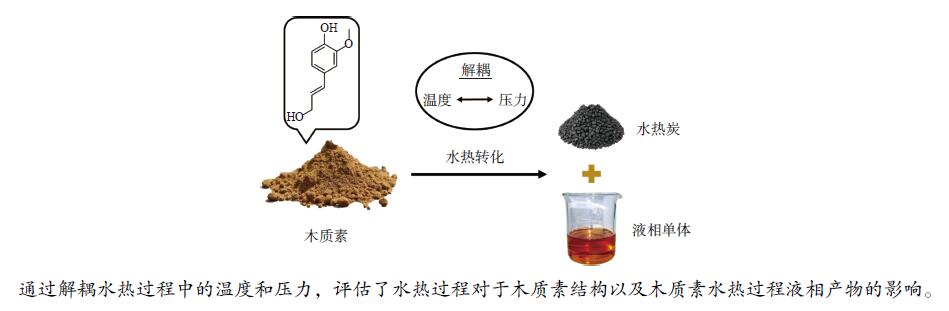
| [1] |
刘宁, 史成香, 潘伦, 张香文, 邹吉军. 生物质替代石油原料合成高密度燃料的研究进展[J]. 燃料化学学报,2021,49(12):1780−1790.LIU Ning, SHI Cheng-xiang, PAN Lun, ZHANG Xiang-wen, ZOU Ji-jun. Progress on using biomass derivatives to replace petroleum for synthesis of high-density fuels[J]. J Fuel Chem Technol,2021,49(12):1780−1790.
|
| [2] |
HICKEL J, KALLIS G. Is green growth possible?[J]. New Polit Econ,2020,25(4):469−486. doi: 10.1080/13563467.2019.1598964
|
| [3] |
ZHANG D H, WANG J Q, LIN Y G, SI Y L, HUANG C, YANG J, HUANG B, LI W. Present situation and future prospect of renewable energy in China[J]. Renewable Sustainable Energ Rev,2017,76:865−871. doi: 10.1016/j.rser.2017.03.023
|
| [4] |
PRASAD S, KUMAR A, MURALIKRISHNA K S. Biofuels production: A sustainable solution to combat climate change[J]. Indian J Agric Sci,2014,84(12):1443−1452.
|
| [5] |
YU S, YANG X, LI Q, ZHANG Y, ZHOU H. Breaking the temperature limit of hydrothermal carbonization of lignocellulosic biomass by decoupling temperature and pressure[J]. Green Energy Environ,2023,8(4):1216−1227.
|
| [6] |
TURSI A. A review on biomass: importance, chemistry, classification, and conversion[J]. Biofuel Res J,2019,6(2):962−979. doi: 10.18331/BRJ2019.6.2.3
|
| [7] |
YU S, YANG X, ZHAO P, LI Q, ZHOU H, ZHANG Y. From biomass to hydrochar: Evolution on elemental composition, morphology, and chemical structure[J]. J Energy Inst,2022,101:194−200. doi: 10.1016/j.joei.2022.01.013
|
| [8] |
LIAO Y H, KOELEWIJN S F, VAN DEN BOSSCHE G, VAN AELST J, VAN DEN BOSCH S, RENDERS T, NAVARE K, NICOLAI T, VAN AELST K, MAESEN M, MATSUSHIMA H, THEVELEIN J M, VAN ACKER K, LAGRAIN B, VERBOEKEND D, SELS B F. A sustainable wood biorefinery for low-carbon footprint chemicals production[J]. Science,2020,367(6484):1385−1390. doi: 10.1126/science.aau1567
|
| [9] |
XU J Y, LI C Y, DAI L, XU C L, ZHONG Y D, YU F X, SI C L. Biomass fractionation and lignin fractionation towards lignin valorization[J]. ChemSusChem,2020,13(17):4284−4295. doi: 10.1002/cssc.202001491
|
| [10] |
YU S, WANG L, LI Q, ZHANG Y, ZHOU H. Sustainable carbon materials from the pyrolysis of lignocellulosic biomass[J]. Mater Today Sustainability,2022,19:100209. doi: 10.1016/j.mtsust.2022.100209
|
| [11] |
KOZLIAK E I, KUBATOVA A, ARTEMYEVA A A, NAGEL E, ZHANG C, RAJAPPAGOWDA R B, SRNIRNOVA A L. Thermal liquefaction of lignin to aromatics: Efficiency, selectivity, and product analysis[J]. ACS Sustainable Chem Eng,2016,4(10):5106−5122. doi: 10.1021/acssuschemeng.6b01046
|
| [12] |
UPTON B M, KASKO A M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective[J]. Chem Rev,2016,116(4):2275−2306. doi: 10.1021/acs.chemrev.5b00345
|
| [13] |
ZHOU H, WANG H, SADOW A D, SLOWING, II. Toward hydrogen economy: Selective guaiacol hydrogenolysis under ambient hydrogen pressure[J]. Appl Catal B: Environ,2020,270:9.
|
| [14] |
ZHU W Z, WESTMAN G, THELIANDER H. Investigation and characterization of lignin precipitation in the LignoBoost process[J]. J Wood Chem Technol,2014,34(2):77−97. doi: 10.1080/02773813.2013.838267
|
| [15] |
BELKHEIRI T, ANDERSSON S I, MATTSSON C, OLAUSSON L, THELIANDER H, VAMLING L. Hydrothermal liquefaction of kraft lignin in subcritical water: Influence of phenol as capping agent[J]. Energy Fuels,2018,32(5):5923−5932. doi: 10.1021/acs.energyfuels.8b00068
|
| [16] |
LIU W J, JIANG H, YU H Q. Thermochemical conversion of lignin to functional materials: A review and future directions[J]. Green Chem,2015,17(11):4888−4907. doi: 10.1039/C5GC01054C
|
| [17] |
SUN R C. Lignin source and structural characterization[J]. ChemSusChem,2020,13(17):4385−4393. doi: 10.1002/cssc.202001324
|
| [18] |
BAJWA D S, POURHASHEM G, ULLAH A H, BAJWA S G. A concise review of current lignin production, applications, products and their environmental impact[J]. Ind Crop Prod,2019,139:111526.
|
| [19] |
SUN Z H, FRIDRICH B, DE SANTI A, ELANGOVAN S, BARTA K. Bright side of lignin depolymerization: Toward new platform chemicals[J]. Chem Rev,2018,118(2):614−678. doi: 10.1021/acs.chemrev.7b00588
|
| [20] |
赵勃, 吴凯, 仲惟鹏, 魏刚, 胡宗华, 郑文广, 阮慧锋, 严新明, 马颖, 王博, 江天霖, 张会岩. 木质素炭与ZSM-5联合催化热解木质素制备芳烃实验研究[J]. 燃料化学学报,2021,49(3):303−310.ZHAO Bo, WU Kai, ZHONG Li-peng, WEI Gang, HU Zong-hua, ZHENG Wen-guang, RUAN Hui-feng, YAN Xin-ming, MA Yin, WANG Bo, JIANG Tian-lin, ZHANG Hui-yan. Experimental study on catalytic pyrolysis of lignin under char and ZSM-5 for preparation of aromatics[J]. J Fuel Chem Technol,2021,49(3):303−310.
|
| [21] |
FAHMY T Y A, FAHMY Y, MOBARAK F, EL-SAKHAWY M, ABOU-ZEID R E. Biomass pyrolysis: Past, present, and future[J]. Environ Dev Sustain,2020,22(1):17−32. doi: 10.1007/s10668-018-0200-5
|
| [22] |
HA J M, HWANG K R, KIM Y M, JAE J, KIM K H, LEE H W, KIM J Y, PARK Y K. Recent progress in the thermal and catalytic conversion of lignin[J]. Renewable Sustainable Energy Rev,2019,111:422−441. doi: 10.1016/j.rser.2019.05.034
|
| [23] |
黄明, 朱亮, 马中青, 周秉亮, 刘晓欢, 叶结旺, 赵超. 金属改性分子筛催化热解木质素制取轻质芳烃[J]. 燃料化学学报,2021,49(3):292−302.HUANG Ming, ZHU Liang, MA Zhong-qing, ZHOU Bing-liang, LIU Xiao-huan, YE Jie-wang, ZHAO Chao. Production of light aromatics from the fast pyrolysis of lignin catalyzed by metal-modified H-ZSM-5 zeolites[J]. J Fuel Chem Technol,2021,49(3):292−302.
|
| [24] |
BELKHEIRI T, MATTSSON C, ANDERSSON S I, OLAUSSON L, AMAND L E, THELIANDER H, VAMLING L. Effect of pH on kraft lignin depolymerisation in subcritical water[J]. Energy Fuels,2016,30(6):4916−4924. doi: 10.1021/acs.energyfuels.6b00462
|
| [25] |
ZHOU H, WANG H, PERRAS F A, NAIK P, PRUSKI M, SADOW A D, SLOWING, II. Two-step conversion of Kraft lignin to nylon precursors under mild conditions[J]. Green Chem,2020,22(14):4676−4682. doi: 10.1039/D0GC01220C
|
| [26] |
娄静, 廖玮婷, 王智玉, 李璐, 李雁, 解新安. 钙钛矿催化木质素水热液化[J]. 燃料化学学报,2022,50(8):984−992. doi: 10.1016/S1872-5813(22)60004-5LOU Jing, LIAO Wei-ting, WANG Zhi-yu, LI Lu, LI Yan, XIE Xin-an. Hydrothermal liquefaction of lignin to aromatics over the perovskite catalysts[J]. J Fuel Chem Technol,2022,50(8):984−992. doi: 10.1016/S1872-5813(22)60004-5
|
| [27] |
OREGUI-BENGOECHEA M, GANDARIAS I, ARIAS P L, BARTH T. Unraveling the role of formic acid and the type of solvent in the catalytic conversion of lignin: A holistic approach[J]. ChemSusChem,2017,10(4):754−766. doi: 10.1002/cssc.201601410
|
| [28] |
YE K, LIU Y, WU S B, ZHUANG J P. A review for lignin valorization: Challenges and perspectives in catalytic hydrogenolysis [J]. Ind Crop Prod, 2021, 172.
|
| [29] |
KUMAR A, BISWAS B, BHASKAR T. Effect of cobalt on titania, ceria and zirconia oxide supported catalysts on the oxidative depolymerization of prot and alkali lignin[J]. Bioresour Technol,2020,299:122589.
|
| [30] |
KIM K H, FAROOQ A, SONG M Y, JUNG S C, JEON K J, SONG J, KO C H, JAE J, PARK Y K. Acetaldehyde removal and increased H-2/CO gas yield from biomass gasification over metal-loaded Kraft lignin char catalyst[J]. J Environ Manage,2019,232:330−335. doi: 10.1016/j.jenvman.2018.11.054
|
| [31] |
AKIYA N, SAVAGE P E. Roles of water for chemical reactions in high-temperature water[J]. Chem Rev,2002,102(8):2725−2750. doi: 10.1021/cr000668w
|
| [32] |
TOOR S S, ROSENDAHL L, RUDOLF A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies[J]. Energy,2011,36(5):2328−2342. doi: 10.1016/j.energy.2011.03.013
|
| [33] |
LAPPALAINEN J, BAUDOUIN D, HORNUNG U, SCHULER J, MELIN K, BJELIC S, VOGEL F, KONTTINEN J, JORONEN T. Sub- and supercritical water liquefaction of kraft lignin and black liquor derived lignin[J]. Energies,2020,13(13):3309.
|
| [34] |
YU S, ZHAO P, YANG X, LI Q, MOHAMED B A, SAAD J M, ZHANG Y, ZHOU H. Low-temperature hydrothermal carbonization of pectin enabled by high pressure[J]. J Anal Appl Pyrolysis,2022,166:105627. doi: 10.1016/j.jaap.2022.105627
|
| [35] |
HEGER K, UEMATSU M, FRANCK E U. The static dielectric-constant of water at high-pressures and temperatures to 500 MPa and 550-degrees-C[J]. Berichte der Bunsengesellschaft für physikalische Chemie,1980,84(8):758−762. doi: 10.1002/bbpc.19800840814
|
| [36] |
YU S, ZHAO P, YANG X, LI Q, ZHANG Y, ZHOU H. Formation and evolution of pectin-derived hydrothermal carbon from pectin[J]. Fuel,2022,326:124997. doi: 10.1016/j.fuel.2022.124997
|
| [37] |
KRUSE A, DINJUS E. Hot compressed water as reaction medium and reactant - Properties and synthesis reactions[J]. J Supercrit Fluids,2007,39(3):362−380. doi: 10.1016/j.supflu.2006.03.016
|
| [38] |
ONWUDILI J A, WILLIAMS P T. Catalytic depolymerization of alkali lignin in subcritical water: Influence of formic acid and Pd/C catalyst on the yields of liquid monomeric aromatic products[J]. Green Chem,2014,16(11):4740−4748. doi: 10.1039/C4GC00854E
|
| [39] |
RANA M, TAKI G, ISLAM M N, AGARWAL A, JO Y T, PARK J H. Effects of temperature and salt catalysts on depolymerization of kraft lignin to aromatic phenolic compounds[J]. Energy Fuels,2019,33(7):6390−6404. doi: 10.1021/acs.energyfuels.9b00808
|
| [40] |
BISWAS B, KUMAR A, SAINI K, RAWAT S, KAUR R, KRISHNA B B, BHASKAR T. Catalytic hydrothermal liquefaction of alkali lignin at low temperature: Effect of acid and base catalysts on phenolic monomers production [J]. Biomass Convers Bior, 2022: 1−10.
|
| [41] |
YU S, DONG X, ZHAO P, LUO Z, SUN Z, YANG X, LI Q, WANG L, ZHANG Y, ZHOU H. Decoupled temperature and pressure hydrothermal synthesis of carbon sub-micron spheres from cellulose[J]. Nat Commun,2022,13(1):3616. doi: 10.1038/s41467-022-31352-x
|
| [42] |
YU S, XIE M, LI Q, ZHANG Y, ZHOU H. Evolution of kraft lignin during hydrothermal treatment under different reaction conditions[J]. J Energy Inst,2022,103:147−153. doi: 10.1016/j.joei.2022.06.005
|
| [43] |
XU C, ARANCON R A D, LABIDI J, LUQUE R. Lignin depolymerisation strategies: towards valuable chemicals and fuels[J]. Chem Soc Rev,2014,43(22):7485−7500. doi: 10.1039/C4CS00235K
|





 下载:
下载: