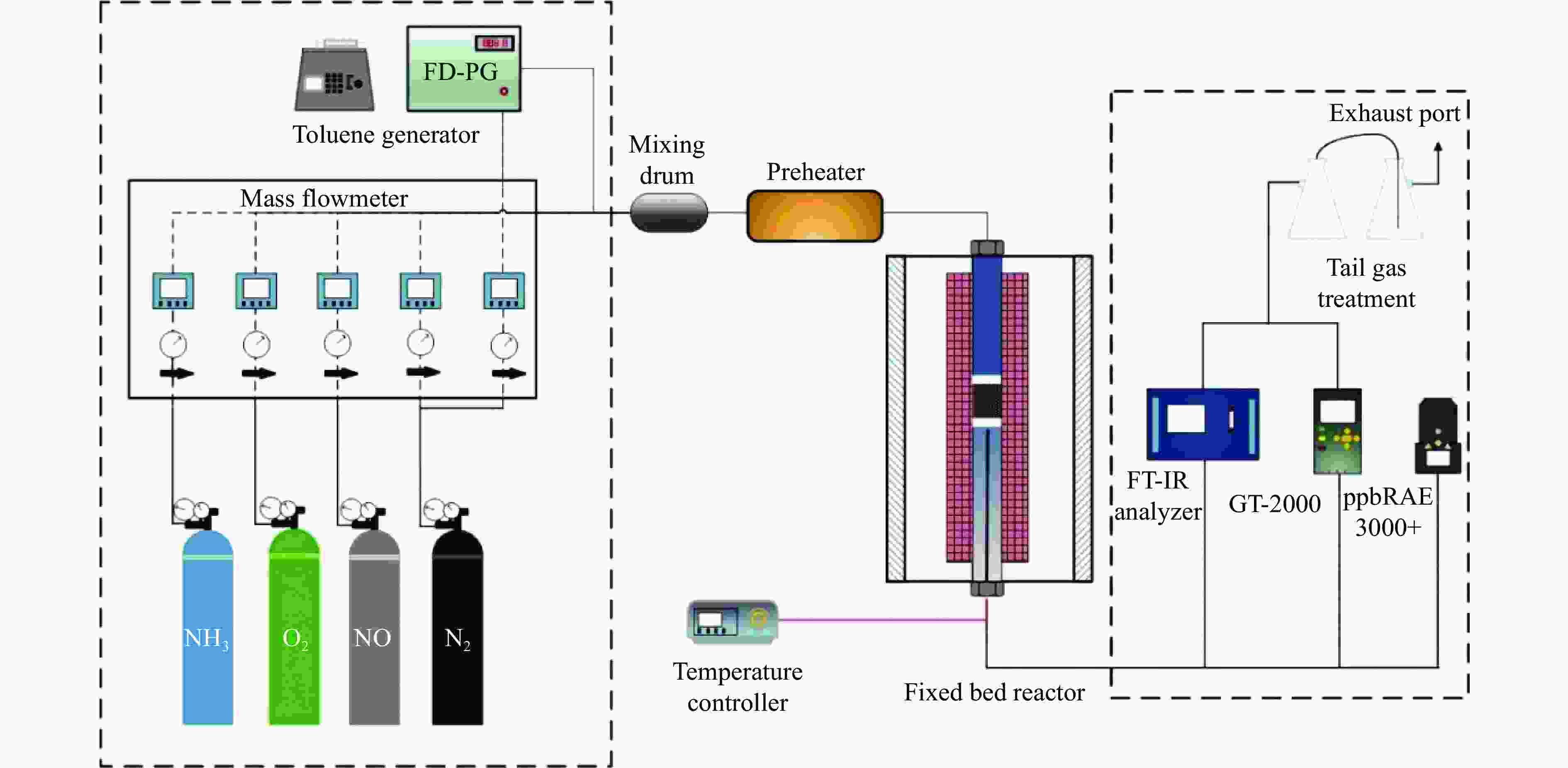
Study on performance and mechanism of CuMn2O4 supported catalyst for simultaneous removal of toluene and NOx
-
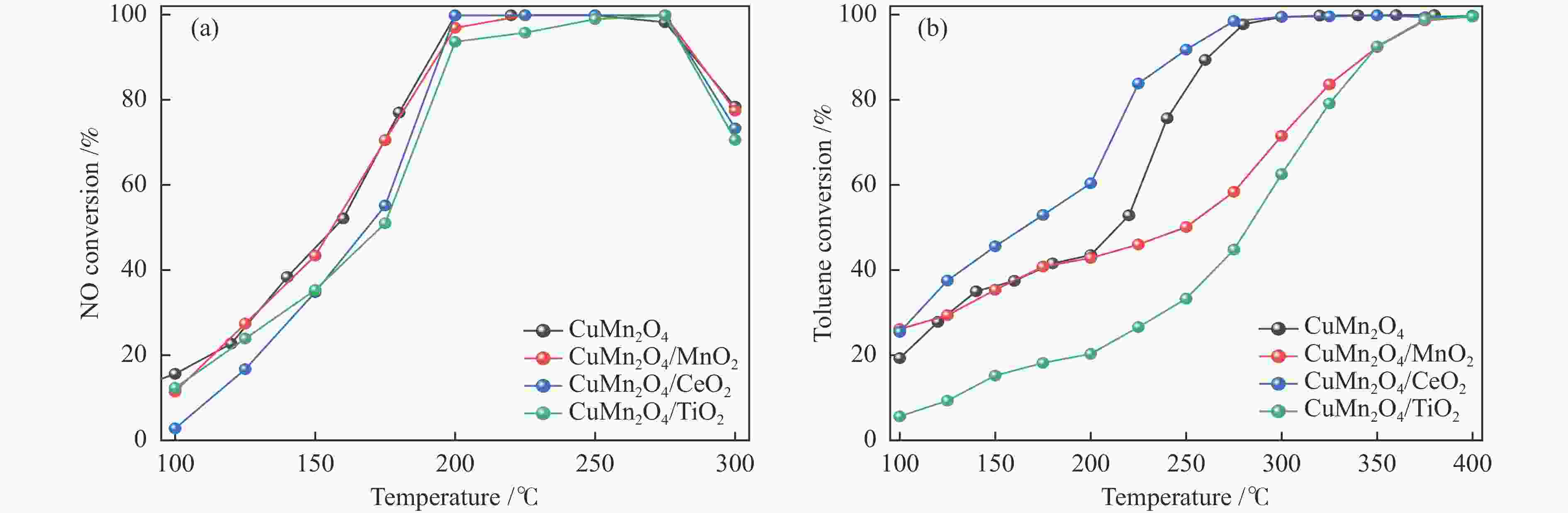
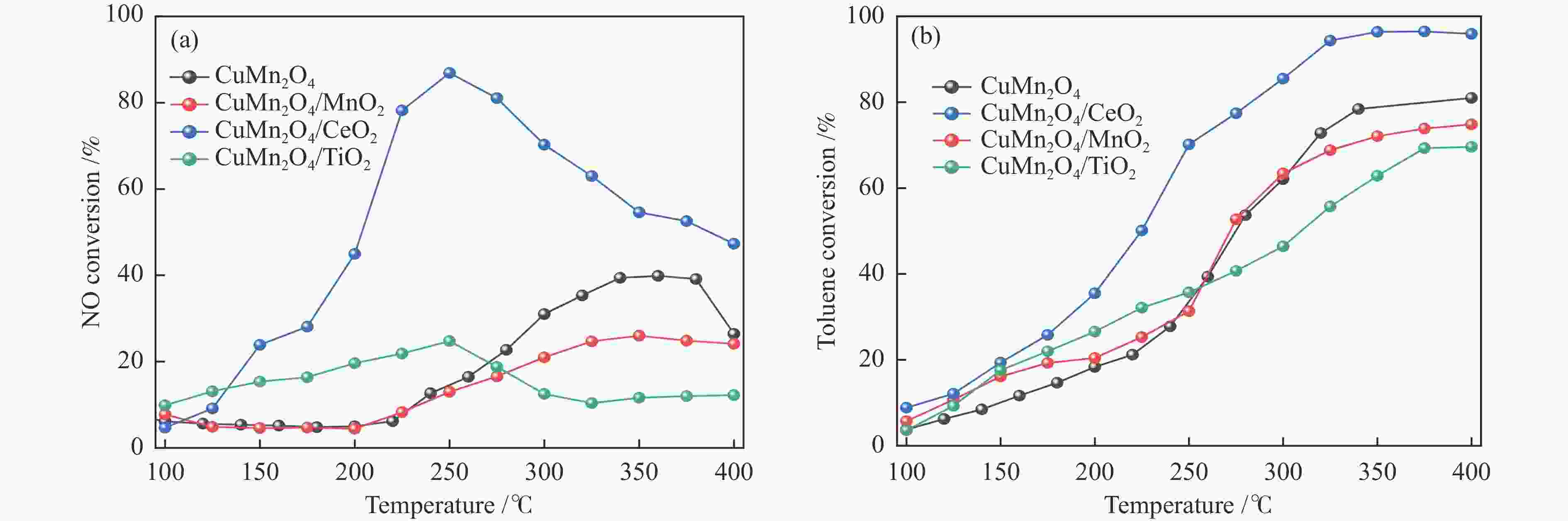
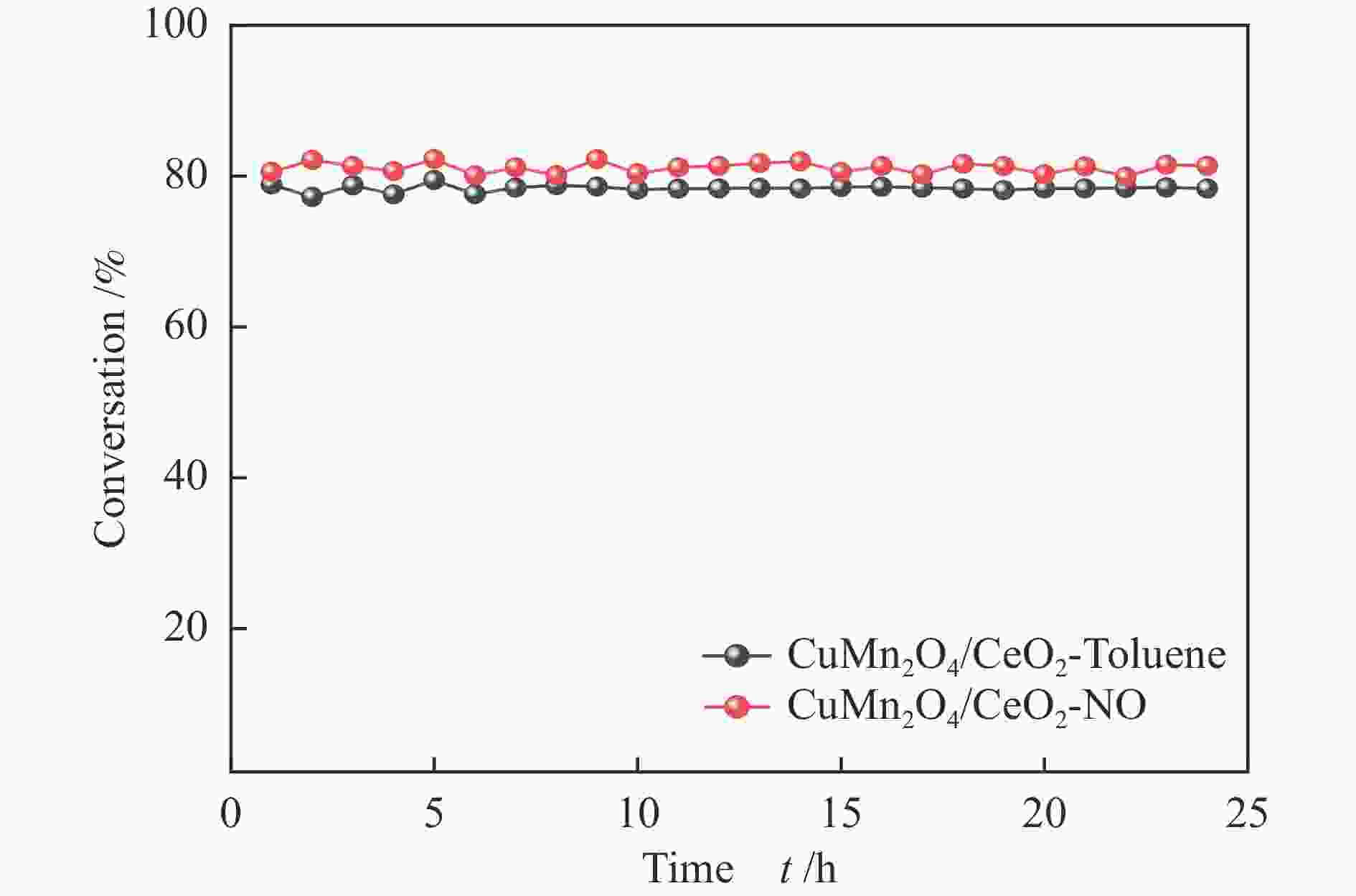
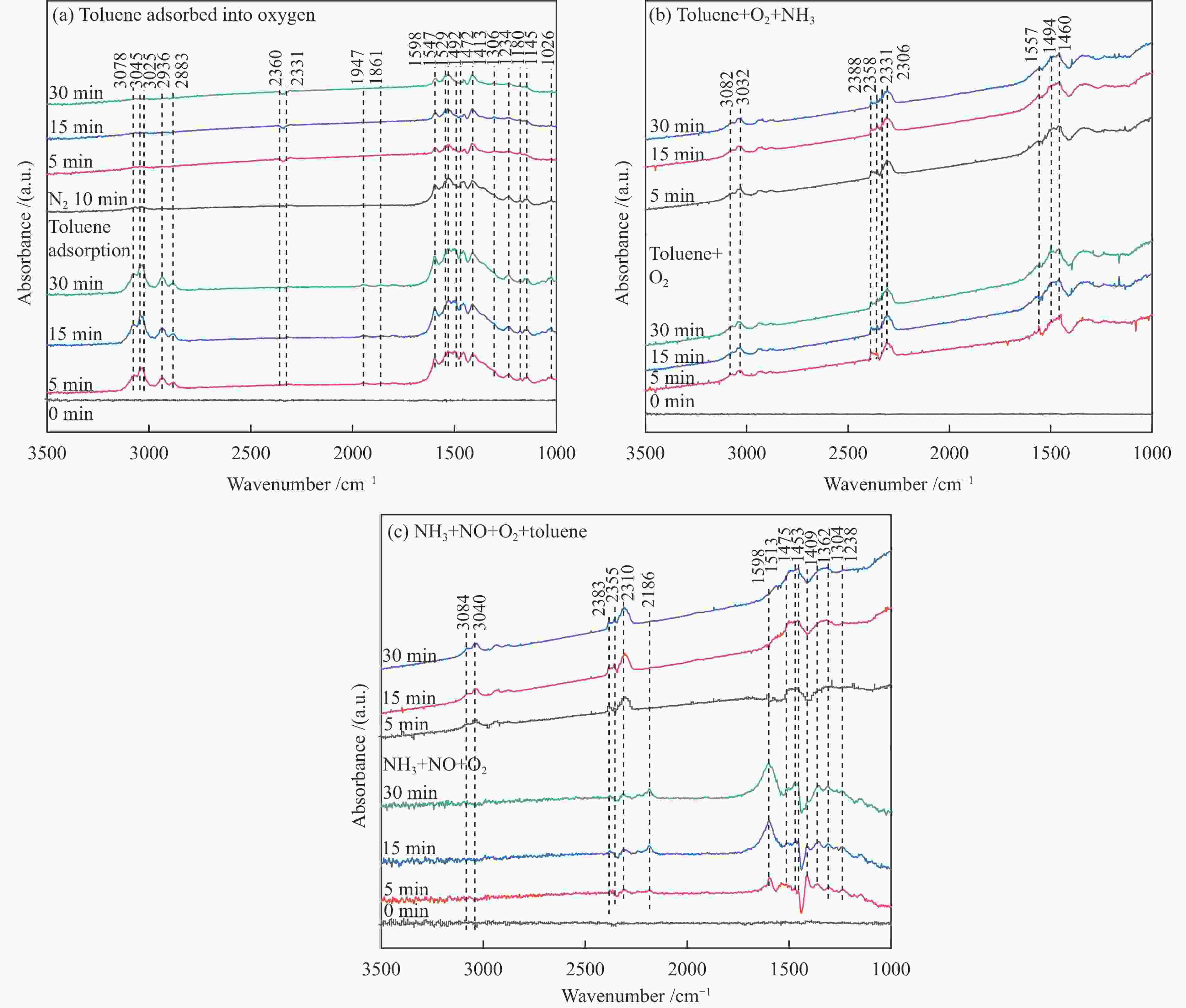
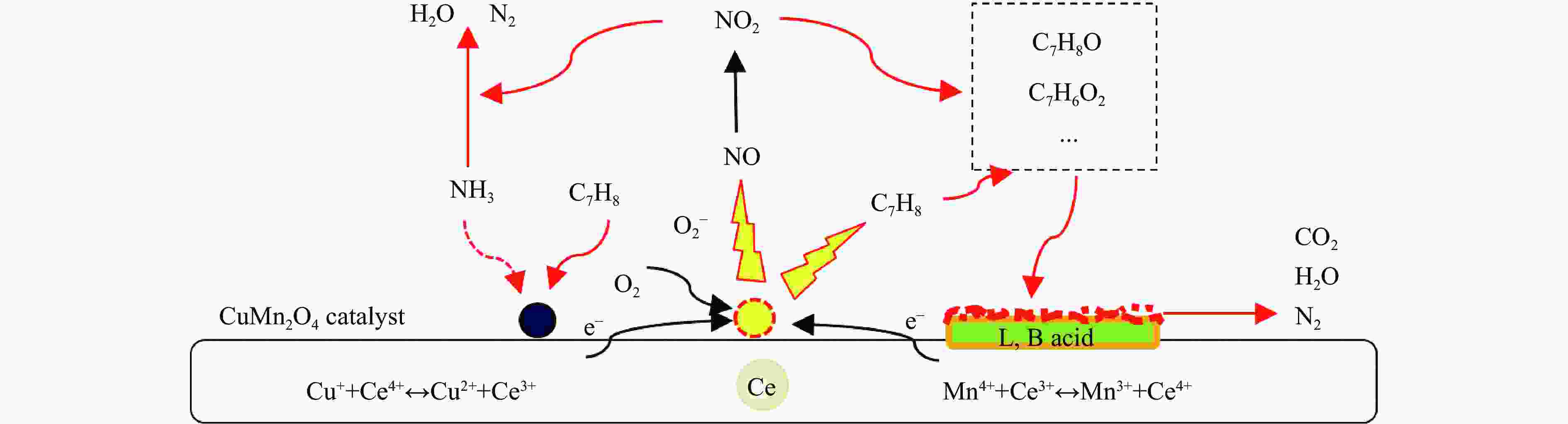
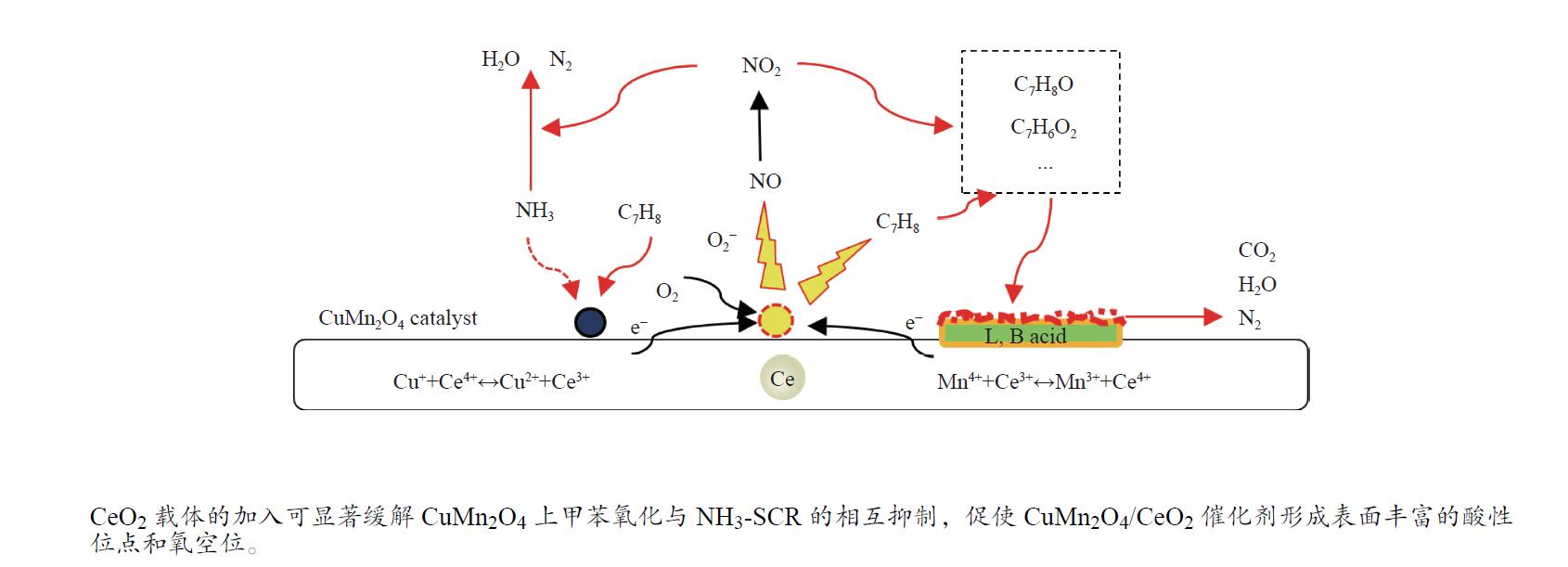
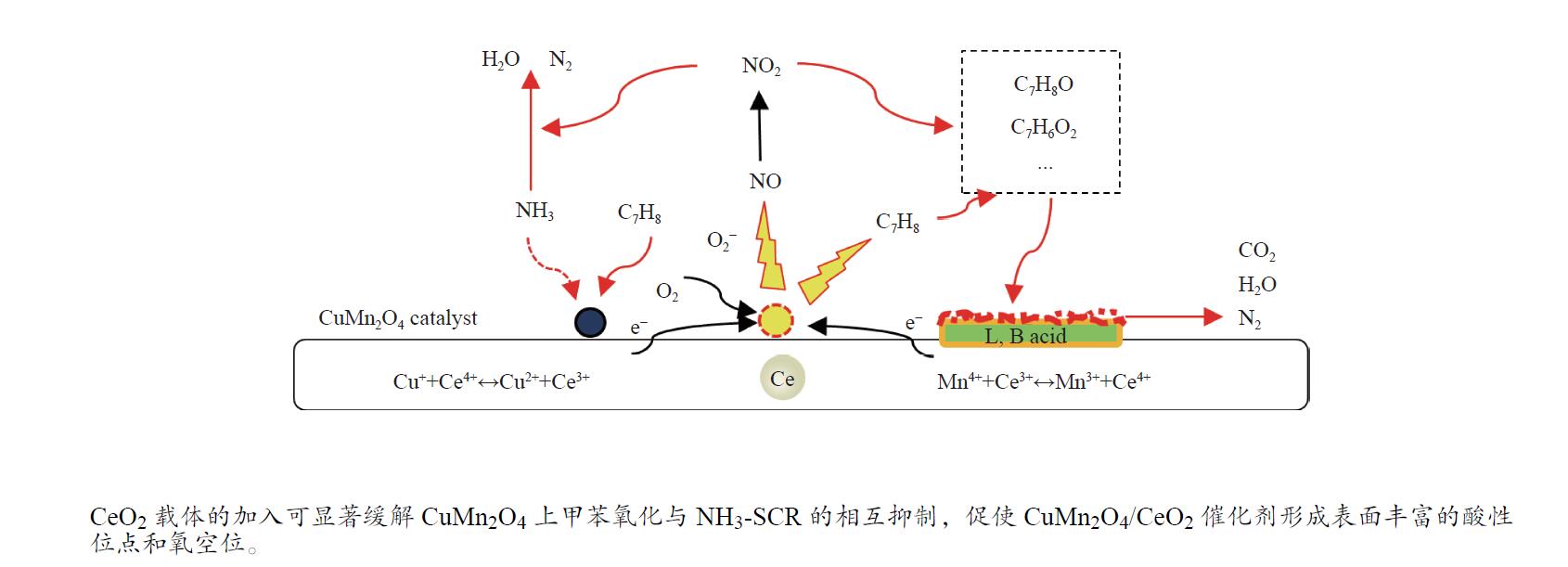
摘要: 本研究使用低温溶胶凝胶自燃法制备了不同载体负载的CuMn2O4/MO2 (M=Mn、Ti、Ce)催化剂,并对其同时去除甲苯和NOx的性能进行了评价。结果表明,CeO2载体的加入可显著缓解CuMn2O4上甲苯氧化与NH3-SCR的相互抑制,因此,CuMn2O4/CeO2催化剂表现出最优异的甲苯与NOx同时去除效率。通过BET、XRD、NH3-TPD、O2-TPD及结合XPS与in-situ DRIFTs对催化剂的物化性质及CuMn2O4同时去除甲苯与NOx的反应机理进行了分析。结果表明,CeO2的引入提高了催化剂中Mn4 + /Mnn + 的占比,促使CuMn2O4/CeO2催化剂形成表面丰富的酸性位点和氧空位。此外,Cu、Mn和Ce之间的强相互作用加速电子转移,增强了Cu + + Ce4 + ↔Cu2 + + Ce3 + 、Mn4 + + Ce3 + ↔Mn3 + + Ce4 + 的氧化还原循环。In-situ DRIFTs证实了CuMn2O4催化剂上NH3-SCR反应遵循Langmuir-Hinshelwood机制,甲苯的氧化遵循Mars-van Krevelen机制。因此,CeO2作为载体的CuMn2O4/CeO2催化剂优异的氧化还原能力促使甲苯的完全氧化,表现出优异的甲苯与NOx同时去除能力。本工作可为同时消除甲苯和NOx的催化剂开发提供指导。Abstract: In this study, CuMn2O4/MO2 (M=Mn, Ti, Ce) catalysts with different support loads were prepared by sol-gel spontaneous combustion at low temperature, and the removal performance of toluene and NOx was evaluated. The results showed that the addition of CeO2 carrier could significantly alleviate the mutual inhibition of toluene oxidation and NH3-SCR over CuMn2O4. Therefore, CuMn2O4/CeO2 catalyst showed the best removal efficiency of toluene and NOx simultaneously. The physicochemical properties of the catalyst and the reaction mechanism of CuMn2O4 were analyzed by BET, XRD, NH3-TPD, O2-TPD and combined XPS and in-situ DRIFTs. The results showed that the introduction of CeO2 increased the proportion of Mn4 + /Mnn + in the catalyst, and promoted the formation of rich acid sites and oxygen vacancies on the surface of CuMn2O4/CeO2 catalyst. In addition, the strong interaction between Cu, Mn and Ce accelerated electron transfer and enhance the redox cycle for Cu + + Ce4 + ↔Cu2 + + Ce3 + 、Mn4 + + Ce3 + ↔Mn3 + + Ce4 + . It has been confirmed by in-situ DRIFTs that NH3-SCR reaction on CuMn2O4 catalyst follows Langmuir-Hinshelwood mechanism and oxidation of toluene follows Mars-van Krevelen mechanism. Therefore, CuMn2O4/CeO2 catalyst with CeO2 as the support has excellent reoxidation ability to promote the complete oxidation of toluene, so it shows excellent removal ability of toluene and NOx simultaneously. This work can provide guidance for the development of catalysts for simultaneous removal of toluene and NOx.
-
Key words:
- supported catalyst /
- catalytic oxidation /
- NH3-SCR /
- NO /
- toluene
-
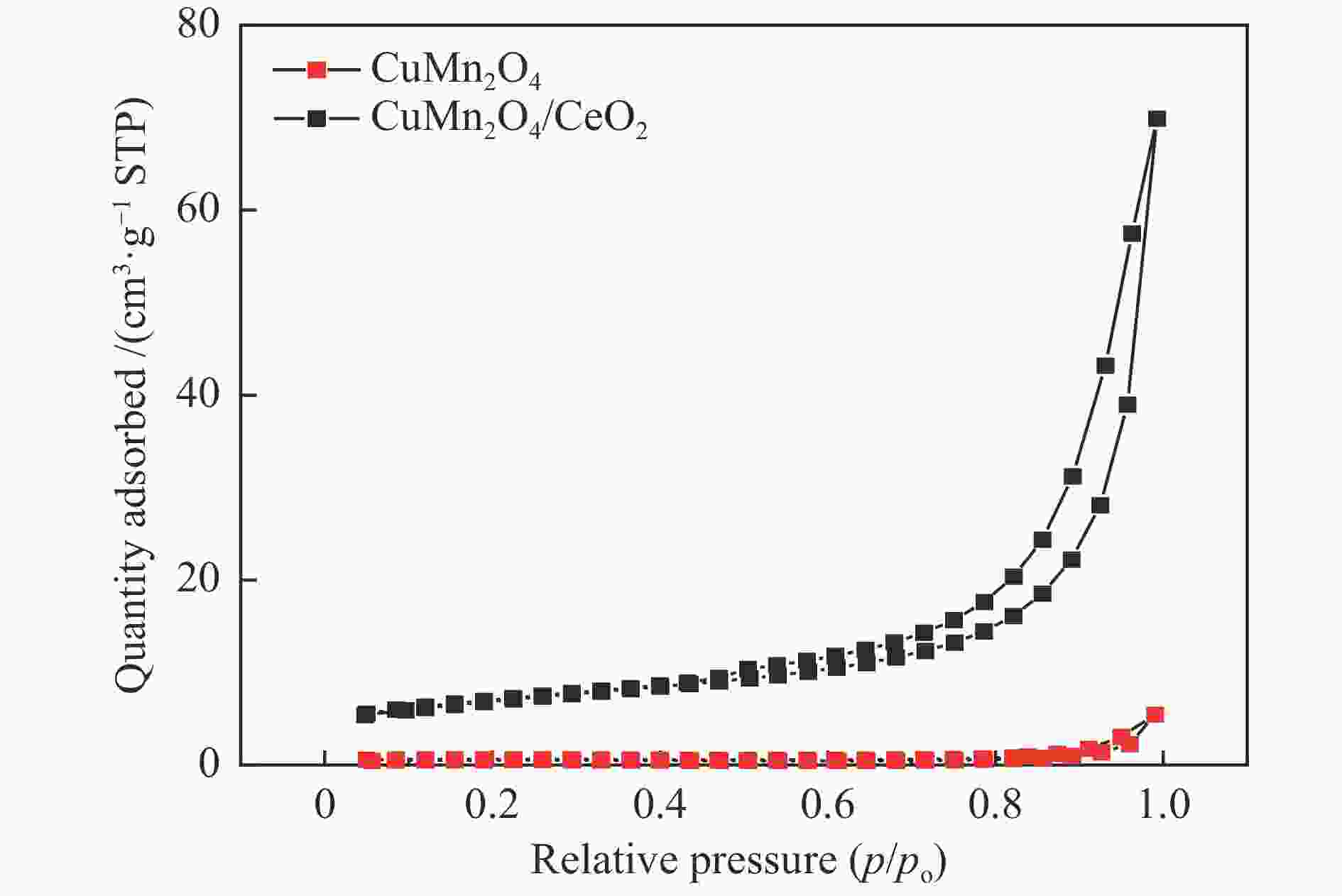
表 1 催化剂的比表面积以及孔容孔径
Table 1 Catalyst specific surface area
Species Surface area
/(m2·g−1)Pore volume
/(cm3·g−1)Mean pore
size /nmCuMn2O4 1.61 0.00249 5.99 CuMn2O4/MnO2 18.52 0.04165 7.89 CuMn2O4/TiO2 19.24 0.04759 8.32 CuMn2O4/CeO2 23.76 0.05247 8.84 表 2 催化剂的XPS表面元素比值
Table 2 XPS of surface element ratio
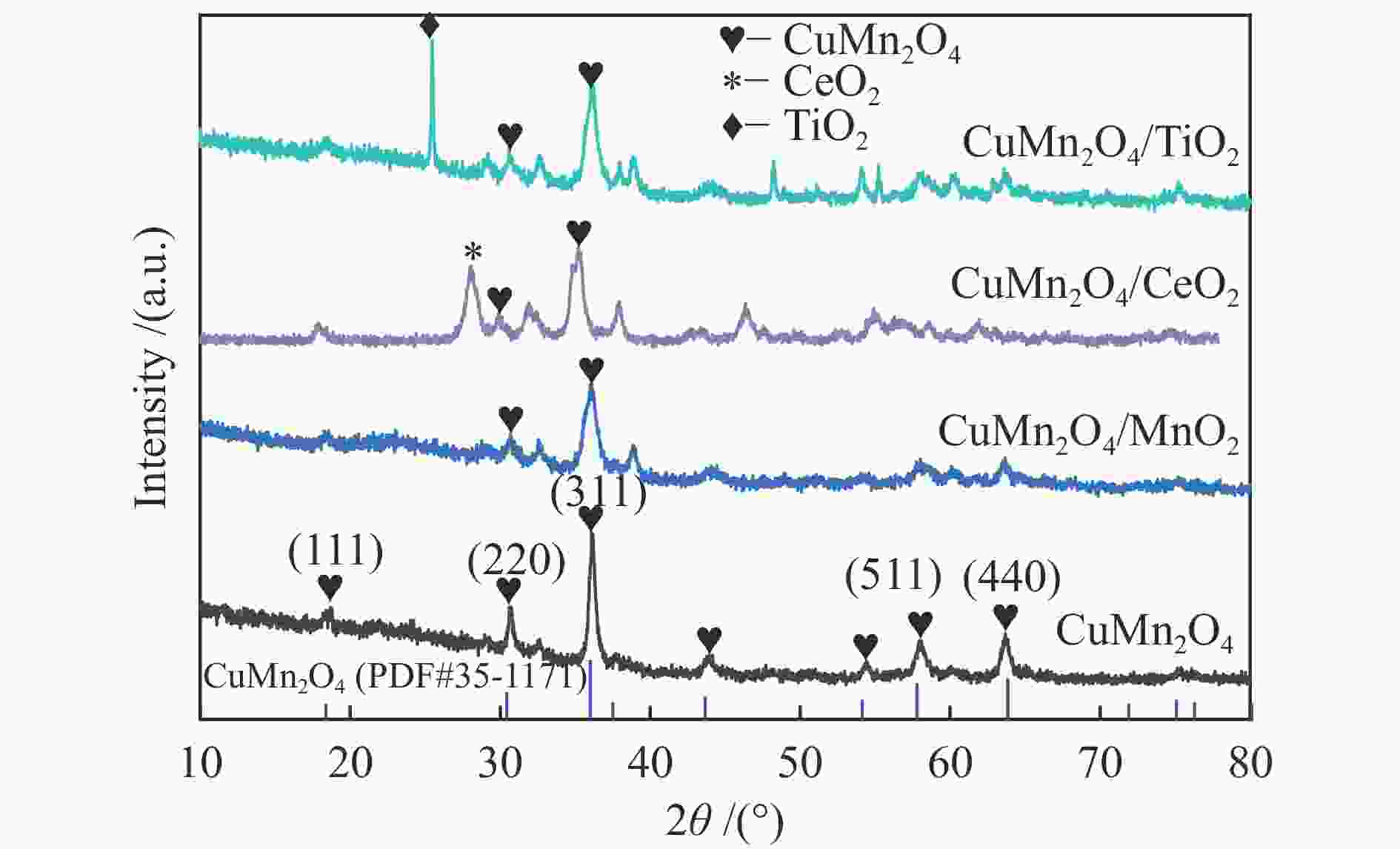
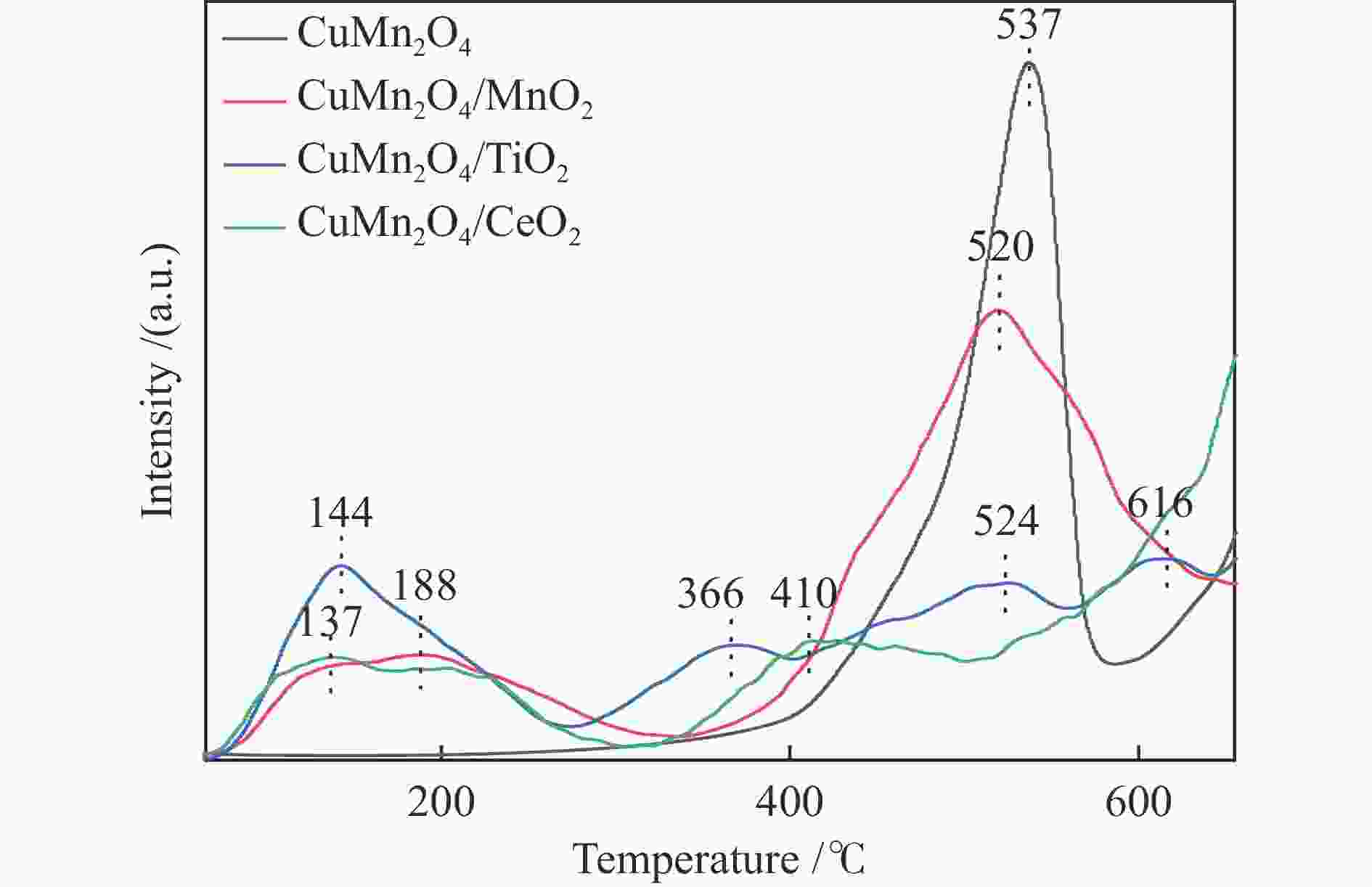
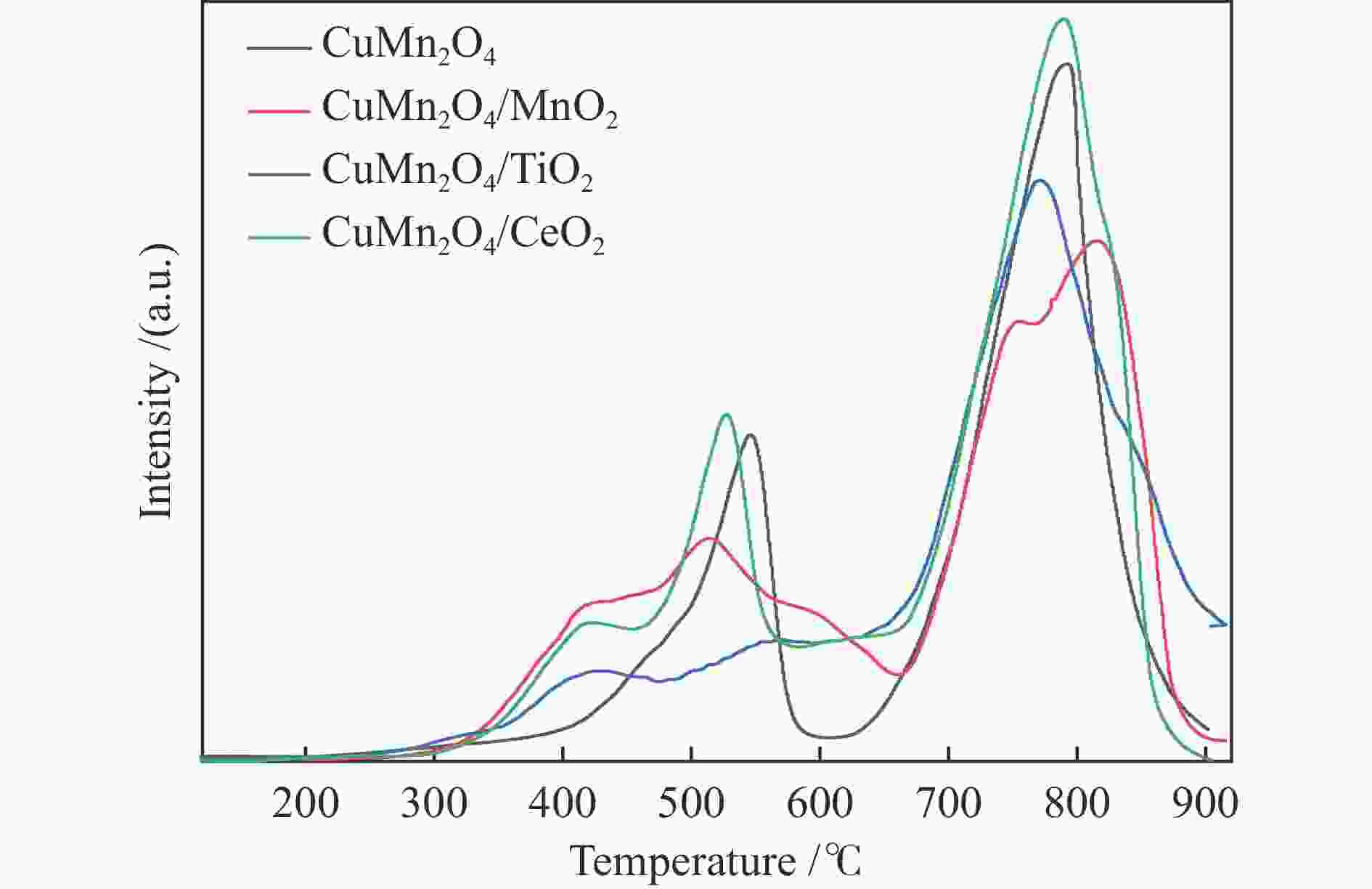
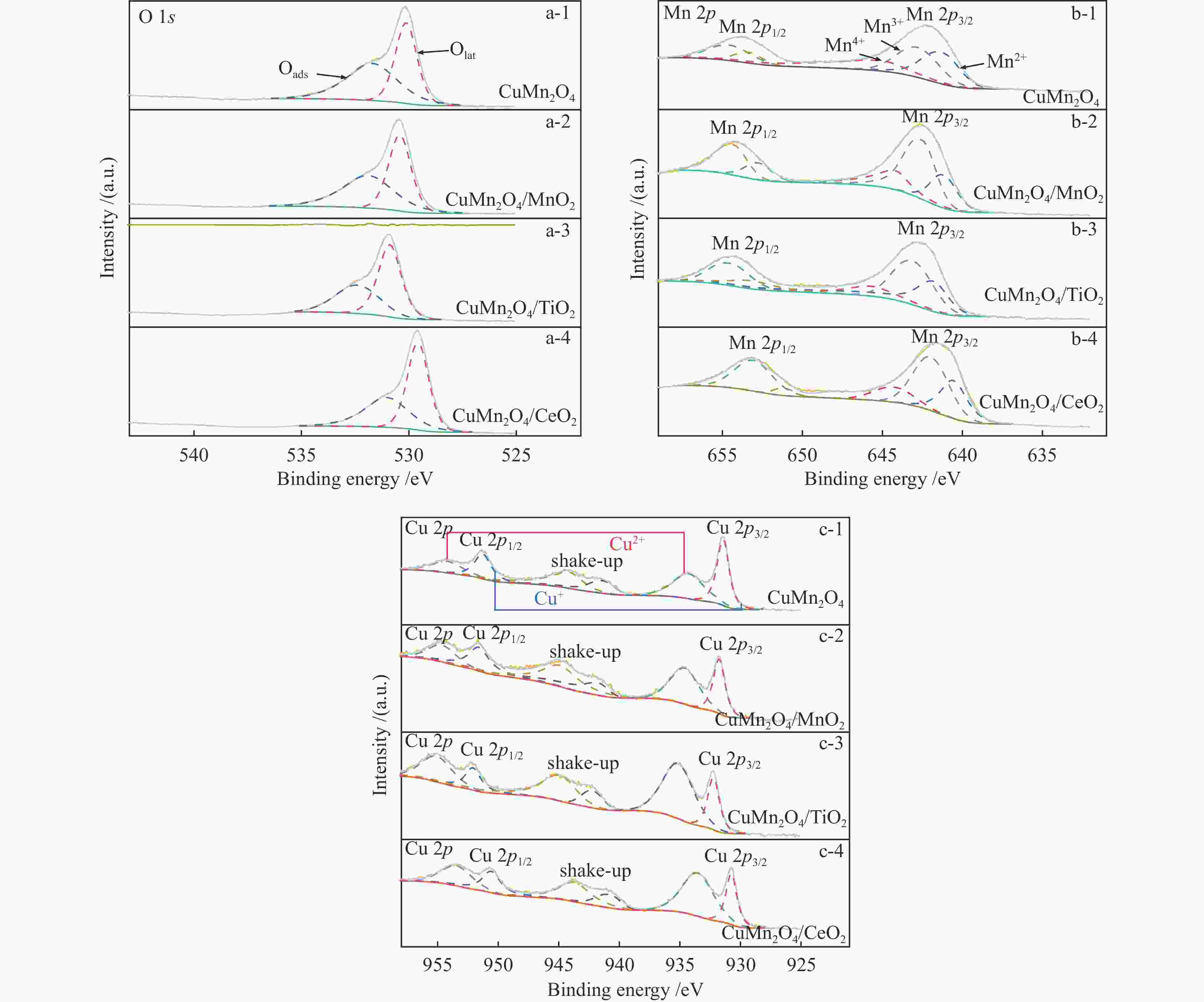
Species O Atomic/% Binding energy/eV Cu + /Cu2 + Mn4 + /Mn3 + Atomic /% Oads Olatt Oads/Olat Oads Olat Cu Mn O M CuMn2O4 52.54 47.46 1.10 531.7 530.1 0.57 0.47 7.16 26.29 66.55 CuMn2O4/MnO2 47.85 52.15 0.91 531.8 530.4 0.82 0.50 6.01 26.81 67.17 CuMn2O4/TiO2 42.20 57.80 0.73 532.4 530.9 0.22 0.22 6.56 23.06 65.24 5.14 CuMn2O4/CeO2 35.11 64.89 0.54 531.0 529.6 0.28 0.78 5.51 23.66 65.75 5.08 -
[1] XIAO G, GUO Z, LIN B. Cu-VWT catalysts for synergistic elimination of NOx and volatile organic compounds from coal-fired flue gas[J]. Environ Sci Technol,2022,56(14):10095−10104. doi: 10.1021/acs.est.2c02083 [2] LIU H, CHEN J, WANG Y, YIN R, YANG W, WANG G, SI W, PENG Y, LI J. Interaction mechanism for simultaneous elimination of nitrogen oxides and toluene over the bifunctional CeO2-TiO2 mixed oxide catalyst[J]. Environ Sci Technol,2022,56(7):4467−4476. doi: 10.1021/acs.est.1c08424 [3] CHEN Y, CHEN Z, ZHANG C, LIN C, TANG J, LIAO Y, MA X. Multiple pollutants control of NO, benzene and toluene from coal-fired plant by Mo/Ni impregnated TiO2-based NH3-SCR catalyst: A DFT supported experimental study[J]. Appl Surf Sci,2022,599:153986. doi: 10.1016/j.apsusc.2022.153986 [4] FANG D, XIE J, MEI D, ZHANG Y, HE F, LIU X, LI Y. Effect of CuMn2O4 spinel in Cu-Mn oxide catalysts on selective catalytic reduction of NOx with NH3 at low temperature[J]. RSC Adv,2014,4(49):25540−25551. [5] ZHANG Y, LI Y, ZENG Z, HU J, HUANG Z. Promotion mechanism of CuMn2O4 modification with NaOH on toluene oxidation: Boosting the ring-opening of benzoate[J]. Fuel,2022,314:122747. [6] PAN H, CHEN Z, MA M, GUO T, LING X, ZHENG Y, HE C, CHEN J. Mutual inhibition mechanism of simultaneous catalytic removal of NOx and toluene on Mn-based catalysts[J]. J Colloid Interface Sci,2022,607:1189−1200. [7] YE L, LU P, CHEN X, FANG X, PENG P, LI Y, HUANG J, BAO H. The deactivation mechanism of toluene on MnOx-CeO2 SCR catalyst[J]. Appl Catal B: Environ,2020,277:119257. [8] LI Z, GAO, WANG Q. The influencing mechanism of NH3 and NOx addition on the catalytic oxidation of toluene over Mn2Cu1Al1Ox catalyst[J]. J Cleaner Prod,2022,348:131152. doi: 10.1016/j.jclepro.2022.131152 [9] ZHOU X, LIAO W, CAI N, ZHANG H, YANG H, SHAO J. Experiment and mechanism investigation on simultaneously catalytic reduction of NOx and oxidation of toluene over MnOx/Cu-SAPO-34[J]. Appl Surf Sci,2023,611:155628. doi: 10.1016/j.apsusc.2022.155628 [10] 张娜, 黄妍, 张俊丰, 赵令葵, 李思密, 陶泓帆, 伍云凡. 负载型LaCoO3/MO2催化氧化甲苯与NO的性能研究[J]. 燃料化学学报,2022,50(7):868−876.ZHANG Na, HUANG Yan, ZHANG Jun-feng, ZHAO Ling-kui, LI Si-mi, TAO Hong-fan, WU Yun-fan. Catalytic oxidation of toluene and NO by supported LaCoO3/MO2[J]. J Fuel Chem Technol,2022,50(7):868−876. [11] WANG Z, LIU J, YANG Y, YU Y, YAN X, ZHANG Z. AMn2O4 (A=Cu, Ni and Zn) sorbents coupling high adsorption and regeneration performance for elemental mercury removal from syngas[J]. J Haz Mater,2020,388:121738. doi: 10.1016/j.jhazmat.2019.121738 [12] KANG M, PARK E D, KIM J M, YIE J. Cu-Mn mixed oxides for low temperature NO reduction with NH3[J]. Catal Today,2006,111(3/4):236−241. doi: 10.1016/j.cattod.2005.10.032 [13] FANG R, LIU F, LIU J, LI Y. Experimental and theoretical insights into the reaction mechanism of spinel CuMn2O4 with CO in chemical-looping combustion[J]. Appl Surf Sci,2021,561:150065. [14] ZHANG Y, LI Y, ZENG Z, HU J, HOU Y, HUANG Z. Synergically engineering Cu + and oxygen vacancies in CuMn2O4 catalysts for enhanced toluene oxidation performance[J]. Mol Catal,2022,517:112043. [15] 蒋露. SO2、H2O对镧铁系钙钛矿催化剂协同催化氧化NO和甲苯的影响机制研究[D]. 湘潭: 湘潭大学, 2021.JIANG Lu. Effect mechanism of SO2 and H2O on synergistic catalytic oxidation of NO and Toluene with lanthanide perovskite catalyst[D]. Xiangtan: Xiangtan University, 2021. [16] LIN B, GUO Z, LI J, XIAO G, YE D, YUN H. V-Cu bimetallic oxide supported catalysts for synergistic removal of toluene and NOx from coal-fired flue gas: The crucial role of support[J]. Chem Eng J,2023,458:141443. [17] YANG J, HU S, FANG Y, HOANG S, GOU Y. Oxygen vacancy promoted O2 activation over perovskite oxide for low-temperature CO oxidation[J]. ACS Catal,2019,9(11):9751−9763. doi: 10.1021/acscatal.9b02408 [18] SHI C, YIN D, LI J. Novel LaMnNi mixed metal oxides catalysts for selective catalytic oxidation of NO[C]//The 2019 North American Catalysis Society Meeting, 2019. NAM, 262019. [19] HEREDIA L, COLOMBO E, QUAINO P, COLLINS S. Toluene adsorption on CeO2 (111) studied by FTIR and DFT[J]. Top Catal,2022,65(7/8):934−943. doi: 10.1007/s11244-022-01625-2 [20] WANG J, XING Y, SU W, LI K, ZHANG W. Bifunctional Mn-Cu-CeOx/γ-Al2O3 catalysts for low-temperature simultaneous removal of NOx and CO[J]. Fuel,2022,321:124050. [21] ZHANG X, LV X, BI F. Highly efficient Mn2O3 catalysts derived from Mn-MOFs for toluene oxidation: The influence of MOFs precursors[J]. Mol Catal,2020,482:110707. [22] LI Z, GAO Y, WANG Q. The influencing mechanism of NH3 and NOx addition on the catalytic oxidation of toluene over Mn2Cu1Al1Ox catalyst [J]. J Clean Prod, 2022, 348: 131152 . [23] ZHONG J, ZENG Y, ZHANG M, FANG W, XIAO D, WU J, CHEN P, FU M, YE D. Toluene oxidation process and proper mechanism over Co3O4 nanotubes: Investigation through in-situ DRIFTS combined with PTR-TOF-MS and quasi in-situ XPS[J]. Chem Eng J,2020,397:125375. doi: 10.1016/j.cej.2020.125375 [24] LU J, ZHONG J, REN Q, LI J, SONG L, MO S, ZHANG M, CHEN P, FU M, YE D. Construction of Cu-Ce interface for boosting toluene oxidation: Study of Cu-Ce interaction and intermediates identified by in situ DRIFTS[J]. Chin Chem Lett,2021,32(11):3435−3439. doi: 10.1016/j.cclet.2021.05.029 [25] BESSELMANN S, LÖFFLER E, MUHLER M. On the role of monomeric vanadyl species in toluene adsorption and oxidation on V2O5/TiO2 catalysts: A Raman and in situ DRIFTS study[J]. J Mol Catal A: Chem,2000,162(1/2):401−411. doi: 10.1016/S1381-1169(00)00307-1 [26] ZHANG X, LI H, SONG Z. In situ DRIFT spectroscopy study into the reaction mechanism of toluene over CeMo catalysts[J]. JECE,2022,10(6):108895. [27] CHEN Y, CHEN Z, ZHANG C, CHEN L, TANG J, LIAO Y, MA X. Multiple pollutants control of NO, benzene and toluene from coal-fired plant by Mo/Ni impregnated TiO2-based NH3-SCR catalyst: A DFT supported experimental study[J]. Appl Surf Sci,2022,599:1539869. [28] JIA Y, JIANG J, ZHENG R, GUO L, GU M. Insight into the reaction mechanism over PMoA for low temperature NH3-SCR: A combined In-situ DRIFTs and DFT transition state calculations[J]. J Haz Mater,2021,412:125258. doi: 10.1016/j.jhazmat.2021.125258 [29] YANG Y, LIU J, LIU F, WANG Z. Reaction mechanism for NH3-SCR of NOx over CuMn2O4 catalyst[J]. Chem Eng J,2019,361:578−587. doi: 10.1016/j.cej.2018.12.103 [30] ZHAO L, YANG Y, LIU J, DING J. Mechanistic insights into benzene oxidation over CuMn2O4 catalyst[J]. J Haz Mater,2022,431:128640. doi: 10.1016/j.jhazmat.2022.128640 -




 下载:
下载: