XAMENA F X L I. Zirconium-containing metal organic frameworks as solid acid catalysts for the esterification of free fatty acids: Synthesis of biodiesel and other compounds of interest[J]. Catal Today,2015,257:213−220. doi: 10.1016/j.cattod.2014.08.015 [9] VALEKAR A H, CHO K-H, CHITALE S K, HONG D-Y, CHA G-Y, LEE U H, HWANG D W, SERRE C, CHANG J-S, HWANG Y K. Catalytic transfer hydrogenation of ethyl levulinate to γ-valerolactone over zirconium-based metal-organic frameworks[J]. Green Chem,2016,18(16):4542−4552. doi: 10.1039/C6GC00524A [10] HAJEK J, VANDICHEL M, VAN DE VOORDE B, BUEKEN B, DE VOS D, WAROQUIER M, VAN SPEYBROECK V. Mechanistic studies of aldol condensations in UiO-66 and UiO-66-NH2 metal organic frameworks[J]. J Catal,2015,331:1−12. doi: 10.1016/j.jcat.2015.08.015 [11] XUE X, YU J, HAN Y, XIAO X, SHI Z, MAO H, MAO D. Zr-based metal-organic frameworks drived Rh-Mn catalysts for highly selective CO hydrogenation to C2 oxygenates[J]. J Ind Eng Chem,2020,86:220−231. doi: 10.1016/j.jiec.2020.03.008 [12] HAN L, MAO D, YU J, GUO Q, LU G. C2-oxygenates synthesis through CO hydrogenation on SiO2-ZrO2 supported Rh-based catalyst: The effect of support[J]. Appl Catal A: Gen,2013,454:81−87. doi: 10.1016/j.apcata.2013.01.008 [13] YU J, MAO D, LU G, GUO Q, HAN L. Enhanced C2 oxygenate synthesis by CO hydrogenation over Rh-based catalyst supported on a novel SiO2[J]. Catal Commun,2012,24:25−29. doi: 10.1016/j.catcom.2012.03.015 [14] HAN L, MAO D, YU J, GUO Q, LU G. Synthesis of C2-oxygenates from syngas over Rh-based catalyst supported on SiO2, TiO2 and SiO2-TiO2 mixed oxide[J]. Catal Commun,2012,23:20−24. doi: 10.1016/j.catcom.2012.02.032 [15] YU J, YU J, SHI Z, GUO Q, XIAO X, MAO H, MAO D. The effects of the nature of TiO2 supports on the catalytic performance of Rh-Mn/TiO2 catalysts in the synthesis of C2 oxygenates from syngas[J]. Catal Sci Technol,2019,9(14):3675−3685. doi: 10.1039/C9CY00406H [16] RAMSAHYE N A, GAO J, JOBIC H, LLEWELLYN P L, YANG Q, WIERSUM A D, KOZA M M, GUILLERM V, SERRE C, ZHONG C L, MAURIN G. Adsorption and diffusion of light hydrocarbons in UiO-66(Zr): A combination of experimental and modeling tools[J]. J Phys Chem C,2014,118(47):27470−27482. doi: 10.1021/jp509672c [17] ØIEN S, WRAGG D, REINSCH H, SVELLE S, BORDIGA S, LAMBERTI C, LILLERUD K P. Detailed structure analysis of atomic positions and defects in zirconium metal-organic frameworks[J]. Cryst Growth Des,2014,14(11):5370−5372. doi: 10.1021/cg501386j [18] SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides[J]. Acta Crystallogr, Sect A,1976,32(5):751−767. doi: 10.1107/S0567739476001551 [19] CAI W, ZHAO Y, CHEN M, JIANG X, WANG H, OU M, WAN S, ZHONG Q. The formation of 3D spherical Cr-Ce mixed oxides with roughness surface and their enhanced low-temperature NO oxidation[J]. Chem Eng J,2018,333:414−422. doi: 10.1016/j.cej.2017.10.002 [20] STAWOWY-KUC M, CIESIELSKI R, MANIECKI T, MATUS K, ŁUŻNY R, TRAWCZYNSKI J, SILVESTRE-ALBERO J, LAMACZ A. CO2 hydrogenation to methanol over Ce and Zr containing UiO-66 and Cu/UiO-66[J]. Catalysts,2019,10:39. doi: 10.3390/catal10010039 [21] TANIGUCHI T, WATANABE T, SUGIYAMA N, SUBRAMANI A K, WAGATA H, MATSUSHITA N, YOSHIMURA M. Identifying defects in ceria-based nanocrystals by UV resonance raman spectroscopy[J]. J Phys Chem C,2009,113:19789−19793. doi: 10.1021/jp9049457 [22] SHEARER G C, FORSELV S, CHAVAN S, BORDIGA S, MATHISEN K, BJØRGEN M, SVELLE S, LILLERUD K P. In situ infrared spectroscopic and gravimetric characterisation of the solvent removal and dehydroxylation of the metal organic frameworks UiO-66 and UiO-67[J]. Top Catal,2013,56(9):770−782. [23] LAMMERT M, GLIßMANN C, REINSCH H, STOCK N. Synthesis and characterization of new Ce(IV)-MOFs exhibiting various framework topologies[J]. Cryst Growth Des,2017,17(3):1125−1131. doi: 10.1021/acs.cgd.6b01512 [24] KATZ M J, BROWN Z J, COLÓN Y J, SIU P W, SCHEIDT K A, SNURR R Q, HUPP J T, FARHA O K. A facile synthesis of UiO-66, UiO-67 and their derivatives[J]. Chem Commun,2013,49(82):9449−9451. doi: 10.1039/c3cc46105j [25] GUO Q, WANG Y, HAN J, ZHANG J, WANG F. Interfacial tandem catalysis for ethylene carbonylation and C–C coupling to 3-pentanone on Rh/ceria[J]. ACS Catal,2022,12(6):3286−3290. doi: 10.1021/acscatal.2c00346 [26] CAO F, GONG N, MA Z, WANG X, TAN M, WU Y, TAN Y. Controlling CO2 hydrogenation selectivity by Rh-based catalysts with different crystalline phases of TiO2[J]. Chem Commun,2022,58(26):4219−4222. doi: 10.1039/D2CC00472K [27] CHEN L, ZHU Y, ZHENG H, ZHANG C, LI Y. Aqueous-phase hydrodeoxygenation of propanoic acid over the Ru/ZrO2 and Ru–Mo/ZrO2 catalysts[J]. Appl Catal A: Gen,2012,411-412:95−104. doi: 10.1016/j.apcata.2011.10.026 [28] LI T, CHEN F, LANG R, WANG H, SU Y, QIAO B, WANG A, ZHANG T. Styrene hydroformylation with in situ hydrogen: Regioselectivity control by coupling with the low-temperature water–gas shift reaction[J]. Angew Chem Int Ed,2020,59(19):7430−7434. doi: 10.1002/anie.202000998 [29] YANG M, YU J, TONG X, SUN X, XU H, SUN J. Flame-made Cu/ZrO2 catalysts with metastable phase and strengthened interactions for CO2 hydrogenation to methanol[J]. Chem Commun,2021,57(61):7509−7512. doi: 10.1039/D1CC02784K -





 下载:
下载: