Preparation of furfural from xylose catalyzed by difunctional carbon-based solid acid
-
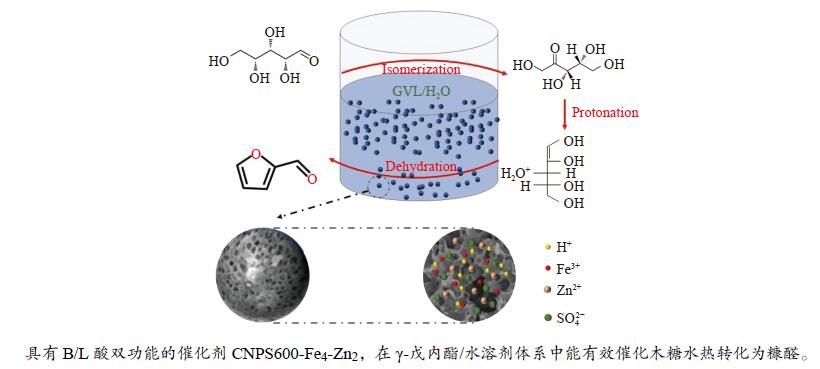
摘要: 本研究以明胶和植酸(PA)为碳源,并掺杂FeCl3·6H2O和ZnCl2,通过硫酸磺化法制备了双功能碳基固体酸催化剂用于高效催化木糖制备糠醛。采用SEM、BET、FT-IR、STEM-EDS等方法对不同炭化温度下制备的催化剂进行了表征,揭示了催化剂的物理化学性质。并考察了催化剂的炭化温度、FeCl3·6H2O与ZnCl2物质的量比、反应温度、反应时间、γ-戊内酯(GVL)与H2O的体积比、催化剂用量对木糖转化为糠醛(FF)的影响。结果表明,600 ℃炭化制备的催化剂CNPS600-Fe4-Zn2催化效果较好,用0.03 g的CNPS600-Fe4-Zn2(FeCl3·6H2O与ZnCl2物质的量比为4∶2)在3 mL的GVL/H2O(体积比为9∶1)溶剂体系中催化0.06 g木糖制备FF,当反应温度为170 ℃、反应时间为120 min时,木糖的转化率为99.6%,FF的摩尔产率可达85.8%。此外,还对催化剂的循环性能进行了测试,经五次循环实验后FF摩尔产率和木糖转化率均能保持在原来的80%以上,表明该催化剂具有较高的催化活性和较好的水热稳定性。Abstract: A bifunctional carbon-based solid acid catalyst was prepared by sulfonation of sulfuric acid to using gelatin and phytic acid (PA) as carbon sources and doping with FeCl3·6H2O and ZnCl2, which was used to efficiently catalyze the preparation of furfural from xylose. The catalysts prepared at different carbonization temperatures were characterized by SEM, BET, FT-IR and STEM-EDS techniques, and the physicochemical properties of the catalysts were revealed. The effects of carbonization temperature of the catalyst, molar ratio of FeCl3·6H2O to ZnCl2, reaction temperature, reaction time, volume ratio of γ-valerolactone (GVL) to H2O and catalyst dosage on the conversion of xylose to furfural (FF) were investigated. The results showed that the catalytic activity of CNPS600-Fe4-Zn2 was higher when the carbonization temperature was 600 ℃. The xylose conversion rate was 99.6%, and the molar yield of FF reached 85.8% when the reaction was carried out at 170 ℃ for 120 min using 0.03 g CNPS600-Fe4-Zn2 (the molar ratio of FeCl3·6H2O to ZnCl2 was 4∶2) as catalyst, 3 mL GVL/H2O (the volume ratio of was 9∶1) as solvent and 0.06 g xylose as raw material. In addition, the cyclic performance of the catalyst was tested. The FF molar yield and xylose conversion rate remained above 80% after 5 cycles, indicating that the catalyst had higher catalytic activity and better hydrothermal stability.
-
Key words:
- carbon-based solid acid catalyst /
- xylose /
- γ-valerolactone /
- furfural
-
表 1 不同催化剂的比表面积和孔道结构
Table 1 Specific surface area and pore structure of different catalysts
Catalyst Specific surface
area /(m2·g−1)Average pore
width /nmTotal pore
volume /
(cm3·g−1)CNPS400-Fe4-Zn2 10.6 3.82 0.0950 CNPS600-Fe4-Zn2 155 3.82 0.745 CNPS800-Fe4-Zn2 18.9 13.0 0.353 表 2 ICP测试结果
Table 2 Results of ICP test
Catalyst Sampling
quality /gConstant
volume /mLTest
elementTest solution element
concentration /(mg·L−1)Multiple of
the dilutionElement content
Cx /(mg·kg−1)Element
content w /%CNPS400-Fe4-Zn2 0.0548 25 Fe 4.92 1 2243.61 0.220 0.0548 25 Zn 2.11 1 962.14 0.100 0.0548 25 S 9.30 20 84863.14 8.49 CNPS600-Fe4-Zn2 0.0536 25 Fe 8.72 20 81324.63 8.13 0.0536 25 Zn 3.30 10 15410.45 1.54 0.0536 25 S 7.42 10 34594.22 3.46 CNPS800-Fe4-Zn2 0.0490 25 Fe 5.68 10 29000.00 2.90 0.0490 25 Zn 3.43 10 17520.41 1.75 0.0490 25 S 4.80 10 24469.39 2.45 表 3 催化剂循环实验中催化剂的回收率*
Table 3 Recovery of catalysts in catalyst cycling experiment
Recycle Fresh 1 2 3 4 5 Amount of recovery /g 0.72 0.63 0.58 0.52 0.46 0.41 Recovery rate /% − 87.5 92.1 89.7 88.5 90.0 Recovery of catalysts in catalyst cycling experiments*: In order to make the remaining catalyst sufficient for the next cycle, the reaction raw material, mixed solvent, catalyst, etc. were expanded by 24 times in the catalyst cycle experiment -
[1] PERALTA-YAHYA P P, ZHANG F, DEL CARDAYRE S B, KEASLING J D. Microbial engineering for the production of advanced biofuels[J]. Nature,2012,488(7411):320−328. doi: 10.1038/nature11478 [2] STEEN E J, KANG Y, BOKINSKY G, HU Z, SCHIRMER A, MCCLURE A, DEL CARDAYRE S B, KEASLING J D. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass[J]. Nature,2010,463(7280):559−U182. doi: 10.1038/nature08721 [3] ZHANG L, YU H, WANG P, LI Y. Production of furfural from xylose, xylan and corncob in gamma-valerolactone using FeCl3·6H2O as catalyst[J]. Bioresour Technol,2014,151:355−360. doi: 10.1016/j.biortech.2013.10.099 [4] LUO Y, LI Z, LI X, LIU X, FAN J, CLARK J H, HU C. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review[J]. Catal Today,2019,319:14−24. doi: 10.1016/j.cattod.2018.06.042 [5] SONG S, DI L, WU G, DAI W, GUAN N, LI L. Meso-Zr-Al-beta zeolite as a robust catalyst for cascade reactions in biomass valorization[J]. Appl Catal B: Environ,2017,205:393−403. doi: 10.1016/j.apcatb.2016.12.056 [6] SONG S, FUNG KIN YUEN V, DI L, SUN Q, ZHOU K, YAN N. Integrating biomass into the organonitrogen chemical supply chain: Production of pyrrole and d-proline from furfural[J]. Angew Chem, Int Edit,2020,59(45):19846−19850. doi: 10.1002/anie.202006315 [7] ZHANG L, TIAN L, SUN R, LIU C, KOU Q, ZUO H. Transformation of corncob into furfural by a bifunctional solid acid catalyst[J]. Bioresour Technol,2019,276:60−64. doi: 10.1016/j.biortech.2018.12.094 [8] SUN K, SHAO Y, LIU P, ZHANG L, GAO G, DONG D, ZHANG S, HU G, XU L, HU X. A solid iron salt catalyst for selective conversion of biomass-derived C5 sugars to furfural[J]. Fuel,2021,300:120990. doi: 10.1016/j.fuel.2021.120990 [9] ZHANG T, LI W, XU Z, LIU Q, MA Q, JAMEEL H, CHANG H, MA L. Catalytic conversion of xylose and corn stalk into furfural over carbon solid acid catalyst in γ-valerolactone[J]. Bioresour Technol,2016,209:108−114. doi: 10.1016/j.biortech.2016.02.108 [10] WANG X, QIU M, TANG Y, YANG J, SHEN F, QI X, YU Y. Synthesis of sulfonated lignin-derived ordered mesoporous carbon for catalytic production of furfural from xylose[J]. Int J Biol Macromol,2021,187:232−239. doi: 10.1016/j.ijbiomac.2021.07.155 [11] DAI Y, YANG S, WANG T, TANG R, WANG Y, ZHANG L. High conversion of xylose to furfural over corncob residue-based solid acid catalyst in water-methyl isobutyl ketone[J]. Ind Crop Prod,2022,180:114781. doi: 10.1016/j.indcrop.2022.114781 [12] LI X, LU X, LIANG M, XU R, YU Z, DUAN B, LU L, SI C. Conversion of waste lignocellulose to furfural using sulfonated carbon microspheres as catalyst[J]. Waste Manage,2020,108:119−126. doi: 10.1016/j.wasman.2020.04.039 [13] XIONG S, LUO C, YU Z, JI N, ZHU L, WANG S. Dual-functional carbon-based solid acid-induced hydrothermal conversion of biomass saccharides: catalyst rational design and kinetic analysis[J]. Green Chem,2021,23(21):8458−8467. doi: 10.1039/D1GC01968F [14] LIU C, WYMAN C E. The enhancement of xylose monomer and xylotriose degradation by inorganic salts in aqueous solutions at 180°C[J]. Carbohydr Res,2006,341(15):2550−2556. doi: 10.1016/j.carres.2006.07.017 [15] KONWAR L J, MÄKI-ARVELA P, MIKKOLA J P. SO3H-containing functional carbon materials: Synthesis, structure, and acid catalysis[J]. Chem Rev,2019,119(22):11576−11630. doi: 10.1021/acs.chemrev.9b00199 [16] YANG T, LI W, SU M, LIU Y, LIU M. Production of furfural from xylose catalyzed by a novel calcium gluconate derived carbon solid acid in 1, 4-dioxane[J]. New J Chem,2020,44(19):7968−7975. doi: 10.1039/D0NJ00619J [17] RUSANEN A, KUPILA R, LAPPALAINEN K, KÄRKKÄINEN J, HU T, LASSI U. Conversion of xylose to furfural over lignin-based activated carbon-supported iron catalysts[J]. Catalysts,2020,10(8):821. doi: 10.3390/catal10080821 [18] WANG S, ZHAO Y, LIN H, CHEN J, ZHU L, LUO Z. Conversion of C5 carbohydrates into furfural catalyzed by a Lewis acidic ionic liquid in renewable γ-valerolactone[J]. Green Chem,2017,19(16):3869−3879. doi: 10.1039/C7GC01298E [19] MELLMER M A, SENER C, GALLO J M R, LUTERBACHER J S, ALONSO D M, DUMESIC J A. Solvent effects in acid-catalyzed biomass conversion reactions[J]. Angew Chem, Int Ed,2014,53(44):11872−11875. doi: 10.1002/anie.201408359 [20] ARAUJO R O, CHAAR J D S, QUEIROZ L S, DA ROCHA FILHO G N, DA COSTA C E F, DA SILVA G C T, LANDERS R, COSTA M J F, GONÇALVES A A S, DE SOUZA L K C. Low temperature sulfonation of acai stone biomass derived carbons as acid catalysts for esterification reactions[J]. Energy Conv Manag,2019,196:821−830. doi: 10.1016/j.enconman.2019.06.059 [21] LAM E, CHONG J H, MAJID E, LIU Y, HRAPOVIC S, LEUNG ACW, LUONG, J H T. Carbocatalytic dehydration of xylose to furfural in water[J]. Carbon,2012,50(3):1033−1043. doi: 10.1016/j.carbon.2011.10.007 [22] GENG L, WANG Y, YU G, ZHU Y. Efficient carbon-based solid acid catalysts for the esterification of oleic acid[J]. Catal Commun,2011,13(1):26−30. doi: 10.1016/j.catcom.2011.06.014 [23] YUAN C, WANG X, YANG X, ALGHAMDI A A, ALHARTHI F A, CHENG X, DENG Y. Sulfonic acid-functionalized core-shell Fe3O4@carbon microspheres as magnetically recyclable solid acid catalysts[J]. Chin Chem Lett,2021,32(6):2079−2085. doi: 10.1016/j.cclet.2020.11.027 [24] PERŠIN Z, MAVER U, PIVEC T, MAVER T, VESEL A, MOZETIČ M, STANA-KLEINSCHEK K. Novel cellulose based materials for safe and efficient wound treatment[J]. Carbohydr Polym,2014,100:55−64. doi: 10.1016/j.carbpol.2013.03.082 [25] QI W, HE C, WANG Q, LIU S, YU Q, WANG W, LEKSAWASDI N, WANG C, YUAN Z. Carbon-based solid acid pretreatment in corncob saccharification: Specific xylose production and efficient enzymatic hydrolysis[J]. ACS Sustainable Chem Eng,2018,6(3):3640−3648. doi: 10.1021/acssuschemeng.7b03959 [26] XU S, YIN C, PAN D, HU F, WU Y, MIAO Y, GAO L, XIAO G. Efficient conversion of glucose into 5-hydroxymethylfurfural using a bifunctional Fe3 + modified Amberlyst-15 catalyst[J]. Sustainable Energy Fuels,2019,3(2):390−395. doi: 10.1039/C8SE00499D [27] YANG H, WANG L, JIA L, QIU C, PANG Q, PAN X. Selective decomposition of cellulose into glucose and levulinic acid over Fe-resin catalyst in NaCl solution under hydrothermal conditions[J]. Ind Eng Chem Res,2014,53(15):6562−6568. doi: 10.1021/ie500318t [28] TIAN S, WU Y, LI K, XIE H, REN H, ZHAO Y, MIAO Z, TAN Y. Isobutanol synthesis from syngas over Zn-Cr catalyst: Effect of Zn/Cr element ratio[J]. Energy Technol,2018,6(9):1805−1812. doi: 10.1002/ente.201700908 [29] GONZALEZ-SERRANO E, CORDERO T, RODRIGUEZ-MIRASOL J, COTORUELO L, RODRIGUEZ J J. Removal of water pollutants with activated carbons prepared from H3PO4 activation of lignin from kraft black liquors[J]. Water Res,2004,38(13):3043−3050. doi: 10.1016/j.watres.2004.04.048 [30] ZHAO Y, LU K, XU H, ZHU L, WANG S. A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion[J]. Renewable Sustainable Energy Rev,2021,139:110706. doi: 10.1016/j.rser.2021.110706 -





 下载:
下载: