Construction of core-shell MOFs@ionic liquid materials and their performance for CO2 cycloaddition reaction under atmospheric pressure
-
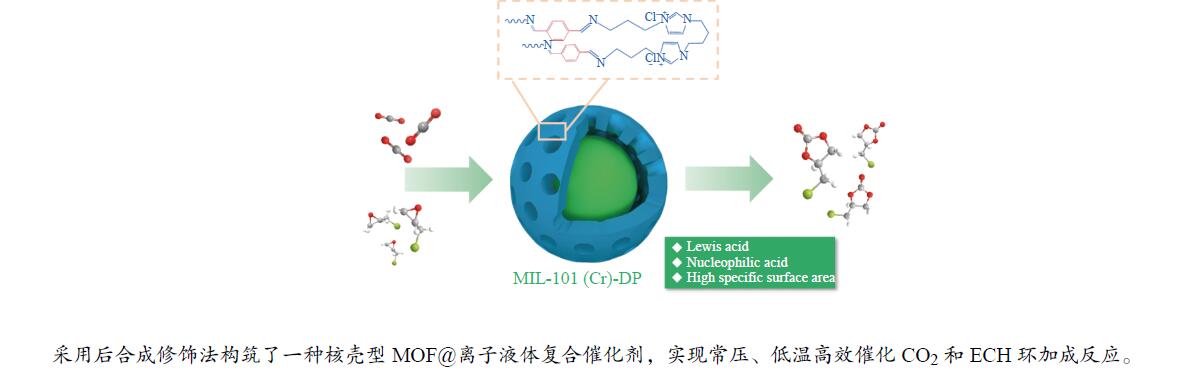
摘要: 通过双氨基功能化离子液体与对苯二甲醛原位共价组装得到柔性聚合物(DP),并采用后合成修饰法将DP包覆于金属有机框架材料MIL-101(Cr)表面,构筑了一种核-壳型复合材料(MIL-101@DP)用于催化CO2和环氧氯丙烷(ECH)环加成反应。MIL-101@DP保留了MIL-101(Cr)高比表面积和高孔隙率的优点,并兼具亲核位点Cl−与Lewis酸性位点Cr3 + 。在Lewis酸碱位点协同作用下,MIL-101@DP可在常压、80 ℃、24 h且无助催化剂的条件下高效催化转化CO2和ECH反应(ECH转化率可达99%),且在循环使用四次后活性未出现明显下降。
-
关键词:
- MOFs@ILs复合材料 /
- 核-壳结构 /
- CO2 /
- 环加成反应 /
- 环氧氯丙烷
Abstract: A flexible polymer (DP) was prepared by in-situ covalent assembly of dual-amino-functionalized ionic liquids and terephthalaldehyde. A core-shell composite (MIL-101@DP) was constructed by coating DP on the surface of metal-organic frame material MIL-101 (Cr) by post-synthesis modification, and was applied to catalyze the cycloaddition reaction of CO2 and epichlorohydrin (ECH). MIL-101@DP retains the advantage of high specific surface area and high porosity of MIL-101 (Cr), and combines the nucleophilic site Cl− and Lewis acidic site Cr3 + . Under the synergistic interaction of Lewis acid sites and Lewis base sites, MIL-101@DP could efficiently catalyze activity the conversion of CO2 and ECH reaction (ECH conversion rate can reach 99%) at atmospheric pressure, 80 ℃, 24 h and without cocatalyst. The activity did not decrease significantly after four cycles.-
Key words:
- MOFs@ILs composites /
- core-shell structure /
- CO2 /
- cycloaddition reaction /
- epichlorohydrin
-
表 1 MIL-101、MIL-101-DAIL、MIL-101@DP-X的比表面积和孔容
Table 1 Specific surface areas and pore volumes of MIL-101, MIL-101-DAIL and MIL-101@DP-X
SBET /(m2·g−1) vtotal /(cm3·g−1) MIL-101 1766 1.07 MIL-101-DAIL 968 0.67 MIL-101@DP-1 1618 1.03 MIL-101@DP-2 1652 1.02 表 2 MIL-101@DP-2催化CO2与各种环氧化合物合成碳酸盐
Table 2 Synthetic carbonates from various epoxides catalyzed by MIL-101@DP-2
Entry Substrate Product Temperature /℃ p /MPa Conversion /% 1 

80 0.1 99 2 

80 0.1 99 3 

80 0.1 59 4 

80 0.1 29 -
[1] KURUPPATHPARAMBIL R R, JOSE T, BABU R, HWANG G Y, KATHALIKKATTIL A C, KIM D W, PARK D W. A room temperature synthesizable and environmental friendly heterogeneous ZIF-67 catalyst for the solvent less and co-catalyst free synthesis of cyclic carbonates[J]. Appl Catal B: Environ,2016,182:562−569. doi: 10.1016/j.apcatb.2015.10.005 [2] ]WU Y, SONG X, XU S, CHEN Y, ODERINDE O, GAO L, WEI R, XIAO G. Chemical fixation of CO2 into cyclic carbonates catalyzed by bimetal mixed MOFs: the role of the interaction between Co and Zn[J]. Dalton Trans,2020,49(2):312−321. doi: 10.1039/C9DT04027G [3] LIU M, WANG X, JIANG Y, SUN J, ARAI M. Hydrogen bond activation strategy for cyclic carbonates synthesis from epoxides and CO2: Current state-of-the art of catalyst development and reaction analysis[J]. Catal Rev,2018,61(2):214−269. [4] ZHANG Z, FAN F, XING H, YANG Q, BAO Z, REN Q. Efficient synthesis of cyclic carbonates from atmospheric CO2 using a positive charge delocalized ionic liquid catalyst[J]. ACS Sustainable Chem Eng,2017,5(4):2841−2846. doi: 10.1021/acssuschemeng.7b00513 [5] HAZRA CHOWDHURY I, HAZRA CHOWDHURY A, SARKAR P, ISLAM S M. Chemical fixation of carbon dioxide by heterogeneous porous catalysts[J]. ChemNanoMat,2021,7(6):580−591. doi: 10.1002/cnma.202100074 [6] BAO C, JIANG Y, ZHAO L, LI D, XU P, SUN J. Aminoethylimidazole ionic liquid-grafted MIL-101-NH2 heterogeneous catalyst for the conversion of CO2 and epoxide without solvent and cocatalyst[J]. New J Chem,2021,45(31):13893−13901. doi: 10.1039/D1NJ02590B [7] LI Z J, SUN J F, XU Q Q, YIN J Z. Homogeneous and heterogeneous ionic liquid system: Promising “ideal catalysts” for the fixation of CO2 into cyclic carbonates[J]. ChemCatChem,2021,13(8):1848−1866. doi: 10.1002/cctc.202001572 [8] SINGH DHANKHAR S, UGALE B, NAGARAJA C M. Co-catalyst-free chemical fixation of CO2 into cyclic carbonates by using metal-organic frameworks as efficient heterogeneous catalysts[J]. Chem Asian J,2020,15(16):2403−2427. doi: 10.1002/asia.202000424 [9] SUN Y, JIA X, HUANG H, GUO X, QIAO Z, ZHONG C. Solvent-free mechanochemical route for the construction of ionic liquid and mixed-metal MOF composites for synergistic CO2 fixation[J]. J Mater Chem A,2020,8(6):3180−3185. doi: 10.1039/C9TA10409G [10] BAHADORI M, MARANDI A, TANGESTANINEJAD S, MOGHADAM M, MIRKHANI V, MOHAMMADPOOR BALTORK I. Ionic liquid-decorated MIL-101(Cr) via covalent and coordination bonds for efficient solvent-free CO2 conversion and CO2 capture at low pressure[J]. J Phys Chem C,2020,124(16):8716−8725. doi: 10.1021/acs.jpcc.9b11668 [11] XIANG W, SHEN C, LU Z, CHEN S, LI X, ZOU R, ZHANG Y, LIU C J. CO2 cycloaddition over ionic liquid immobilized hybrid zeolitic imidazolate frameworks: Effect of Lewis acid/base sites[J]. Chem Eng Sc,2021,233:116429. doi: 10.1016/j.ces.2020.116429 [12] QU Y, ZHAO Y, LI D, SUN J. Task-specific ionic liquids for carbon dioxide absorption and conversion into value-added products[J]. Curr Opin Green Sustain Chem,2022,34:100599. doi: 10.1016/j.cogsc.2022.100599 [13] LIU D, LI G, LIU H. Functionalized MIL-101 with imidazolium-based ionic liquids for the cycloaddition of CO2 and epoxides under mild condition[J]. Appl Surf Sc,2018,428:218−225. doi: 10.1016/j.apsusc.2017.09.040 [14] LONG J, DAI W, ZOU M, LI B, ZHANG S, YANG L, MAO J, MAO P, LUO S, LUO X. Chemical conversion of CO2 into cyclic carbonates using a versatile and efficient all-in-one catalyst integrated with DABCO ionic liquid and MIL-101 (Cr)[J]. Microporous Mesoporous Mater,2021,318:111027. doi: 10.1016/j.micromeso.2021.111027 [15] DAS S, PEREZ RAMIREZ J, GONG J, DEWANGAN N, HIDAJAT K, GATES B C, KAWI S. Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2[J]. Chem Soc Rev,2020,49(10):2937−3004. doi: 10.1039/C9CS00713J [16] ZHANG Y, LIU L, XU W G, HAN Z B. MOF@POP core-shell architecture as synergetic catalyst for high-efficient CO2 fixation without cocatalyst under mild conditions[J]. J CO2 Util,2021,46:101463. doi: 10.1016/j.jcou.2021.101463 [17] TSAI C Y, CHEN Y H, LEE S, LIN C H, CHANG C H, DAI W T, LIU W L. Uniform core-shell microspheres of SiO2@MOF for CO2 cycloaddition reactions[J]. Inorg Chem,2022,61(6):2724−2732. doi: 10.1021/acs.inorgchem.1c01570 [18] ZHAO M, BAN Y, YANG W. Assembly of ionic liquid molecule layers on metal-organic framework-808 for CO2 capture[J]. Chem Eng J,2022,439:135650. doi: 10.1016/j.cej.2022.135650 [19] SHI S, WU Y, ZHANG M, ZHANG Z, ODERINDE O, GAO L, XIAO G. Direct conversion of cellulose to levulinic acid using SO3H-functionalized ionic liquids containing halogen-anions[J]. J Mol Liq,2021,339:117278. doi: 10.1016/j.molliq.2021.117278 [20] GUO Z, CAI X, XIE J, WANG X, ZHOU Y, WANG J. Hydroxyl-exchanged nanoporous ionic copolymer toward low-temperature cycloaddition of atmospheric carbon dioxide into carbonates[J]. ACS Appl Mater Interfaces,2016,8(20):12812−12821. doi: 10.1021/acsami.6b02461 [21] HONG D Y, HWANG Y K, SERRE C, FéREY G, CHANG J S. Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis[J]. Adv Funct Mater,2009,19(10):1537−1552. doi: 10.1002/adfm.200801130 [22] OLANIYAN B, SAHA B. Comparison of catalytic activity of ZIF-8 and Zr/ZIF-8 for greener synthesis of chloromethyl ethylene carbonate by CO2 utilization[J]. Energies,2020,13(3):521. doi: 10.3390/en13030521 [23] NGUYEN Q T, DO X H, CHO K Y, LEE Y R, BAEK K Y. Amine-functionalized bimetallic Co/Zn-zeolitic imidazolate frameworks as an efficient catalyst for the CO2 cycloaddition to epoxides under mild conditions[J]. J CO2 Util,2022,61:102061. doi: 10.1016/j.jcou.2022.102061 [24] CHEN Y, ZHANG C, XIE J, LI H, DAI W, DENG Q, WANG S. Covalent organic frameworks as a sensing platform for water in organic solvent over a broad concentration range[J]. Anal Chem Acta,2020,1109:114−121. doi: 10.1016/j.aca.2020.03.003 [25] JIANG Y, WANG Z, XU P, SUN J. Dicationic ionic liquid @MIL-101 for the cycloaddition of CO2 and epoxides under cocatalyst-free conditions[J]. Cryst Growth Des,2021,21(7):3689−3698. doi: 10.1021/acs.cgd.0c01666 [26] RASTKARI N, AKBARI S, BRAHMAND M B, TAKHVAR A, AHMADKHANIHA R. Synthesis and characterization of tetraethylene pentamine functionalized MIL-101 (Cr) for removal of metals from water[J]. J Environ Health Sci Eng,2021,19(2):1735−1742. doi: 10.1007/s40201-021-00728-4 [27] WANG Y, ZHANG Y, JIANG Z, JIANG G, ZHAO Z, WU Q, LIU Y, XU Q, DUAN A, XU C. Controlled fabrication and enhanced visible–light photocatalytic hydrogen production of Au@CdS/MIL-101 heterostructure[J]. Appl Catal B: Environ,2016,185:307−314. doi: 10.1016/j.apcatb.2015.12.020 [28] THOMMES M, KANEKO K, NEIMARK A V, OLIVIER J P, RODRIGUEZ-REINOSO F, ROUQUEROL J, SING K S W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report) [J]. Pure Appl Chem, 2015, 87(9/10): 1051–1069. [29] ZIMPEL A, PREIß T, RÖDER R, ENGELKE H, INGRISCH M, PELLER M, RÄDLER J O, WAGNER E, BEIN T, LÄCHELT U, WUTTKE S. Imparting functionality to MOF nanoparticles by external surface selective covalent attachment of polymers[J]. Chem Mater,2016,28(10):3318−3326. doi: 10.1021/acs.chemmater.6b00180 [30] VYAS V S, VISHWAKARMA M, MOUDRAKOVSKI I, HAASE F, SAVASCI G, OCHSENFELD C, SPATZ J P, LOTSCH B V. Exploiting noncovalent interactions in an imine-based covalent organic framework for quercetin delivery[J]. Adv Mater,2016,28(39):8749−8754. doi: 10.1002/adma.201603006 [31] SOGUKOMEROGULLARI H G, ZIREK A G, AYTAR E, KöSE M, SöNMEZ M. Pd, Ni, Fe and Cu complexes containing a novel SNS-pincer ligand bearing pyridine: Synthesis and catalytic application to form cyclic carbonates[J]. J Mol Struct,2022,1263:133074. doi: 10.1016/j.molstruc.2022.133074 [32] BHIN K M, THARUN J, ROSHAN K R, KIM D W, CHUNG Y, PARK D W. Catalytic performance of zeolitic imidazolate framework ZIF-95 for the solventless synthesis of cyclic carbonates from CO2 and epoxides[J]. J CO2 Util,2017,17:112−118. doi: 10.1016/j.jcou.2016.12.001 [33] FERREIRA A, FERREIRA C, TEIXEIRA J A, ROCHA F. Temperature and solid properties effects on gas–liquid mass transfer[J]. Chem. Eng. J,2010,162(2):743−752. doi: 10.1016/j.cej.2010.05.064 [34] LIU C, LIU X H, LI B, ZHANG L, MA J G, CHENG P. Salen-Cu(II)@MIL-101 (Cr) as an efficient heterogeneous catalyst for cycloaddition of CO2 to epoxides under mild conditions[J]. J Energy Chem,2017,26(5):821−824. doi: 10.1016/j.jechem.2017.07.022 [35] ZALOMAEVA O V, CHIBIRYAEV A M, KOVALENKO K A, KHOLDEEVA O A, BALZHINIMAEV B S, FEDIN V P. Cyclic carbonates synthesis from epoxides and CO2 over metal-organic framework Cr-MIL-101[J]. Chin J Catal,2013,298:179−185. doi: 10.1016/j.jcat.2012.11.029 [36] CHEN Y, CHEN C, LI X, FENG N, WANG L, WAN H, GUAN G. Hydroxyl-ionic liquid functionalized metalloporphyrin as an efficient heterogeneous catalyst for cooperative cycloaddition of CO2 with epoxides[J]. J CO2 Util,2022,62:102107. doi: 10.1016/j.jcou.2022.102107 [37] CHEN Y, XU P, ARAI M, SUN J. Cycloaddition of carbon dioxide to epoxides for the synthesis of cyclic carbonates with a mixed catalyst of layered double hydroxide and tetrabutylammonium bromide at ambient temperature[J]. Adv Synth Catal,2019,361(2):335−344. doi: 10.1002/adsc.201801223 [38] JIANG Y, ZHAO Y, LIANG L, ZHANG X, SUN J. Imidazolium-based poly (ionic liquid) s@MIL-101 for CO2 adsorption and subsequent catalytic cycloaddition without additional cocatalyst and solvent[J]. New J Chem,2022,46(5):2309−2319. doi: 10.1039/D1NJ05358B -





 下载:
下载: