Research on the migration and transformation mechanism of nitrogen during biomass pyrolysis
-
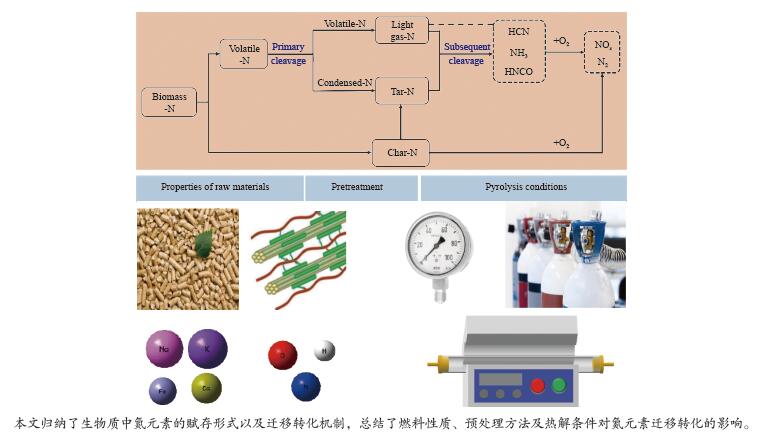
摘要: 利用热解技术将生物质转化为高值含氮化学品或含氮炭材料能够显著提升生物质的利用价值,且明显降低含氮物质带来的环境污染风险。因此,明晰热解过程中氮元素的迁移转化机制对促进生物质热解技术的开发具有重要意义。本研究归纳了不同种类生物质中氮元素的赋存形式及含量;以固、液、气相热解产物中氮元素的分布为基础,概述了氮元素从生物质向热解产物迁移转化的反应机制;总结了燃料性质、预处理方法及热解条件对氮元素迁移转化的影响,并对未来生物质热解过程中氮元素迁移转化机制的研究方向进行了展望。Abstract: Biomass can be converted into high-value nitrogen-containing chemicals and nitrogen-containing carbon materials by pyrolysis technique, which significantly increases the value of biomass and lowers the risk of environmental pollution by nitrogen-containing pollutants. Therefore, a good understanding of the migration and conversion mechanisms of nitrogen during pyrolysis is critical for the advancement of biomass pyrolysis technique. Herein, the forms and contents of nitrogen in biomass were first summarized. Afterward, the transformation process of nitrogen from biomass to pyrolysis products was discussed based on the distribution of nitrogen in the solid, liquid, and gaseous pyrolysis products. Finally, the effects of fuel properties, pretreatment methods and pyrolysis conditions on the migration and transformation of nitrogen were discussed carefully. In addition, an outlook for future research on nitrogen migration in biomass pyrolysis process was provided.
-
Key words:
- biomass /
- pyrolysis /
- nitrogen-containing compounds /
- migration and transformation
-
表 1 生物质中常见氮的赋存形式
Table 1 Forms of nitrogen in biomass
N-functionalitie Structure N-functionalities Structure Protein 
Alkaloids (caffeine) 
Free amino acids 
Inorganic-N ${\bf{NO}}_3^- $,${\bf{NO}}_2^- $,${\bf{NH}}_4^+ $ Nucleic acids and mononucleotides 
Chlorophyll 
表 2 矿物成分对含氮化合物生成的影响
Table 2 Effect of mineral composition on nitrogen-containing compounds formation
Biomas Temperature /℃ Mineral components Mineral components:
Raw materialsEffects on nitrogenous
compoundsRef. Microalgae 450–600 Li2CO3-Na2CO3-K2CO3 − amines↓ [59] N-heterocycles↓ nitriles↑ Bean 400–800 Fe2O3 1∶10 amines↑ [60] N-heterocycles↓ nitriles↓ Al2O3 1∶10 amines↓ N-heterocycles↓ nitriles (unchanged) CaO 1∶10 amines↓ N-heterocycles↓ nitriles (first↑, then↓) Protein 600 Ca(OH)2 1∶1 amines↓
N-heterocycles↓
nitriles↓[58] Fe2(SO4)3 1∶1 amines↓
N-heterocycles (unchanged)
nitriles↑Ca(OH)2 + Fe2(SO4)3 1∶1∶1 amines↓
N-heterocycles↓
nitriles↑Sludge sewage 600 CaO 3:10 amines↓
N-heterocycles↓
nitriles↑[56] FeSO4 0.4∶10 amines↑,
N-heterocycles↓
nitriles↑CaO + FeSO4 3∶0.4 amines↓
N-heterocycles↓
nitriles↑Sludge sewage 100–900 MgO 1∶10 All↓ [61] -
[1] OKUDA M. World energy trends seen in BP Statistics 2020[J]. Haikan Gijutsu,2020,62(2):1−11. [2] 刘宁, 史成香, 潘伦, 张香文, 邹吉军. 生物质替代石油原料合成高密度燃料的研究进展[J]. 燃料化学学报,2021,49(12):1780−1790. doi: 10.19906/j.cnki.jfct.2021076LIU Ning, SHI Cheng-xiang, PAN Lun, ZHANG Xiang-wen, ZOU Ji-jun. Progress on using biomass derivatives to replace petroleum for synthesis of high-density fuels[J]. J Fuel Chem Technol,2021,49(12):1780−1790. doi: 10.19906/j.cnki.jfct.2021076 [3] 李承宇, 张军, 袁浩然, 王树荣, 陈勇. 纤维素热解转化的研究进展[J]. 燃料化学学报,2021,49(12):1733−1751. doi: 10.1016/S1872-5813(21)60134-2LI Cheng-yu, ZHANG Jun, YUAN Hao-ran, WANG Shu-rong, CHEN Yong. Advance on the pyrolytic transformation of cellulose[J]. J Fuel Chem Technol,2021,49(12):1733−1751. doi: 10.1016/S1872-5813(21)60134-2 [4] 中国生物质能源网. 《3060零碳生物质能发展潜力蓝皮书》全文发布[EB/OL]. http://www.cnbioenergy.com/laws/2171.html, 2022-01-06.China Biomass Energy Network.《3060 Blue Book on the Development Potential of Zero Carbon Biomass Energy》Full text publishing[EB/OL]. http://www.cnbioenergy.com/laws/2171.html, 2022-01-06. [5] 王俊丽, 赵强, 郝晓刚, 黄伟, 赵建国. 低阶煤与生物质混合低温共热解特性分析及对产物组成的影响[J]. 燃料化学学报,2021,49(1):37−46. doi: 10.19906/j.cnki.JFCT.2021003WANG Jun-li, ZHAO Qiang, HAO Xiao-gang, HUANG Wei, ZHAO Jian-guo. Low temperature co-pyrolysis of low rank coal with biomass and its influence on pyrolysis-derived products[J]. J Fuel Chem Technol,2021,49(1):37−46. doi: 10.19906/j.cnki.JFCT.2021003 [6] XU L, YAO Q, DENG J, HAN Z, ZHANG Y, HUBER W, GUO Q. Renewable N-heterocycles production by thermocatalytic conversion and ammonization of biomass over ZSM-5[J]. ACS Sustainable Chem Eng,2015,3(11):2890−2899. doi: 10.1021/acssuschemeng.5b00841 [7] 陈伟. 生物质富氮热解过程中氮的迁移转化及含氮目标产物调控研究[D]. 武汉: 华中科技大学, 2018.CHEN Wei. Research on the nitrogen transformation and N-containing target products control during biomass nitrogen-enriched pyrolysis[D]. Wuhan: Huazhong University of Science and Technology, 2018. [8] ZHONG G, MENG Z, XU M, XIE H, XU S, FU X, LIAO W, ZHENG S, XU Y. Self-nitrogen-doped porous carbon prepared via pyrolysis of grass-blade without additive for oxygen reduction reaction[J]. Diamond Relat Mater,2022,121:108742. doi: 10.1016/j.diamond.2021.108742 [9] THYGESEN A, TSAPEKOS P, ALVARADO-MORALES M, ANGELIDAKI I. Valorization of municipal organic waste into purified lactic acid[J]. Bioresour Technol,2021,342:125933. doi: 10.1016/j.biortech.2021.125933 [10] 周建强, 高攀, 董长青, 杨勇平. 固体生物质燃烧中氮氧化物产生机理综述[J]. 热力发电,2018,47(12):1−9. doi: 10.19666/j.rlfd.201802073ZHOU Jian-qiang, GAO Pan, DONG Chang-qing, YANG Yong-ping. Formation mechanism of nitrogen oxides during solid biomass fuel buring: A review[J]. Therm Power Conf,2018,47(12):1−9. doi: 10.19666/j.rlfd.201802073 [11] STUBENBERGER G, SCHARLER R, ZAHIROVIC S, OBERNBERGER I. Experimental investigation of nitrogen species release from different solid biomass fuels as a basis for release models[J]. Fuel,2008,87(6):793−806. doi: 10.1016/j.fuel.2007.05.034 [12] TAN X, ZHANG Y, YANG L, CHU H, GUO J. Outdoor cultures of Chlorella pyrenoidosa in the effluent of anaerobically digested activated sludge: the effects of pH and free ammonia[J]. Bioresour Technol,2016,200:606−615. doi: 10.1016/j.biortech.2015.10.095 [13] FONT-PALMA C. Methods for the treatment of cattle manure—a review[J]. C,2019,5(2):27. [14] PARK C, LEE N, KIM J, LEE J. Co-pyrolysis of food waste and wood bark to produce hydrogen with minimizing pollutant emissions[J]. Environ Pollut,2021,270:116045. doi: 10.1016/j.envpol.2020.116045 [15] 王宗华, 张军营, 赵永椿, 李扬, 郑楚光. 生物质热解过程中NO、NH3和HCN的释放特性[J]. 燃料化学学报,2011,39(2):99−102. doi: 10.3969/j.issn.0253-2409.2011.02.005WANG Zong-hua, ZHANG Jun-ying, ZHAO Yong-chun, LI Yang, ZHENG Chu-guang. Formation of NO, NH3 and HCN during pyrolysis of biomass[J]. J Fuel Chem Technol,2011,39(2):99−102. doi: 10.3969/j.issn.0253-2409.2011.02.005 [16] 孙志向. 生物质热解过程中燃料氮转化及碱/碱土金属离子催化转化的实验研究[D]. 北京: 华北电力大学, 2014.SUN Zhi-xiang. Experimental study on the fuel-nitrogen transformation and the alkali/alkali-earth metal ions catalysis during the biomass pyrolysis[D]. Beijing: North China Electric Power University, 2014. [17] PLEISSNER D, LAM W, SUN Z, LIN C. Food waste as nutrient source in heterotrophic microalgae cultivation[J]. Biosour Technol, 2013, 137: 139−146. [18] TONG B, HOU Y, WANG S, MA W. Partial substitution of urea fertilizers by manure increases crop yield and nitrogen use efficiency of a wheat-maize double cropping system[J]. Nutr Cycling Agroecosyst, 2022: 1–11. [19] BROER K M, BROWN R C. The role of char and tar in determining the gas-phase partitioning of nitrogen during biomass gasification[J]. Appl Energy,2015,158:474−483. doi: 10.1016/j.apenergy.2015.08.100 [20] YU Q, BRAGE C, CHEN G, SJOSTROM K. The fate of fuel-nitrogen during gasification of biomass in a pressurised fluidised bed gasifier[J]. Fuel,2007,86(4):611−618. doi: 10.1016/j.fuel.2006.08.007 [21] ZHAN H, ZHUANG X, SONG Y, CHANG G, WANG Z, YIN X, WANG X, WU C. Formation and regulatory mechanisms of N-containing gaseous pollutants during stage-pyrolysis of agricultural biowastes[J]. J Cleaner Prod,2019,236:117706. doi: 10.1016/j.jclepro.2019.117706 [22] ZHANG J, TIAN Y, CUI Y, ZUO W, TAN T. Key intermediates in nitrogen transformation during microwave pyrolysis of sewage sludge: a protein model compound study[J]. Bioresour Technol,2013,132:57−63. doi: 10.1016/j.biortech.2013.01.008 [23] DEBONO O, VILLOT A. Nitrogen products and reaction pathway of nitrogen compounds during the pyrolysis of various organic wastes[J]. J Anal Appl Pyrolysis,2015,114:222−234. doi: 10.1016/j.jaap.2015.06.002 [24] LIU T, GUO Y, PENG N, LANG Q, XIA Y, GAI C, LIU Z. Nitrogen transformation among char, tar and gas during pyrolysis of sewage sludge and corresponding hydrochar[J]. J Anal Appl Pyrolysis,2017,126:298−306. doi: 10.1016/j.jaap.2017.05.017 [25] ANCA-COUCE A, SOMMERSACHER P, EVIC N, MEHRABIAN R, SCHARLER R. Experiments and modelling of NOx precursors release (NH3 and HCN) in fixed-bed biomass combustion conditions[J]. Fuel,2018,222:529−537. doi: 10.1016/j.fuel.2018.03.003 [26] NEVES D, THUNMAN H, MATOS A, TARELHO L, GOMEZ-BAREA A. Characterization and prediction of biomass pyrolysis products[J]. Prog Energy Combust Sci,2011,37(5):611−630. doi: 10.1016/j.pecs.2011.01.001 [27] CHEN H, WANG Y, XU G, YOSHIKAWA K. Fuel-N evolution during the pyrolysis of industrial biomass wastes with high nitrogen content[J]. Energies,2012,5(12):5418−5438. doi: 10.3390/en5125418 [28] 武桐. 生活垃圾典型组分热解过程中氮的迁移和转化规律研究[D]. 上海: 上海交通大学, 2015.WU Tong. Research on nitrogen transfer in typical municial solid waste constituents pyrolysis[D]. Shanghai: Shanghai Jiao Tong University, 2015. [29] 李祺. 不同垃圾组分在热转化过程中NOx释放规律实验研究[D]. 杭州: 浙江大学, 2019.LI Qi. Experimental study on NOx release rule of different waste components during thermal conversion[D]. Hangzhou: Zhejiang University, 2019. [30] GUO S, LIU T, HUI J, CHE D, LI X, SUN B, LI S. Effects of calcium oxide on nitrogen oxide precursor formation during sludge protein pyrolysis[J]. Energy,2019,189:116217. doi: 10.1016/j.energy.2019.116217 [31] ZHAN H, ZHUANG X, SONG Y, YIN X, WU C. Insights into the evolution of fuel-N to NOx precursors during pyrolysis of N-rich nonlignocellulosic biomass[J]. Appl Energy,2018,219:20−33. doi: 10.1016/j.apenergy.2018.03.015 [32] CHENG F, BAYAT H, JENA U, BREWER C. Impact of feedstock composition on pyrolysis of low-cost, protein-and lignin-rich biomass: A review[J]. J Anal Appl Pyrolysis,2020,147:104780. doi: 10.1016/j.jaap.2020.104780 [33] MALIUTINA K, TAHMASEBI A, YU J. The transformation of nitrogen during pressurized entrained-flow pyrolysis of Chlorella vulgaris[J]. Bioresour Technol,2018,262:90−97. doi: 10.1016/j.biortech.2018.04.073 [34] LI C, TAN L. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part III. Further discussion on the formation of HCN and NH3 during pyrolysis[J]. Fuel,2000,79(15):1899−1906. doi: 10.1016/S0016-2361(00)00008-9 [35] LENG L, YANG L, CHEN J, LENG S, LI H, LI H, YUAN X, ZHOU W, HUANG H. A review on pyrolysis of protein-rich biomass: Nitrogen transformation[J]. Bioresour Technol,2020,315:123801. doi: 10.1016/j.biortech.2020.123801 [36] ADAMCZYK W, WERLE S, RYFA A. Application of the computational method for predicting NOx reduction within large scale coal-fired boiler[J]. Appl Therm Eng,2014,73(1):343−350. doi: 10.1016/j.applthermaleng.2014.07.045 [37] MACKIE J C, COLKET Iii M B, NELSON P F, ESLER M. Shock tube pyrolysis of pyrrole and kinetic modeling[J]. Int J Chem Kinet,1991,23(8):733−760. doi: 10.1002/kin.550230807 [38] NINOMIYA Y, DONG Z, SUZUKI Y, KOKETSU J. Theoretical study on the thermal decomposition of pyridine[J]. Fuel, 2000, 79(3/4): 449–457. [39] HAN X, CHEN B, LI Q, TONG J, JIANG X. Organic nitrogen conversion during the thermal decomposition of huadian oil shale of China[J]. Oil Shale, 2017, 34(2): 97–109. [40] LIU J, ZHANG X, HU B, LU Q, LIU D, DONG C, YANG Y. Formation mechanism of HCN and NH3 during indole pyrolysis: A theoretical DFT study[J]. J Energy Inst,2020,93(2):649−657. doi: 10.1016/j.joei.2019.05.015 [41] LIU J, ZHANG X, LU Q, SHAW A, HU B, JIANG X, DONG C. Mechanism study on the effect of alkali metal ions on the formation of HCN as NOx precursor during coal pyrolysis[J]. J Energy Inst,2019,92(3):604−612. doi: 10.1016/j.joei.2018.03.012 [42] LIU J, FAN X, ZHAO W, YANG S, XIE W, HU B, LU Q. A theoretical investigation on the thermal decomposition of pyridine and the effect of H2O on the formation of NOx precursors[J]. Front Chem Sci Eng,2021,15(5):1217−1228. doi: 10.1007/s11705-020-2024-8 [43] KHAVANI M, IZADYAR M, MEHRANFAR A. A DFT study on the kinetics and mechanism of cyclodiglycine thermal decomposition in the gas phase[J]. Prog React Kinet Mech,2016,41(2):205−213. doi: 10.3184/146867816X14651390195612 [44] ZHOU J, GAO P, DONG C, YANG Y. Effect of temperature and mineral matter on the formation of NOx precursors during fast pyrolysis of 2, 5-diketopiperazine[J]. Energies,2018,11(3):629. doi: 10.3390/en11030629 [45] HANSSON K-M, SAMUELSSON J, TULLIN C, AMAND L-E. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds[J]. Combust Flame,2004,137(3):265−277. doi: 10.1016/j.combustflame.2004.01.005 [46] LIU J, ZHAO W, YANG S, HU B, XU M, MA S, LU Q. Formation mechanism of NOx precursors during the pyrolysis of 2, 5-diketopiperazine based on experimental and theoretical study[J]. Sci Total Environ,2021,801:149663. doi: 10.1016/j.scitotenv.2021.149663 [47] WU L, TIAN Z, WANG D, ZHENG Z, JIN K, LIU B, WANG Z. Dinitriles and nitriles are common intermediates of pyrrole pyrolysis[J]. Combust Flame,2022,245:112358. doi: 10.1016/j.combustflame.2022.112358 [48] METCALFE E, BOOTH D, MCANDREW H, WOOLEY W D. The pyrolysis of organic nitriles[J]. Fire Mater,1983,7(4):185−192. doi: 10.1002/fam.810070406 [49] REN Q, ZHAO C, WU X, LIANG C, CHEN X, SHEN J, WANG Z. Formation of NOx precursors during wheat straw pyrolysis and gasification with O2 and CO2[J]. Fuel,2010,89(5):1064−1069. doi: 10.1016/j.fuel.2009.12.001 [50] VERAS C, SAASTAMOINEN J, De CARVALHO J. Effect of particle size and pressure on the conversion of fuel N to no in the boundary layer during devolatilization stage of combustion[C]//Twenty-Seventh Symposium (International) on Combustion. Pittsburgh: The Combustion Institute, 1998: 3019–3025. [51] TULLIN C J, GOEL S, MORIHARA A, SAROFIM A F, BEER J M. Nitrogen oxide (NO and N2O) formation for coal combustion in a fluidized bed: Effect of carbon conversion and bed temperature[J]. Energy Fuels,1993,7(6):796−802. doi: 10.1021/ef00042a015 [52] YUAN S, ZHOU Z, LI J, CHEN X, WANG F. HCN and NH3 released from biomass and soybean cake under rapid pyrolysis[J]. Energy fuels,2010,24(11):6166−6171. doi: 10.1021/ef100959g [53] RAVEENDRAN K, GANESH A, KHILAR K C. Influence of mineral matter on biomass pyrolysis characteristics[J]. Fuel,1995,74(12):1812−1822. doi: 10.1016/0016-2361(95)80013-8 [54] ENEJI A E, HONNAT, T, YAMAMOTO S, MASUDA T, ENDO T, IRSHAD M. The relationship between total and available heavy metals in composted manure[J]. J Sustain Agr,2003,23(1):125−134. doi: 10.1300/J064v23n01_09 [55] ZHANG Q, LIU H, LU G, YI L, HU H, CHI H, YAO H. Mechanism of conditioner CaO on NOx precursors evolution during sludge steam gasification[J]. Proc Combust Inst,2017,36(3):4003−4010. doi: 10.1016/j.proci.2016.09.006 [56] LIU H, YI L, HU H, XU K, ZHANG Q, LU G, YAO H. Emission control of NOx precursors during sewage sludge pyrolysis using an integrated pretreatment of Fenton peroxidation and CaO conditioning[J]. Fuel,2017,195:208−216. doi: 10.1016/j.fuel.2017.01.067 [57] LIN Y, WANG J, WANG H, GU M, ZHANG C, CHU H. Effects of Fe2O3 on pyrolysis characteristics of soybean protein and release of NOx precursors[J]. Energy Sources, Part A,2018,40(4):459−465. doi: 10.1080/15567036.2017.1423417 [58] YI L, LIU H, LU G, ZHANG Q, WANG J, HU H, YAO H. Effect of mixed Fe/Ca additives on nitrogen transformation during protein and amino acid pyrolysis[J]. Energy Fuels,2017,31(9):9484−9490. doi: 10.1021/acs.energyfuels.7b01413 [59] XU K, LI J, ZENG K, ZHONG D, PENG J, QIU Y, FLAMANT G, YANG H, CHEN H. The characteristics and evolution of nitrogen in bio-oil from microalgae pyrolysis in molten salt[J]. Fuel,2023,331:125903. doi: 10.1016/j.fuel.2022.125903 [60] XIAO K, GUAN R, YANG J, LI H, YU Z, LIANG S, YU W, HU J, HOU H, LIU B. Effects of red mud on emission control of NOx precursors during sludge pyrolysis: A protein model compound study[J]. Wastes Manage,2019,85:452−463. doi: 10.1016/j.wasman.2019.01.014 [61] TANG S, ZHENG C, YAN F, SHAO N, TANG Y, ZHANG Z. Product characteristics and kinetics of sewage sludge pyrolysis driven by alkaline earth metals[J]. Energy,2018,153:921−932. doi: 10.1016/j.energy.2018.04.108 [62] MEENAKSHISUNDARAM S, FAYEULLE A, LEONARD E, CEBALLOS C, PAUSS A. Fiber degradation and carbohydrate production by combined biological and chemical/physicochemical pretreatment methods of lignocellulosic biomass–A review[J]. Bioresour Technol,2021,331:125053. doi: 10.1016/j.biortech.2021.125053 [63] LI M, WU J, GUAN Z. Effect of physical osmosis methods on quality of tilapia fillets processed by heat pump drying[J]. Pol J Food Nutr Sci, 2017, 67 (2): 145–150. https://doi.org/10.1515/pjfns-2016-0016. [64] ABRAHAM A, MATHEW A K, PARK H, CHOI O, SINDHU R, PARAMESWARAN B, PANDEY A, PARK J, SANG B. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass[J]. Bioresour Technol,2020,301:122725. doi: 10.1016/j.biortech.2019.122725 [65] 胡秋龙, 熊兴耀, 谭琳, 苏小军, 贺应龙, 刘祥华, 易锦琼. 木质纤维素生物质预处理技术的研究进展[J]. 中国农学通报,2011,27(10):1−7.HU Qiu-long, XIONG Xing-yao, TAN Lin, SU Xiao-jun, HE Ying-long, LIU Xiang-hua, YI Jin-qiong. Advances in pretreatment technologies of lignocellulosic biomass[J]. Chin Agr Sci Bull,2011,27(10):1−7. [66] SHARMA H K, XU C, QIN W. Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview[J]. Waste Biomass Valorization,2019,10(2):235−251. doi: 10.1007/s12649-017-0059-y [67] KAYA H, TUFEKCI P, UZUN E. Predicting CO and NOx emissions from gas turbines: novel data and a benchmark PEMS[J]. Turk J Elec Eng Comp Sci,2019,27(6):4783−4796. [68] FAN T, WANG X, XU T, WANG C. A novel sludge Acidification combined Multistage Elutriation (AME) pretreatment strategy for sludge dewaterability improvement, inorganic components separation and heavy metals removal[J]. Resour, Conserv Recycl,2022,185:106498. doi: 10.1016/j.resconrec.2022.106498 [69] SHARMILA V G, KUMAR G, SIVASHANMUGHAM P, PIECHOTA G, PARK J, KUMAR S A, BANU J R. Phase separated pretreatment strategies for enhanced waste activated sludge disintegration in anaerobic digestion: An outlook and recent trends[J]. Bioresour Technol, 2022, 363: 127985. [70] HUANG Q, LIU Y, DHAR BIPRO R. A critical review of microbial electrolysis cells coupled with anaerobic digester for enhanced biomethane recovery from high-strength feedstocks[J]. Crit Rev Environ Sci Technol,2022,52(1):50−89. doi: 10.1080/10643389.2020.1813065 [71] SHAH A, SEEHAR T, SHARMA K, TOOR S. Biomass pretreatment technologies[M]//Hydrocarbon Biorefinery. Elsevier: Amsterdam, The Netherlands, 2022: 203–228. [72] LEE B, SH L, LEE D, JEON C. Effect of torrefaction and ashless process on combustion and NOx emission behaviors of woody and herbaceous biomass[J]. Biomass Bioenergy,2021,151:106133. doi: 10.1016/j.biombioe.2021.106133 [73] JONES JM, BRIDGEMAN TG, DARVELL L, GUDKA B, SADDAWI A, WIILIAMS A. Combustion properties of torrefied willow compared with bituminous coals[J]. Fuel Process Technol,2012,101:1−9. doi: 10.1016/j.fuproc.2012.03.010 [74] SCHMID D, KARLSTROM O, YRJAS P. Release of NH3, HCN and NO during devolatilization and combustion of washed and torrefied biomass[J]. Fuel,2020,280:118583. doi: 10.1016/j.fuel.2020.118583 [75] ZHUANG X, SONG Y, WANG X, ZHAN H, YIN X, WU C, WANG P. Pyrolysis of hydrothermally pretreated biowastes: The controllability on the formation of NOx precursors[J]. Chem Eng J,2020,393:124727. doi: 10.1016/j.cej.2020.124727 [76] ZHANG J, TIAN Y, ZHU J, ZUO W, YIN L. Characterization of nitrogen transformation during microwave-induced pyrolysis of sewage sludge[J]. J Anal Appl Pyrolysis,2014,105:335−341. doi: 10.1016/j.jaap.2013.11.021 [77] ZHAN H, ZHUANG X, SONG Y, HUANG Y, LIU H, YIN X, WU C. Evolution of nitrogen functionalities in relation to NOx precursors during low-temperature pyrolysis of biowastes[J]. Fuel,2018,218:325−334. doi: 10.1016/j.fuel.2018.01.049 [78] TIAN F, YU J, MCKENZIE L J, HAYASHI J, LI C. Conversion of fuel-N into HCN and NH3 during the pyrolysis and gasification in steam: a comparative study of coal and biomass[J]. Energy Fuels,2007,21(2):517−521. doi: 10.1021/ef060415r [79] CHEN H, SI Y, CHEN Y, YANG H, CHEN D, CHEN W. NOx precursors from biomass pyrolysis: Distribution of amino acids in biomass and Tar-N during devolatilization using model compounds[J]. Fuel,2017,187:367−375. doi: 10.1016/j.fuel.2016.09.075 [80] CHEN Y, ZHANG L, ZHANG Y, LI A. Pressurized pyrolysis of sewage sludge: Process performance and products characterization[J]. J Anal Appl Pyrolysis,2019,139:205−212. doi: 10.1016/j.jaap.2019.02.007 [81] KURKELA E, STAHLBERG P. Air gasification of peat, wood and brown coal in a pressurized fluidized-bed reactor. II. Formation of nitrogen compounds[J]. Fuel Process Technol,1992,31(1):23−32. doi: 10.1016/0378-3820(92)90039-S [82] HAMALAINEN J P, AHO MARTTI J. Conversion of fuel nitrogen through HCN and NH3 to nitrogen oxides at elevated pressure[J]. Fuel,1996,75(12):1377−1386. doi: 10.1016/0016-2361(96)00100-7 [83] CHEN Z, YUAN S, LIANG Q, WANG F, YU Z. Distribution of HCN, NH3, NO and N2 in an entrained flow gasifier[J]. Chem Eng J, 2009, 148(2–3): 312–318. [84] HAYNES B. Reactions of ammonia and nitric oxide in the burnt gases of fuel-rich hydrocarbon-air flames[J]. Combust Flame,1977,28:81−91. doi: 10.1016/0010-2180(77)90010-4 [85] SMITH P J, HILL S C, Smoot L D. Theory for NO formation in turbulent coal flames[C]// Nineteenth Symposium (International) on Combustion, Israel: Technion-Israel Intitute of Technology, 1982: 1263–1270. [86] CHANG L, XIE Z, XIE K, PRATT K C, HAYASHI J, CHIBA TADATOSHI, LI C. Formation of NOx precursors during the pyrolysis of coal and biomass. Part VI. Effects of gas atmosphere on the formation of NH3 and HCN[J]. Fuel,2003,82(10):1159−1166. doi: 10.1016/S0016-2361(03)00024-3 [87] DUAN L, ZHAO C, REN Q, WU Z, CHEN X. NOx precursors evolution during coal heating process in CO2 atmosphere[J]. Fuel,2011,90(4):1668−1673. doi: 10.1016/j.fuel.2010.12.014 -





 下载:
下载: