Study on the mechanism of CO2 adsorption by metal oxide coupled with pyrrole nitrogen biochar
-
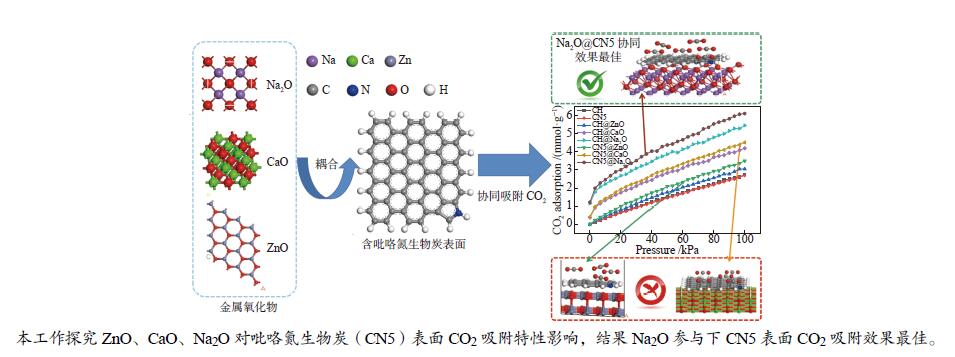
摘要: 本研究采用密度泛函理论,研究含吡咯氮生物质炭(CN5)及其耦合不同金属氧化物(ZnO、CaO、Na2O)对CO2吸附特性的影响机理。计算CO2在不同金属氧化物耦合含吡咯氮生物质炭(CN5@MOx∶CN5@ZnO、CaO、Na2O)上的吸附量,并结合吸附热对吸附量差异进行分析,发现CO2在CN5@Na2O表面发生多层吸附,相较于CN5@ZnO及CN5@CaO,CO2在CN5@Na2O上吸附热与吸附量均较高,100 kPa、20 ℃时达到6.11 mmol/g,相互作用更强,更有利于吸附。进一步考察了CN5@MOx吸附能,计算结果表明,CN5@Na2O对CO2吸附能高于CN5@CaO和CN5@ZnO,与吸附量一致。然后对其开展电荷差分密度及态密度分析,电荷差分密度表明CN5@Na2O吸附能高是由于Na2O中Na参与吸附,与CO2中O之间发生电荷转移,态密度分析结果表明CO2在CN5@Na2O表面吸附更稳定。Abstract: In this study, density functional theory was used to study the influence mechanism of pyrroleaze-containing biochar (CN5) and its coupling of different metal oxides (ZnO, CaO, Na2O) on the adsorption characteristics of CO2. Calculating the adsorption capacity of CO2 on different metal oxides coupled with pyrrole nitrogen-containing biochar (CN5@MOx∶CN5@ZnO, CN5@CaO, CN5@Na2O), and analyzing the difference in adsorption capacity combined with adsorption heat, it was found that CO2 multi-layer adsorption occurred on the CN5@Na2O surface, compared with CN5@ZnO and CN5@CaO, CO2 adsorption heat and adsorption capacity on CN5@Na2O were higher, reaching 6.11 mmol/g at 100 kPa and 20 ℃, with stronger interaction and more utilization of adsorption. The CN5@MOx adsorption energy was further investigated, and the calculation results showed that the CN5@Na2O adsorption energy for CO2 was higher than that of CN5@CaO and CN5@ZnO, which was consistent with the adsorption capacity. The charge differential density and state density analysis were carried out, and the charge differential density showed that due to the participation of Na in Na2O in adsorption, charge transfer occurred between O in CO2, and the state density analysis showed that CO2 was more stable adsorption on the CN5@Na2O surface.
-
Key words:
- biochar /
- pyrrole-containing groups /
- metal oxides /
- CO2 /
- adsorption characteristics
-
图 12 (a)、(c)、(e)分别为CO2在不同生物质炭上吸附电荷差分密度图(蓝色、黄色区域分别为得到和失去电子);(b)、(d)、(f)分别为其剖面图(蓝色、红色区域分别为得到和失去电子)
Figure 12 (a), (c) and (e) were the differential density maps of CO2 adsorption charges on different biochars (the blue and yellow regions gained and lost electrons, respectively); (b), (d), (f) are their profiles (blue and red areas gain and loss of electrons, respectively)
表 1 CH、CN5构型比表面积和孔容
Table 1 CH, CN5 configuration specific surface area and pore volume
Sample BET surface
area/(m2·g−1)Total pore
volume/(cm3·g−1)CH 1556.34 0.69 CN5 1803.37 0.70 表 2 CN5与ZnO不同耦合位点优化平衡能量
Table 2 CN5 and ZnO have different coupling sites to optimize the balance energy
Coupling site Balance energy /eV Top −74207.0723 Bridge −74207.1353 Hollow −74207.2676 表 3 CN5与ZnO不同耦合位点对CO2吸附能
Table 3 CN5 and ZnO have different coupling sites for CO2 adsorption energy
Coupling site Adsorption
energy /eVAdsorption energy /
(kJ·mol−1)Top −1.23 −119.05 Bridge −1.20 −116.19 Hollow −1.15 −111.05 表 4 100 kPa时不同温度下CO2在生物质炭上吸附量
Table 4 At 100 kPa, the amount of CO2 adsorbed on biochar at different temperature strips
Structure Uptake /(mmol·g−1) 0 ℃ 20 ℃ 50 ℃ CH 4.41 2.73 1.46 CN5 4.45 2.69 1.42 CH@ZnO 4.95 3.05 1.62 CN5@ZnO 5.30 3.51 1.88 CH@CaO 6.17 4.19 2.64 CN5@CaO 6.61 4.51 2.82 CH@Na2O 7.68 5.45 3.62 CN5@Na2O 8.86 6.11 4.10 表 5 20 ℃下各吸附模型拟合常数
Table 5 Fitting constants for each adsorption model at 20 ℃
Structure Langmuir Freundlich Qm AL R2 AF n R2 CH 12.877 0.003 0.999 0.050 1.148 0.999 CN5 15.709 0.002 0.999 0.043 1.116 0.999 CH@ZnO 11.132 0.004 0.999 0.070 1.211 0.999 CN5@ZnO 9.713 0.005 0.998 0.099 1.293 0.999 CH@CaO 6.057 0.020 0.962 0.381 1.928 0.993 CN5@CaO 6.345 0.020 0.963 0.414 1.947 0.994 CH@Na2O 6.429 0.037 0.876 0.870 2.557 0.966 CN5@Na2O 7.252 0.036 0.889 0.956 2.524 0.972 表 6 吸附能计算
Table 6 Result of the adsorption energy calculation
Structure Epro /eV Eslab /eV Eadsorbate /eV Eads /eV Eads /(kJ·mol−1) CN5@ZnO −74207.29 −75241.52 −1032.98 −1.25 −120.88 CN5@CaO −55031.81 −56066.17 −1032.98 −1.38 −133.04 CN5@Na2O −75948.70 −76983.19 −1032.98 −1.51 −145.86 -
[1] bp Statistical Review of World Energy. 2022: 39. [2] bp Statistical Review of World Energy. 2022: 51. [3] 赵震宇, 姚舜, 杨朔鹏, 王小龙. “双碳”目标下: 中国CCUS发展现状、存在问题及建议[J]. 环境科学, 2022, 44(2): 1–15.ZHAO Zhen-yu, YAO Shun, YANG Shuo-peng, WANG Xiao-long. Under the "dual carbon" goal: the development status, existing problems and suggestions of CCUS in China[J]. Environ Sci, 2022, 44(2): 1–15. [4] KOU J, SUN L. Nitrogen-doped porous carbons derived from carbonization of a nitrogen-containing polymer: Efficient adsorbents for selective CO2 capture[J]. Ind Eng Chem Res, 2016, 55(41): 10916–10925. [5] JIANG Q, RENTSCHLER J, SETHIA G. Synthesis of T-type zeolite nanoparticles for the separation of CO2/N2 and CO2/CH4 by adsorption process[J]. Chem Eng J,2013,230(16):380−388. [6] SUMIDA K, ROGOW D, MASON J, MCDONALD T, BLOCH E, HERM Z, BAE T, LONG J. Carbon dioxide capture by metal organic frameworks[J]. Indian J Chem,2012,51(9):1223−1230. [7] SUN L, KANG Y, SHI Y. Highly selective capture of the greenhouse gas CO2 in polymers[J]. ACS Sustainable Chem Eng,2015,3(12):3077−3085. doi: 10.1021/acssuschemeng.5b00544 [8] XIN H, RADOSZ M, CYCHOSZ K, THOMMES M. CO2-filling capacity and selectivity of carbon nanopores: Synthesis, texture, and pore-size distribution from quenched-solid density functional theory (QSDFT)[J]. Environ Sci Technol,2011,45(16):7068−7074. doi: 10.1021/es200782s [9] JIMENEZ V, RAMIREZ-LUCAS A, DÍAZ J-A, SANCHEZ P, ROMERO A. CO2 capture in different carbon materials[J]. Environ Sci Technol,2012,46(13):7407−7414. doi: 10.1021/es2046553 [10] ZHANG XU, GAO B, CREAMER A, CAO C, LI Y. Adsorption of VOCs onto engineered carbon materials: A review[J]. J Hazard Mater,2017,338:102−123. doi: 10.1016/j.jhazmat.2017.05.013 [11] QI J, LI Y, WEI G, LI J, SUN X, SHEN J, HAN W, WANG L. Nitrogen doped porous hollow carbon spheres for enhanced benzene removal[J]. Sep Purif Technol,2017,188:112−118. doi: 10.1016/j.seppur.2017.07.021 [12] KIM K-J, KANG C-S, YOU Y-J, CHUNG M-C, WOO M-W. Adsorption-desorption characteristics of VOCs over impregnated activated carbons[J]. Catal Today,2006,111:223−228. doi: 10.1016/j.cattod.2005.10.030 [13] ZHOU K, MA W, ZENG Z, CHEN R, LI L. Waste biomass-derived oxygen and nitrogen co-doped porous carbon/MgO composites as superior acetone adsorbent: Experimental and DFT study on the adsorption behavior[J]. Chem Eng J,2020,387:124173. doi: 10.1016/j.cej.2020.124173 [14] XING W, LIU C, ZHOU Z, ZHANG L, ZHOU J, ZHUO S, YAN Z, GAO H, WANG G, QIAO S. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction[J]. Energy Environ Sci,2012,5(6):7323−7327. doi: 10.1039/c2ee21653a [15] MA X, CAO M, HU C. Bifunctional HNO3 catalytic synthesis of N-doped porous carbons for CO2 capture[J]. J Mater Chem A,2012,1(3):913−918. [16] YUE L, XIA Q, WANG L. CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell[J]. J Colloid Interf Sci,2018,511:259−267. doi: 10.1016/j.jcis.2017.09.040 [17] SETHIA G, SAYARI A. Comprehensive study of ultra-microporous nitrogen-doped activated carbon for CO2 capture[J]. Carbon,2015,93:68−80. doi: 10.1016/j.carbon.2015.05.017 [18] WANG Y, XU X, HAO J, MA R, BAI H. Nitrogen and oxygen codoped porous carbon with superior CO2 adsorption performance: A combined experimental and DFT calculation study[J]. Ind Eng Chem Res,2019,58(29):13390−13400. doi: 10.1021/acs.iecr.9b01454 [19] 陈伟. 生物质富氮热解过程中氮的迁移转化及含氮目标产物调控研究[D]. 武汉: 华中科技大学, 2018.CHEN Wei. Research on nitrogen migration and transformation and regulation of nitrogen-containing target products during nitrogen-rich pyrolysis of biomass[D]. Wuhan: Huazhong University of Science and Technology, 2018. [20] XU L, GUO L, HU G, CHEN J, HU X, WANG S, DAI W, FAN M. Nitrogen-doped porous carbon spheres derived from D-glucose as highly-efficient CO2 sorbents[J]. RSC Adv, 2015, 5(48): 37964−37969. [21] SANCHEZ Á, SUAREZ-GARCIA F, MARTINEZ-ALONSO A, TASCON J-M-D. Influence of porous texture and surface chemistry on the CO adsorption capacity of porous carbons: acidic and basic site interactions.[J]. ACS Appl Mater Inter,2014,6(23):21237. doi: 10.1021/am506176e [22] NG S-W-L, YILMAZ G, ONG W-L, HO G-W. One-step activation towards spontaneous etching of hollow and hierarchical porous carbon nanospheres for enhanced pollutant adsorption and energy storage[J]. Appl Catal B: Environ,2018,220:533−541. doi: 10.1016/j.apcatb.2017.08.069 [23] ZENG S, YAO Y, HUANG L, WU H, PENG B, ZHANG Q, LI X, YU L, LIU S, TU W. Facile synthesis of ultrahigh-surface-area hollow carbon nanospheres and theirapplication in lithium-sulfur batteries[J]. Chem Eur J,2018,24:1988−1997. doi: 10.1002/chem.201705211 [24] LI P, LIU W, DENNIS J-S, ZENG H. Synthetic architecture of MgO/C nanocomposite from hierarchical-structured coordination polymer toward enhanced CO2 capture[J]. ACS Appl Mater Inter,2017,9:9592−9602. doi: 10.1021/acsami.6b14960 [25] SHEN F, LIU J, WU D, DONG Y, HUANG H. Design of O2/SO2 dual-doped porous carbon as superior sorbent for elemental mercury removal from flue gas[J]. J Hazard Mater,2019,366:321−328. doi: 10.1016/j.jhazmat.2018.12.007 [26] WANG L, RAO L, XIA B, LIN L, YUE, LI M, LIANG. Highly efficient CO2 adsorption by nitrogen-doped porous carbons synthesized with lowtemperature sodium amide activation[J]. Carbon,2018,130:31−40. doi: 10.1016/j.carbon.2018.01.003 [27] MA X, LI L, CHEN R, WANG C, ZHOU K, LI H. Doping of alkali metals in carbon frameworks for enhancing CO2 capture: a theoretical study[J]. Fuel,2019,236:942−948. doi: 10.1016/j.fuel.2018.08.166 [28] ZHAO B, WANG J, ZHU D, SONG G, XIE X. Adsorption characteristics of gas molecules (H2O, CO2, CO, CH4, and H2) on CaO-based catalysts during biomass thermal conversion with in situ CO2 capture[J]. Catalysts,2019,9(9):757. doi: 10.3390/catal9090757 [29] REDDY E P, SMIMIOTIS P G. High-temperature sorbents for CO2 made of alkali metals doped on CaO supports[J]. J Phys Chem B,2004,108(23):7794−7800. doi: 10.1021/jp031245b [30] WANG W, FAN L, WANG G, LI Y. CO2 and SO2 sorption on the alkali metals doped CaO (100) surface: A DFT-D study[J]. Appl Surf Sci,2017,425:972−977. doi: 10.1016/j.apsusc.2017.07.158 [31] LAHIJANI P, MOHAMMADI M, MOHAMED A R. Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition[J]. J CO2 Util,2018,26:281−293. doi: 10.1016/j.jcou.2018.05.018 [32] YU F, TIAN F, ZOU H, YE Z, PENG C, HUANG J, ZHENG Y, ZHANG Y, YANG Y, WEI X, GAO B. ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue[J]. J Hazard Mater,2021,415:125511. doi: 10.1016/j.jhazmat.2021.125511 [33] LI R, WANG J. , GASTON L, ZHOU B, LI M, XIAO R, WANG Q, ZHANG Z, HUANG H, LIANG W, HUANG H, ZHANG X. An overview of carbothermal synthesis of metal-biochar composites for the removal of oxyanion contaminants from aqueous solution[J]. Carbon,2018,129:674−687. doi: 10.1016/j.carbon.2017.12.070 [34] ZHOU S, GUO C, WU Z, WANG M, WANG Z, WEI S, LI S, LU X. Edge-functionalized nanoporous carbons for high adsorption capacity and selectivity of CO2 over N2[J]. Appl Surf Sci,2017,410:259−266. doi: 10.1016/j.apsusc.2017.03.136 [35] CAI T, CHEN X, TANG H, ZHOU W, WU Y, ZHAO C. Unraveling the disparity of CO2 sorption on alkali carbonates under high humidity[J]. J CO2 Util,2021,53:101737. [36] MA X, LI Y, ZHANG W, WANG Z, ZHAO J. DFT study of CO2 adsorption across a CaO/Ca12Al14O33 sorbent in the presence of H2O under calcium looping conditions[J]. Chem Eng J,2019,370:10−18. doi: 10.1016/j.cej.2019.03.176 [37] FAN Y, ZHUO Y, ZHU Z, WEN D, LI L. Zerovalent selenium adsorption mechanisms on CaO surface: DFT calculation and experimental study[J]. J Phys Chem A,2017,121(39):7385−7392. doi: 10.1021/acs.jpca.7b04672 [38] KUMAR K V, PREUSS K, LU L, GUO Z, TITIRICI M M. Effect of nitrogen doping on the CO2 adsorption behavior in nanoporous carbon structures: A molecular simulation study[J]. J Phys Chem C,2015,119(39):22310−22321. doi: 10.1021/acs.jpcc.5b06017 [39] ZHOU X, YI H, TANG X, DENG H, LIU H. Thermodynamics for the adsorption of SO2, NO and CO2 from flue gas on activated carbon fiber[J]. Chem Eng J,2012,200:399−404. [40] PERRY S T, HAMBLY E M, FLETCHER T H, SOLUM M S, PUGMIRE R J. Solid-state 13C NMR characterization of matched tars and chars from rapid coal devolatilization[J]. Proc Combust Inst,2000,28(2):2313−2319. doi: 10.1016/S0082-0784(00)80642-6 [41] ZHANG X, XIE M, WU H, LV X, ZHOU Z. DFT study of the effect of Ca on NO heterogeneous reduction by char[J]. Fuel,2020,265:116995. doi: 10.1016/j.fuel.2019.116995 [42] ZHANG X, LÜ X, WU H, MIAO X, LIN R, ZHOU Z. Microscopic mechanism for effect of sodium on NO heterogeneous reduction by char[J]. J Fuel Chem Technol,2020,48(6):663−673. doi: 10.1016/S1872-5813(20)30050-5 [43] ZHANG H, JIANG X, LIU J, SHEN J. Application of density functional theory to the nitric oxide heterogeneous reduction mechanism in the presence of hydroxyl and carbonyl groups[J]. Energy Convers Manage,2014,83:167−176. doi: 10.1016/j.enconman.2014.03.067 [44] FENG K, HU Y, CAO T. Mechanism of fuel gas denitration on the KOH-activated biochar surface[J]. J Phys Chem A,2022,126(2):296−305. doi: 10.1021/acs.jpca.1c09518 [45] LI Q, LIU S, PENG W, ZHU W, WANG L, CHEN F, SHAO J, HU X. Preparation of biomass-derived porous carbons by a facile method and application to CO2 adsorption[J]. J Taiwan Inst Chem E,2020,116:128−136. doi: 10.1016/j.jtice.2020.11.001 [46] FAN Y, ZHUO Y, LOU Y, ZHU Z, LI L. SeO2 adsorption on CaO surface: DFT study on the adsorption of a single SeO2 molecule[J]. Appl Surf Sci,2017,413:366−371. doi: 10.1016/j.apsusc.2017.03.196 [47] NORSKOV J K, STUDT F, ABILD-PEDERSEN F, BLIGAARD T, FUNDAMENTAL T. Poisoning and promotion of catalysts[C]//Fundamental Concepts in Heterogeneous Catalysis. Hoboken: Wiley, 2014: 150–154. -





 下载:
下载: