Performance study of Pt-CeO2/ZSM-22 catalyzed n-heptane isomerization reaction
-
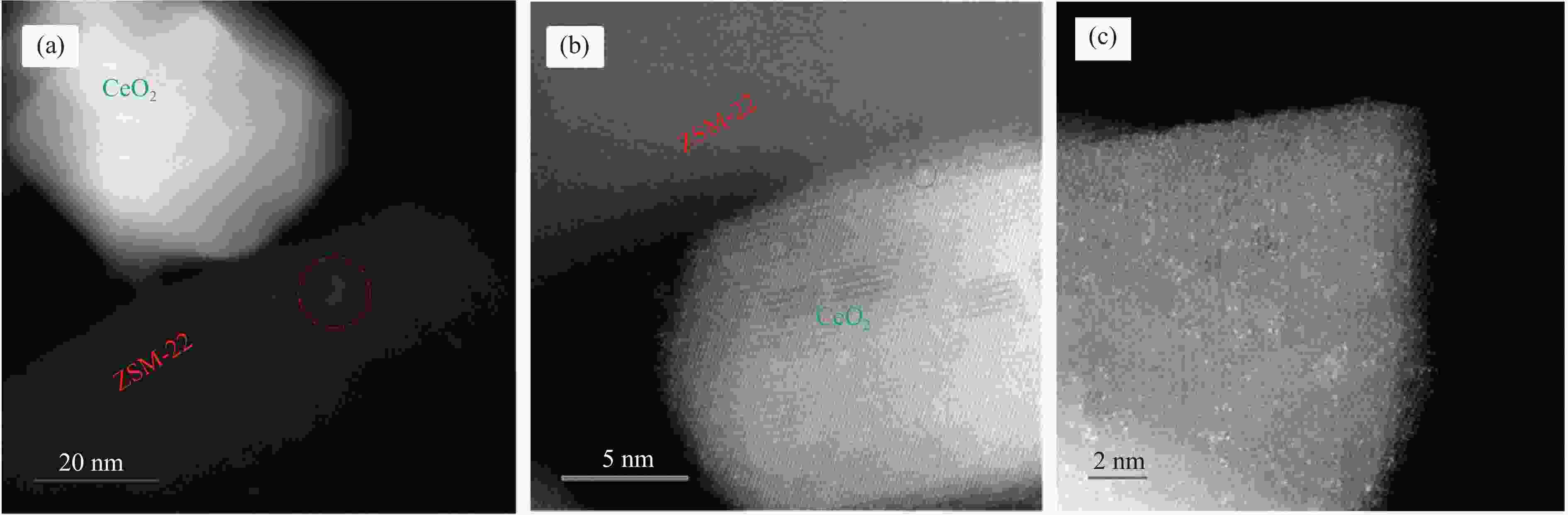
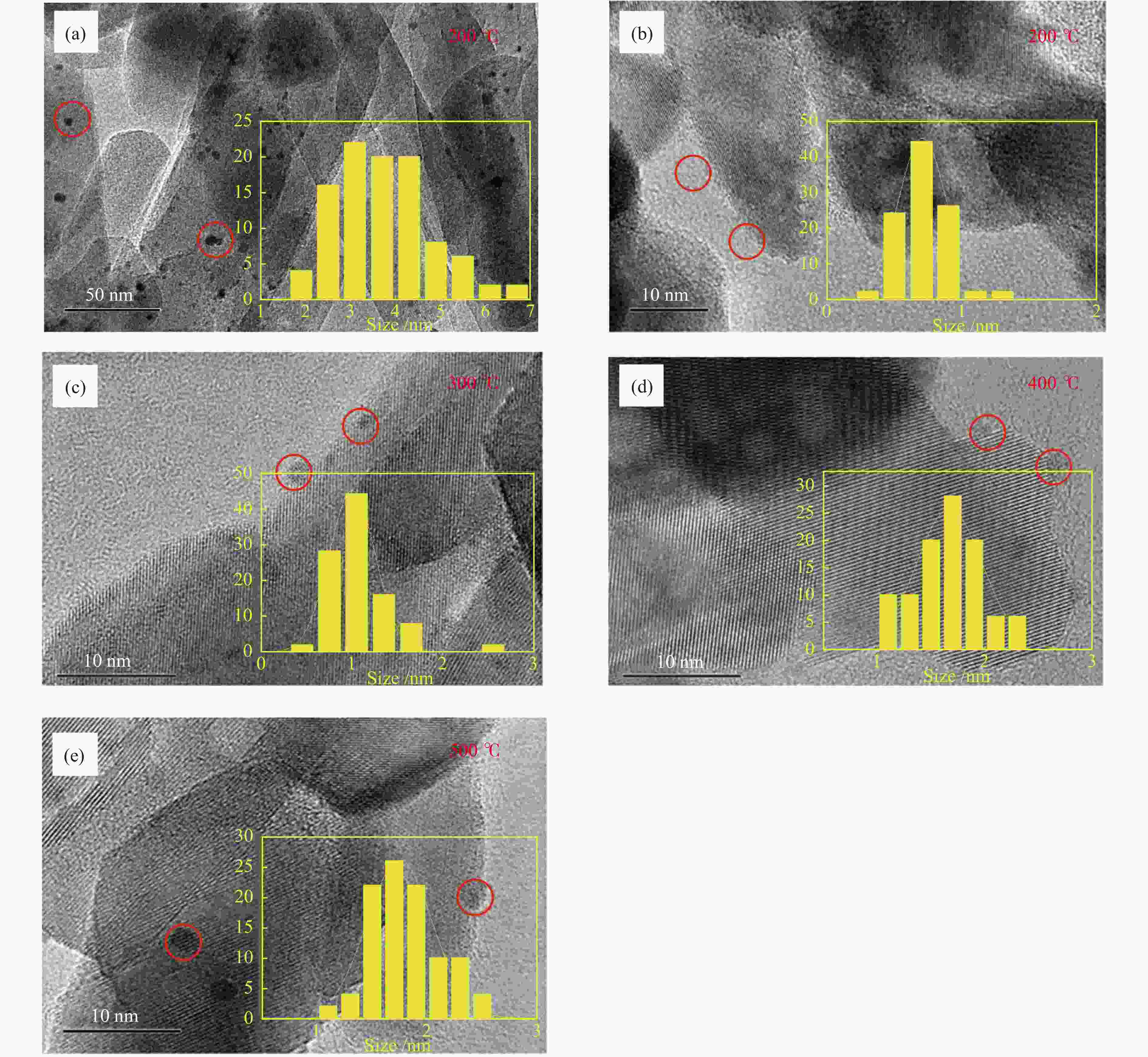
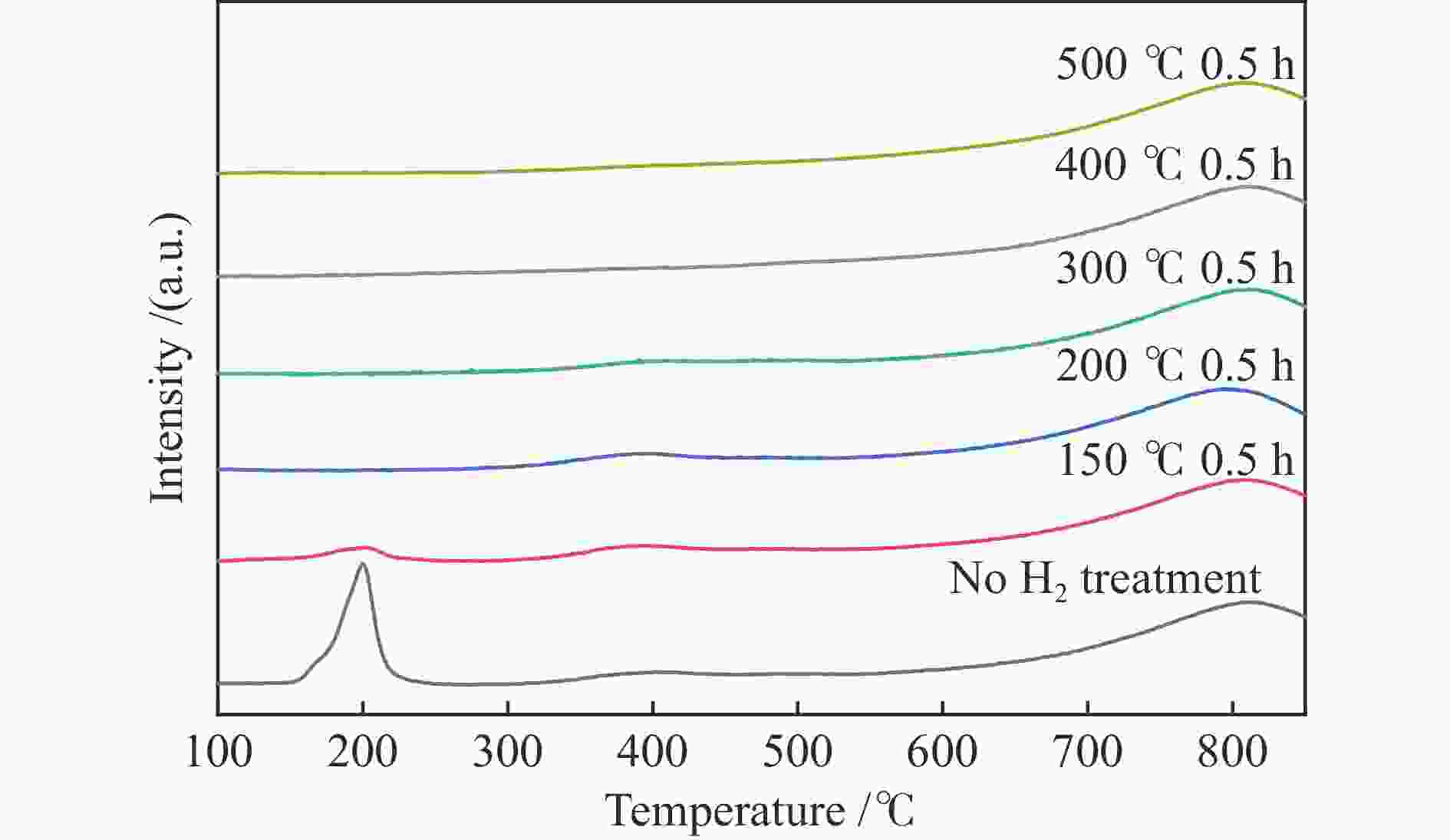
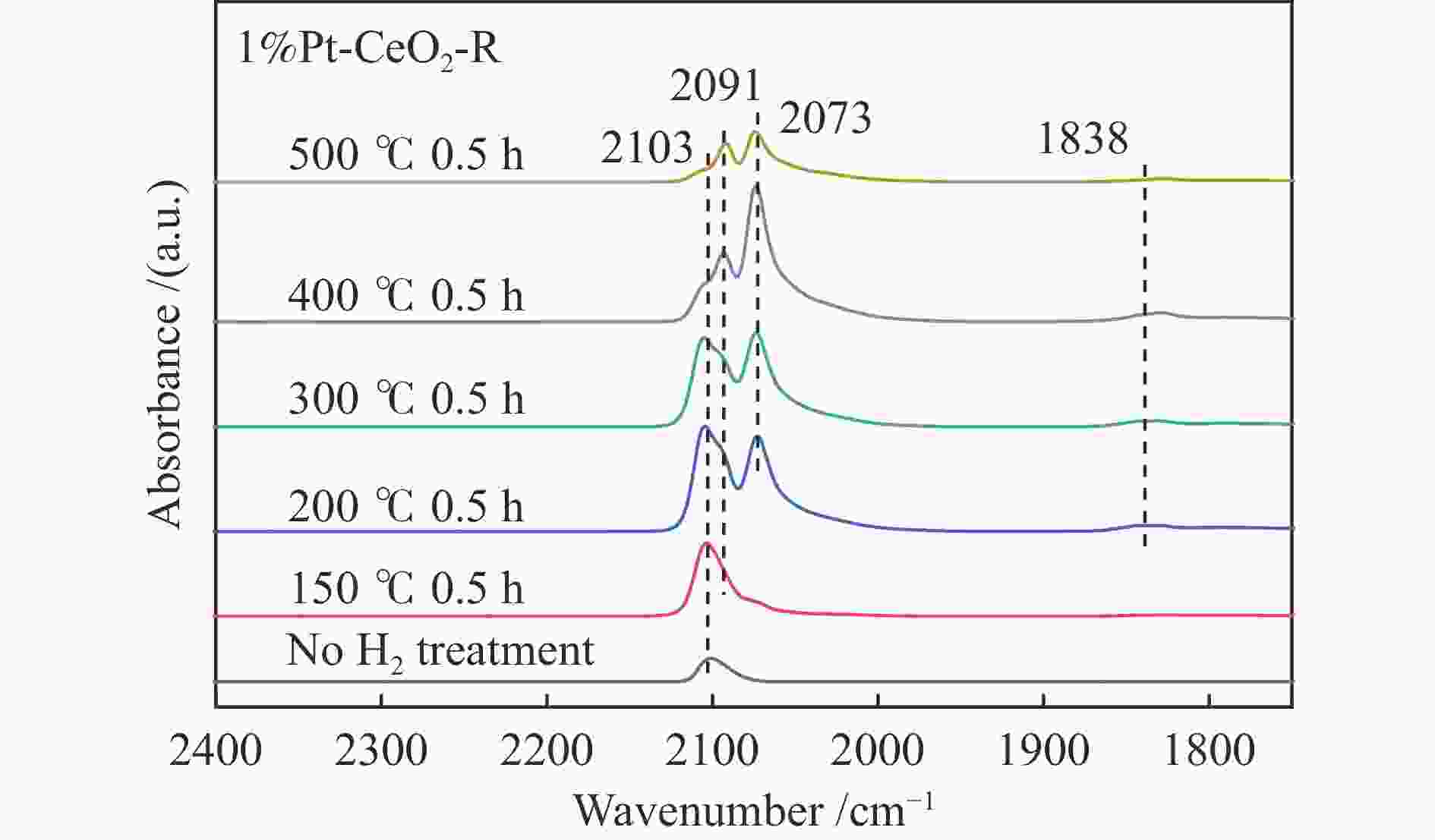
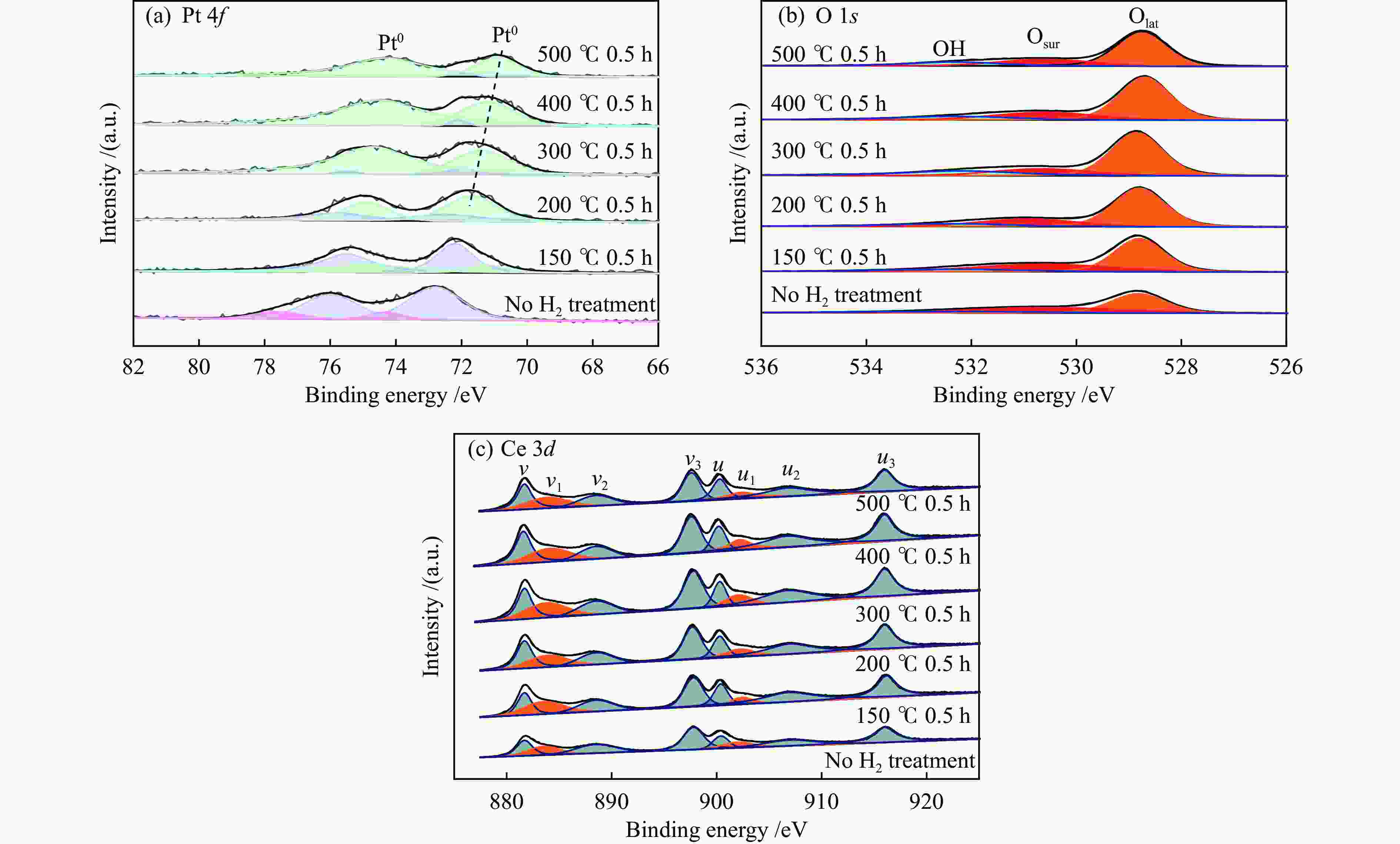
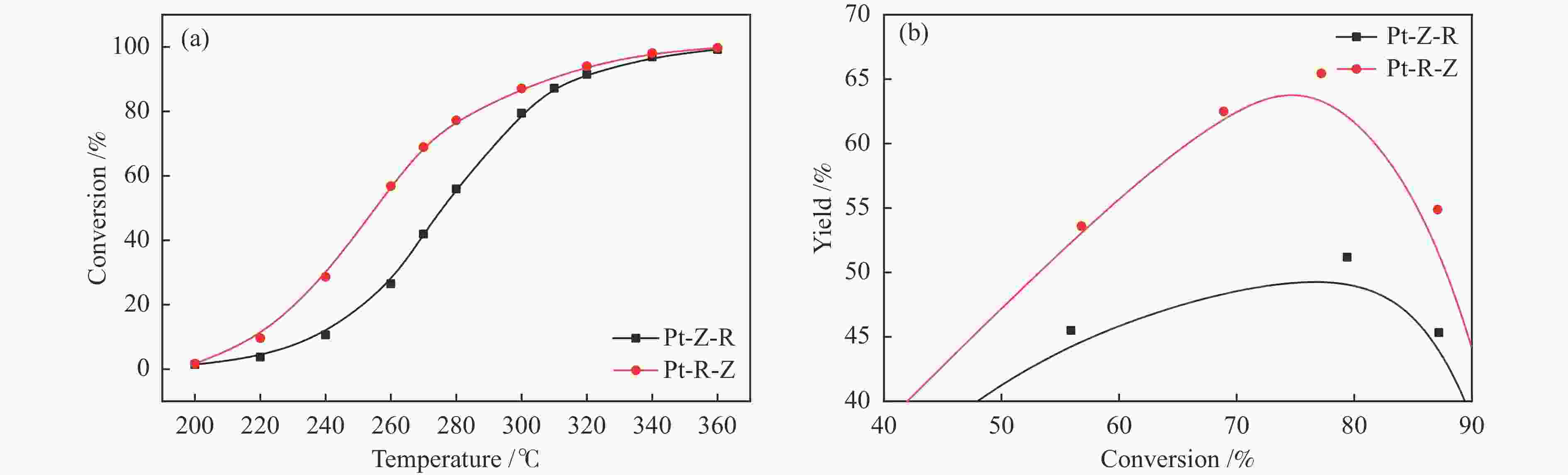
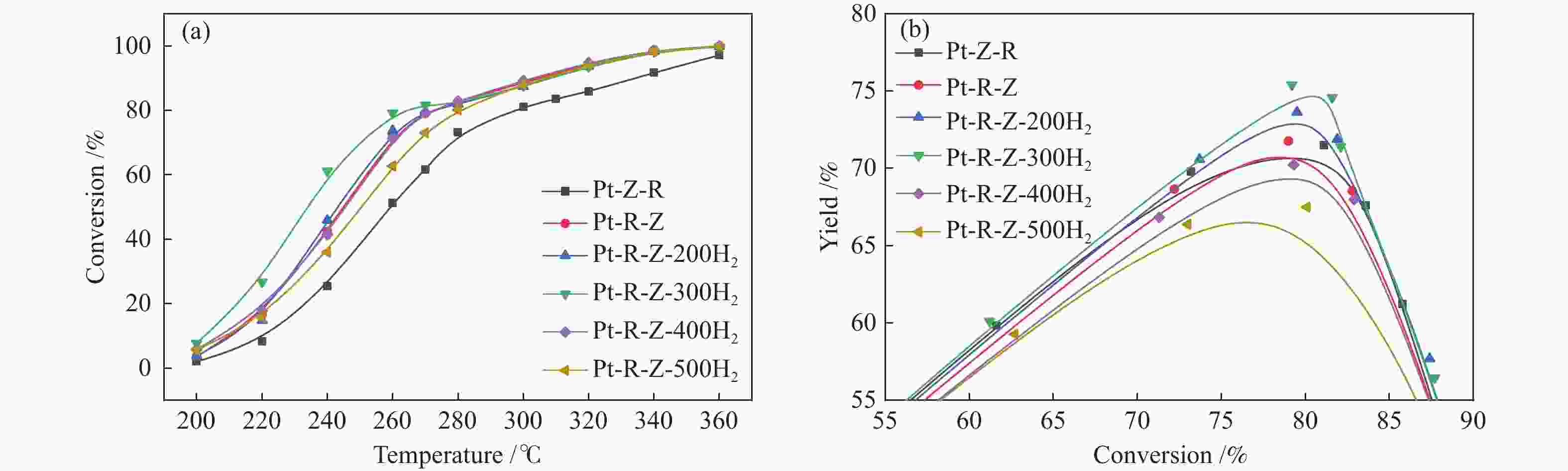
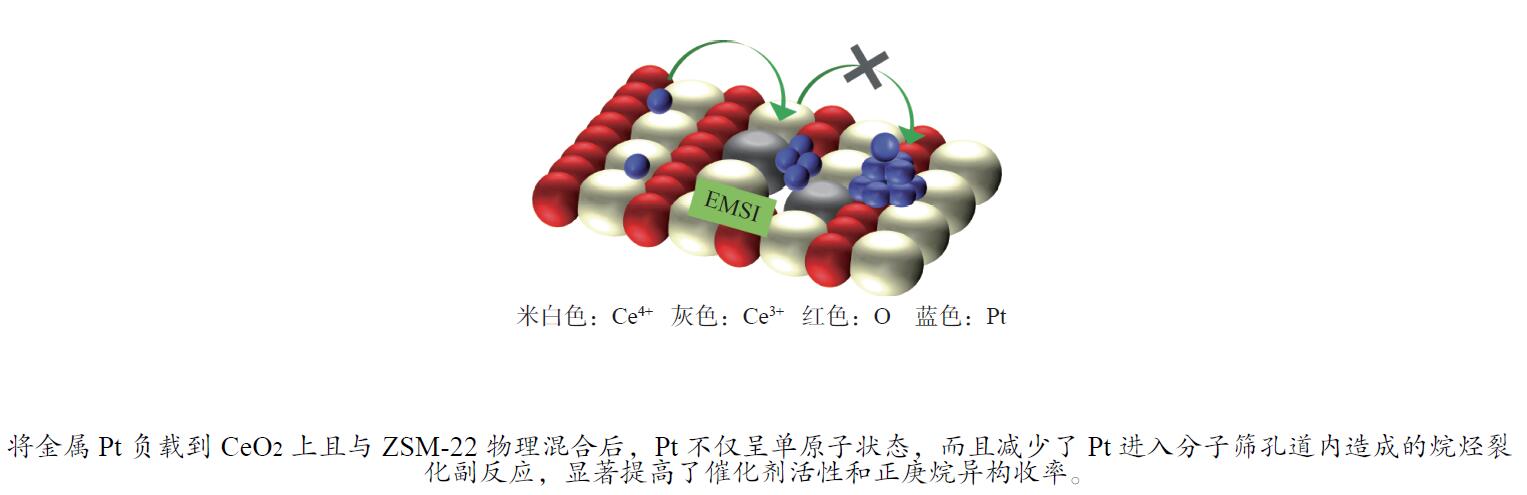
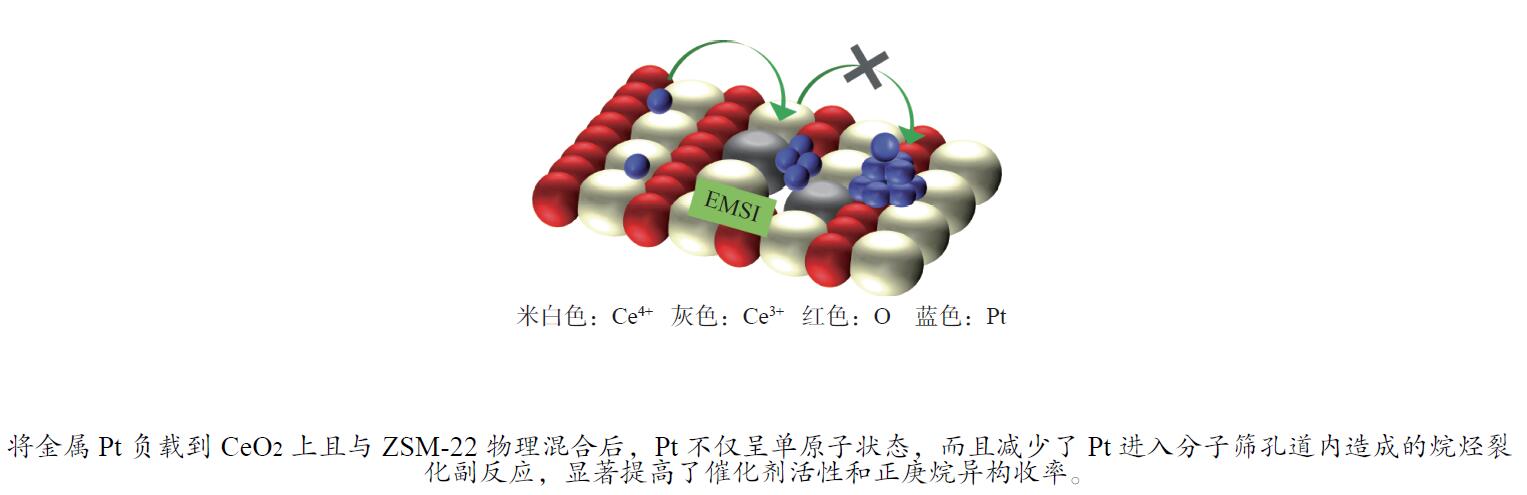
摘要: 结合浸渍法和物理混合法调控金属活性中心Pt的位点,使其单独落位到ZSM-22分子筛或氧化铈载体上,从而分别得到金属-酸双中心位点的间距达到原子级接触的Pt-ZSM-22/CeO2催化剂和保持纳米级间距的Pt-CeO2/ZSM-22催化剂。利用球差电镜、XRD、BET、H2-TPR和XPS等手段,表征了两种催化剂的物化性质,并对其正庚烷异构化反应性能进行了研究。另外,考察了不同还原温度下Pt-CeO2/ZSM-22催化剂的物化性质和反应性能变化。结果显示,金属-酸中心保持纳米级间距Pt-CeO2/ZSM-22催化剂具有更高的正庚烷异构化活性和异构烃收率,这是由于Pt在CeO2载体上呈原子级分散的原因。在Pt-CeO2/ZSM-22催化剂还原过程中,CeO2载体释放更多的氧空位有助于延缓金属Pt的聚集且有利于庚烷分子的吸附。当还原预处理温度为300 ℃时,庚烷转化率和庚烷异构烃收率分别为79.2%和75.4%,异构化选择性达到95.2%。Abstract: A combination of impregnation and physical mixing methods was used to modulate the sites of the metal active center Pt, which were individually settled onto ZSM-22 molecular sieves or cerium oxide carriers, resulting in Pt-ZSM-22/CeO2 catalysts with atomic-level contacts at the spacing of the metal-acid bicenter sites and Pt-CeO2/ZSM-22 catalysts that maintained nanoscale spacing, respectively. The physical and chemical properties of the two catalysts were characterized by means of spherical differential electron microscopy, XRD, BET, H2-TPR and XPS, and their n-heptane isomerization reaction performance was investigated. In addition, the changes of the physicochemical properties and reaction performance of Pt-CeO2/ZSM-22 catalysts at different reduction temperatures were investigated. The results showed that the metal-acid center maintained nanoscale spacing Pt-CeO2/ZSM-22 catalyst had higher n-heptane isomerization activity and isomeric hydrocarbon yield, which could be attributed to the atomic-level dispersion of Pt on the CeO2 carrier. During the reduction of Pt-CeO2/ZSM-22 catalyst, the release of more oxygen vacancies from the CeO2 carrier helps to retard the aggregation of metal Pt and facilitates the adsorption of heptane molecules. When the reduction pretreatment temperature was 300 ℃, the heptane conversion and heptane isomerization hydrocarbon yield were 79.2% and 75.4%, respectively, and the isomerization selectivity reached 95.2%.
-
Key words:
- n-heptane /
- isomerization /
- bifunctional catalyst /
- intimacy /
- CeO2
-
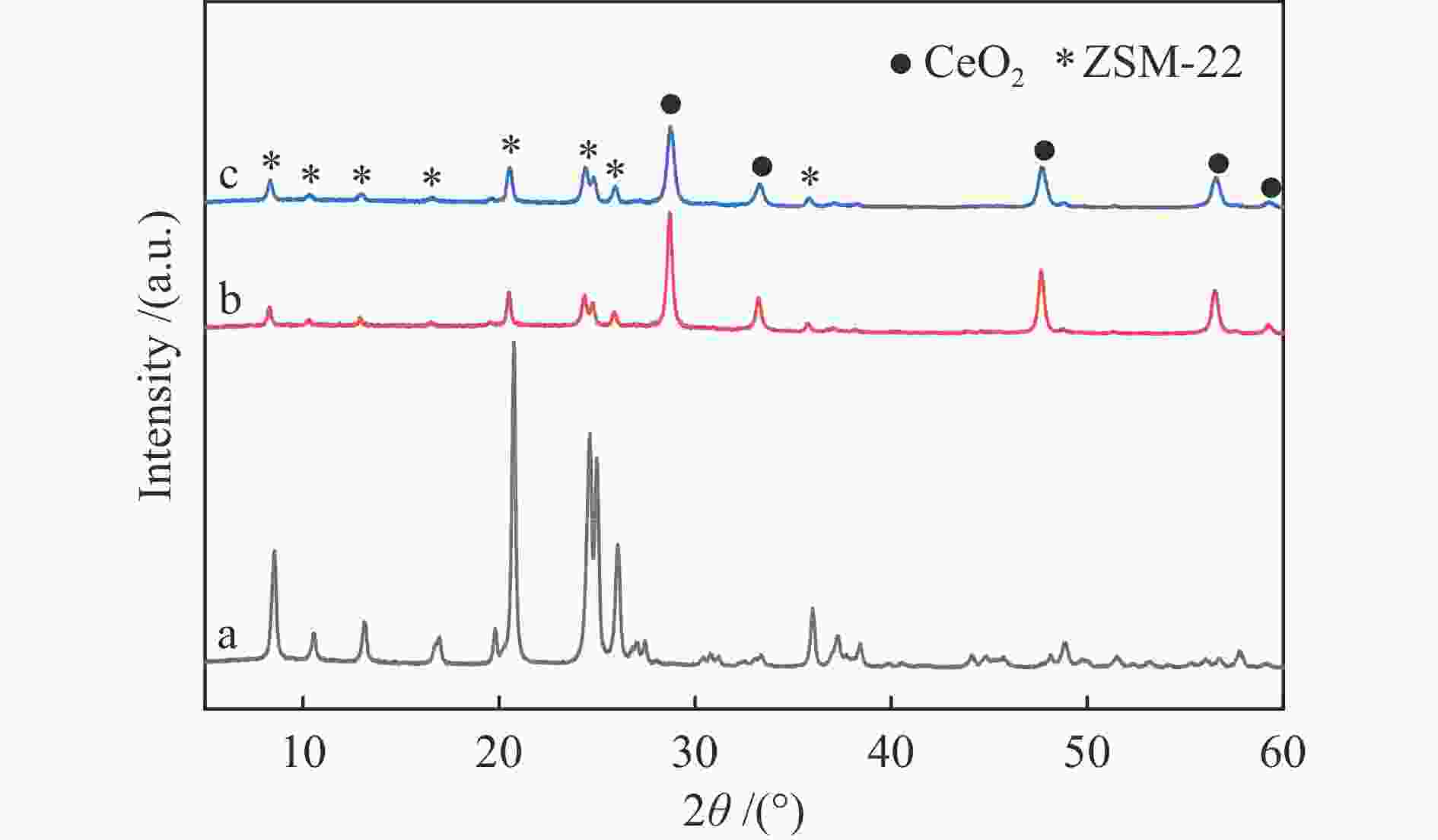
表 1 两种催化剂的织构性质
Table 1 Texture properties of two types of catalysts
Sample SBETa /(m2·g−1) dporeb /nm vporec /(cm3·g−1) vmicrod /(cm3·g−1) Pt-R-Z 196.0 5.9 0.29 0.06 Pt-Z-R 193.9 6.4 0.31 0.06 a: BET surface area, b: average pore diameter, c: total pore volume, d: t-plot micropore volume 表 2 不同温度还原预处理后Pt-CeO2的织构性质
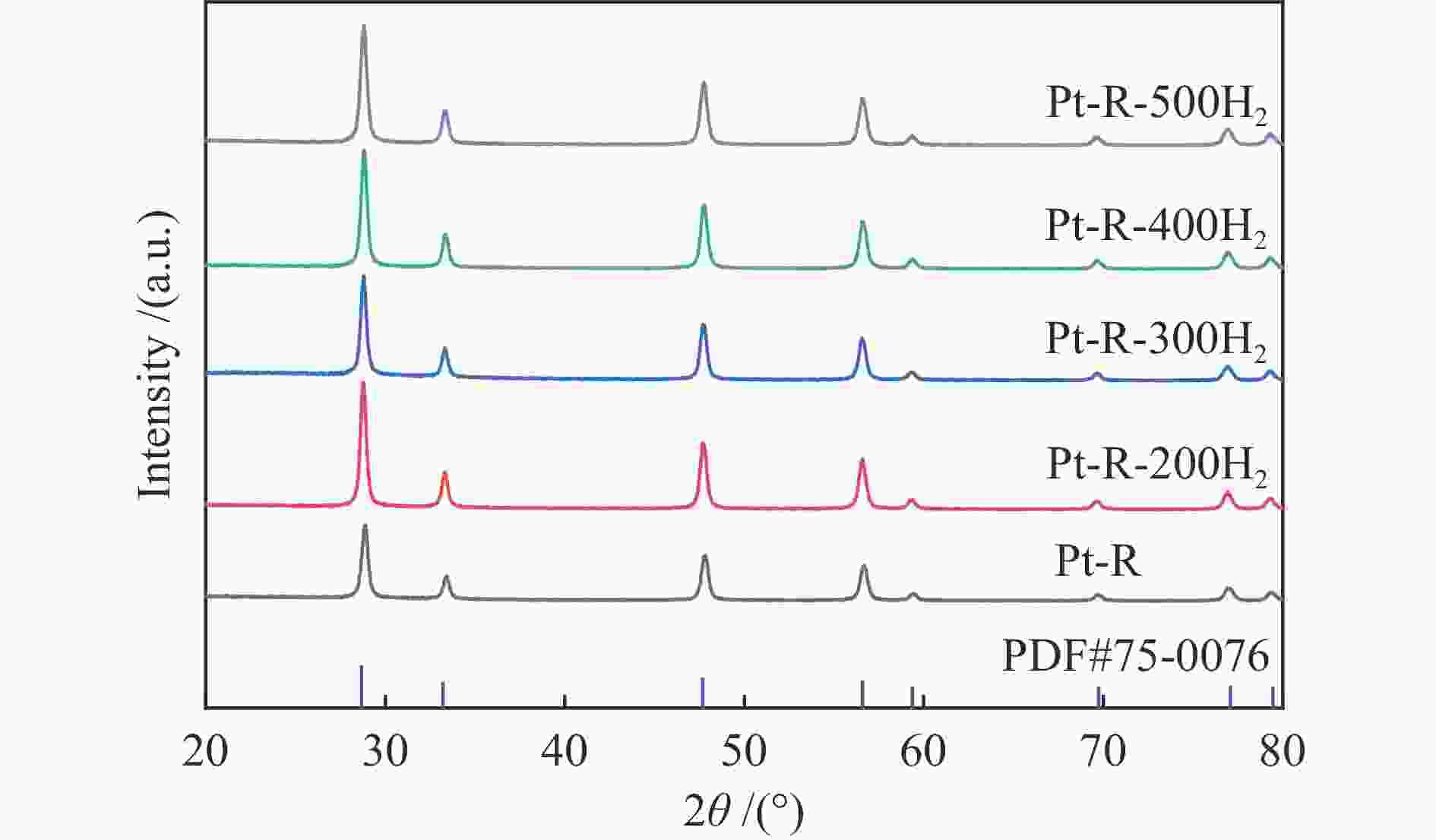
Table 2 Texture properties of samples after reduction pretreatment at different temperatures
Sample SBETa /(m2·g−1) dporeb /nm vporec /(cm3·g−1) Ptd /% Pt-ZSM-22 – – – 0.83 Pt-CeO2 23.3 19.9 0.12 0.79 Pt-CeO2-H2-200 22.3 19.1 0.11 - Pt-CeO2-H2-300 22.2 18.1 0.10 – Pt-CeO2-H2-400 24.7 18.2 0.11 – Pt-CeO2-H2-500 23.8 18.5 0.11 – a: BET surface area, b: average pore diameter, c: total pore volume, d: determined by ICP -
[1] ANDERSEN M, MEDFORD A J, NORSKOV J K, REUTER, K. Analyzing the case for bifunctional catalysis[J]. Angew Chem Int Ed,2016,55(17):5210−5214. doi: 10.1002/anie.201601049 [2] GUISNET M. "Ideal" bifunctional catalysis over Pt-acid zeolites[J]. Catal Today,2013,218:123−134. [3] WANG D X, LIU J C, CHENG X S, KONG X, WU A P, TIAN C G, FU H G. Trace Pt clusters dispersed on SAPO-11 promoting the synergy of metal sites with acid sites for high-effective hydroisomerization of n-alkanes[J]. Small Methods,2019,3(5):10. [4] LYU Y C, ZHAN W L, YU Z M, LIU X M, YANG Y, WANG X X, SONG C S, YAN Z F. One-pot synthesis of the highly efficient bifunctional Ni-SAPO-11 catalyst[J]. J Mater Sci Technol,2021,76:86−94. doi: 10.1016/j.jmst.2020.10.033 [5] SONG Z, ZHANG Z, CHEN J, ZHU L, XIANG Y, XIA D. Progresses in Pt-M bimetallic bifunctional catalysts for isomerization of light alkanes[J]. Petrochem Technol,2017,46(1):1−8. [6] ZECEVIC J, VANBUTSELE G, DE JONG K P, MARTENS J A. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons[J]. Nature,2015,528(7581):245−248. doi: 10.1038/nature16173 [7] NAGAI Y, HIRABAYASHI T, DOHMAE K, TAKAGI N, MINAMI T, SHINJOH H, MATSUMOTO S. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide-support interaction[J]. J Catal,2006,242(1):103−109. doi: 10.1016/j.jcat.2006.06.002 [8] MA Y Y, GAO W, ZHANG Z Y, ZHANG S, TIAN Z M, LIU Y X, HO J C, QU Y Q. Regulating the surface of nanoceria and its applications in heterogeneous catalysis[J]. Surf Sci Rep,2018,73(1):1−36. doi: 10.1016/j.surfrep.2018.02.001 [9] YE X X, WANG H W, LIN Y, LIU X Y, CAO L N, GU J, LU J L. Insight of the stability and activity of platinum single atoms on ceria[J]. Nano Res,2019,12(6):1401−1409. doi: 10.1007/s12274-019-2351-6 [10] MAI H X, SUN L D, ZHANG Y W, SI R, FENG W, ZHANG H P, LIU H C, YAN C H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes[J]. J Phys Chem B,2005,109(51):24380−24385. doi: 10.1021/jp055584b [11] LEE J, RYOU Y, CHAN X, KIM T J, KIM D H. How Pt interacts with CeO2 under the reducing and oxidizing environments at elevated temperature: The origin of improved thermal stability of Pt-CeO2 compared to CeO2[J]. J Phys Chem C,2016,120(45):25870−25879. doi: 10.1021/acs.jpcc.6b08656 [12] SANCHEZ M G, GAZQUEZ J L. Oxygen vacancy model in strong metal support interaction[J]. J Catal,1987,104(1):120−135. doi: 10.1016/0021-9517(87)90342-3 [13] QIAO B T, WANG A Q, YANG X F, ALLARD L F, JIANG Z, CUI Y T, LIU J Y, LI J, ZHANG T. Single-atom catalysis of CO oxidation using Pt-1/FeOx[J]. Nat Chem,2011,3(8):634−641. [14] WANG C L, GU X K, YAN H, LIN Y, LI J J, LIU D D, LI W X, LU J L. Water-mediated Mars-Van Krevelen mechanism for CO oxidation on ceria-supported single-atom Pt-1 catalyst[J]. ACS Catal,2017,7(1):887−891. doi: 10.1021/acscatal.6b02685 [15] WANG B F, CHEN B X, SUN Y H, XIAO H L, XU X X, FU M L, WU J L, CHEN L M, YE D Q. Effects of dielectric barrier discharge plasma on the catalytic activity of Pt-CeO2 catalysts[J]. Appl Catal B: Environ,2018,238:328−38. [16] ZHANG Q S, BU J H, WANG J D, SUN C Y, ZHAO D Y, SHENG G Z, XIE X W, SUN M, YU L. Highly efficient hydrogenation of nitrobenzene to aniline over Pt-CeO2 catalysts: The shape effect of the support and key role of additional Ce3 + sites[J]. ACS Catal,2020,10(18):10350−63. -




 下载:
下载: