Study on the reaction characteristics of tar model compounds with an Fe-based oxygen carrier
-
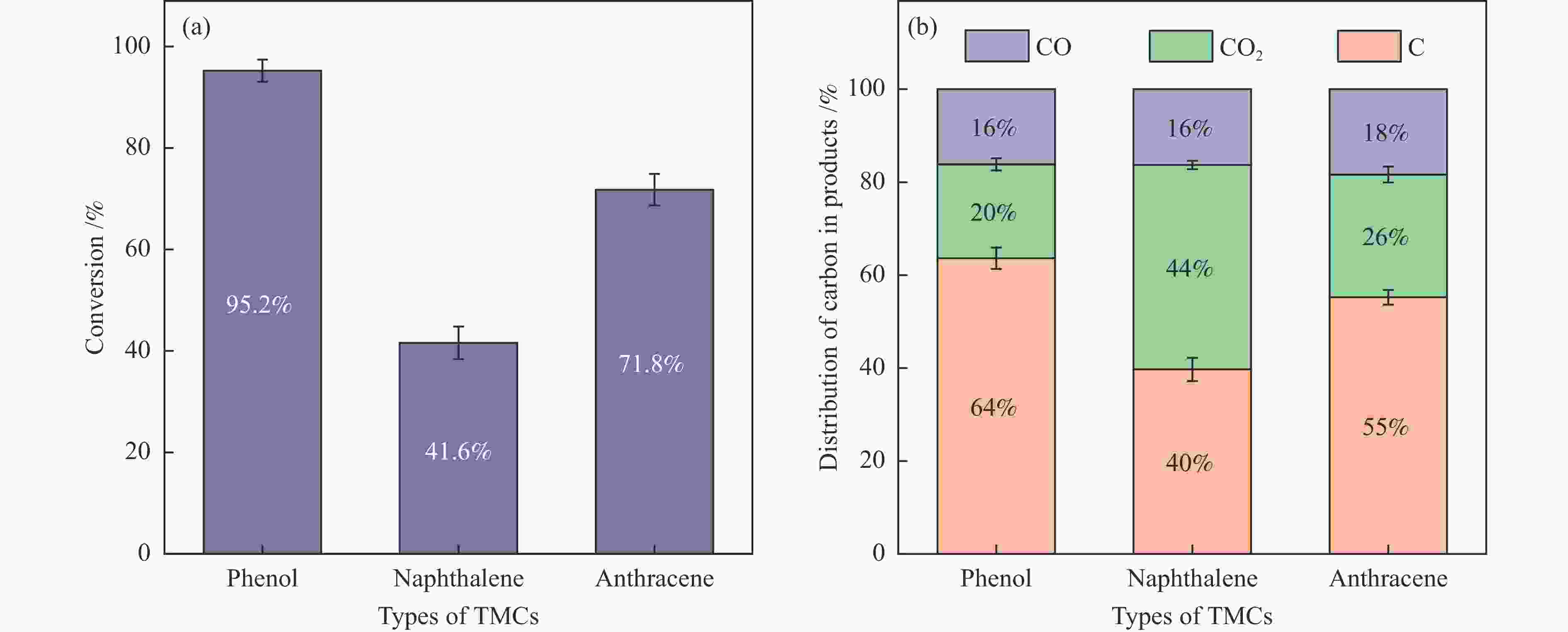
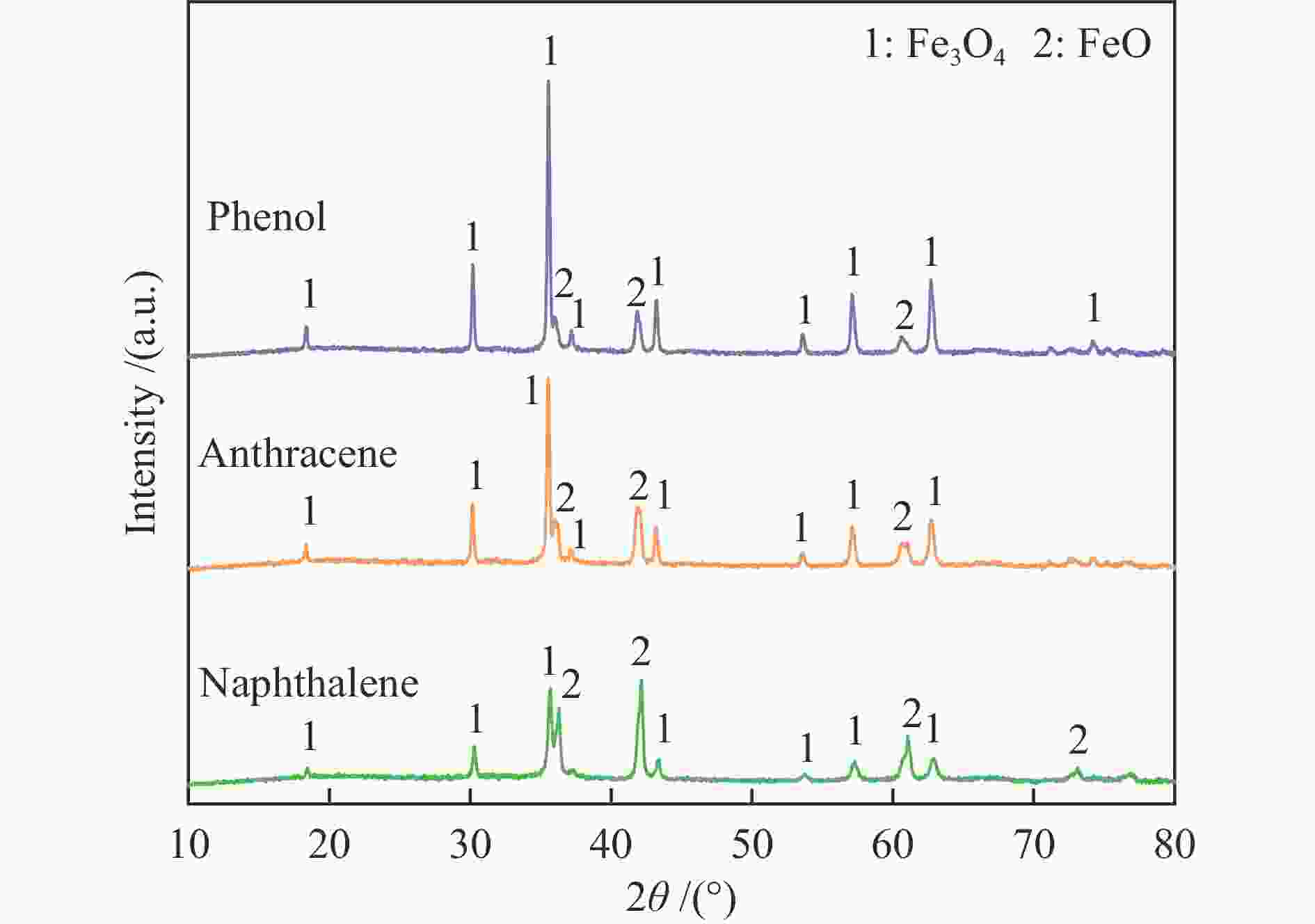
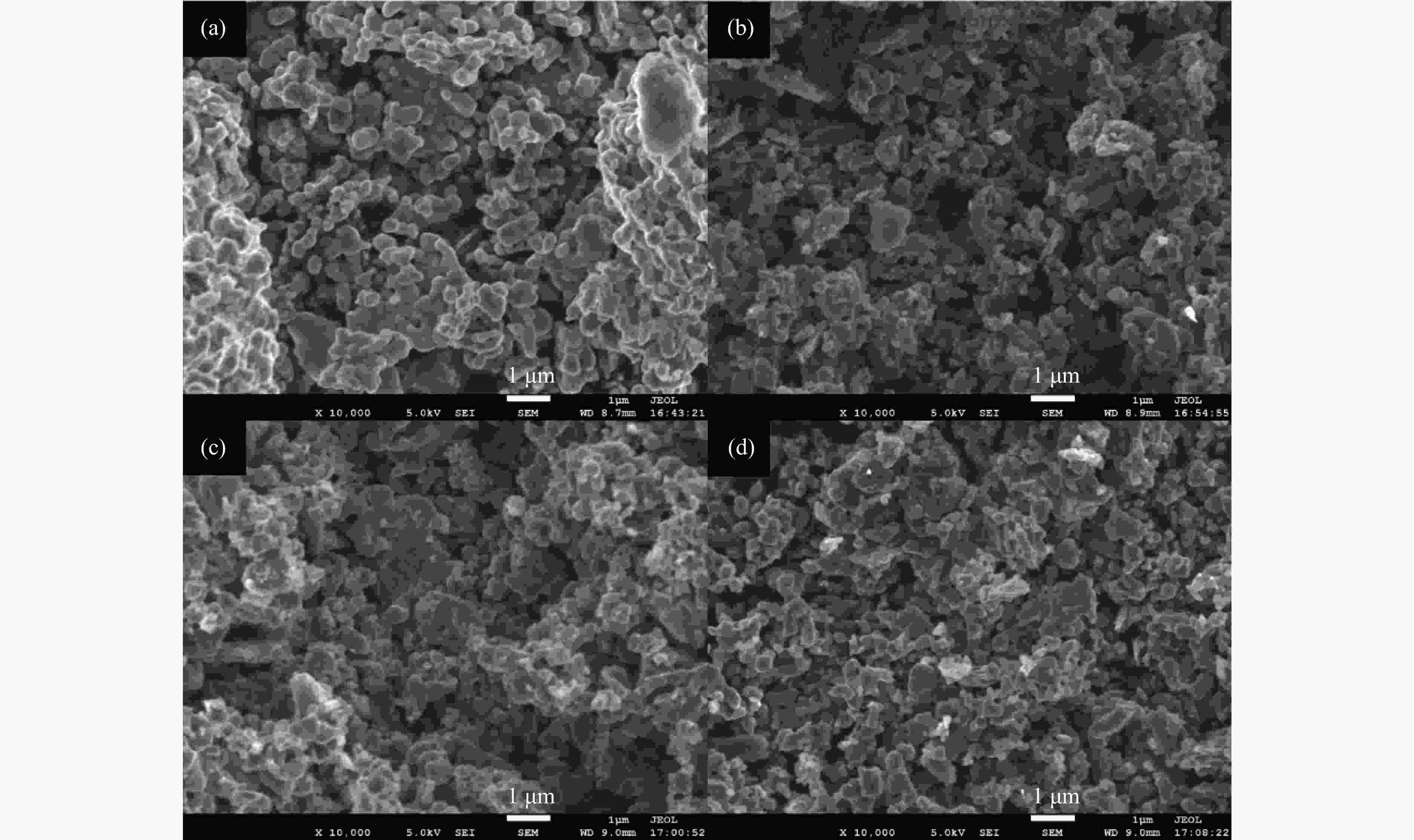
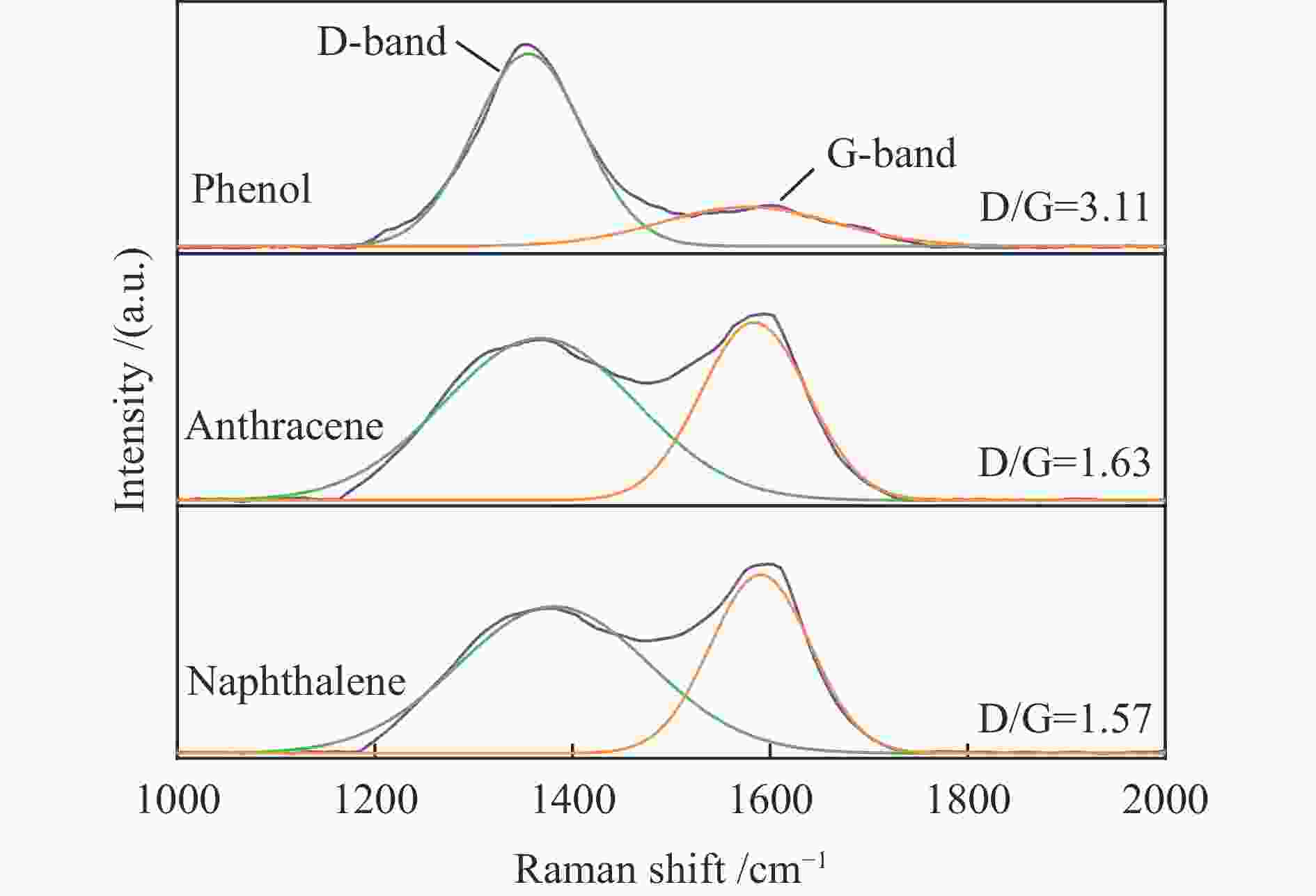
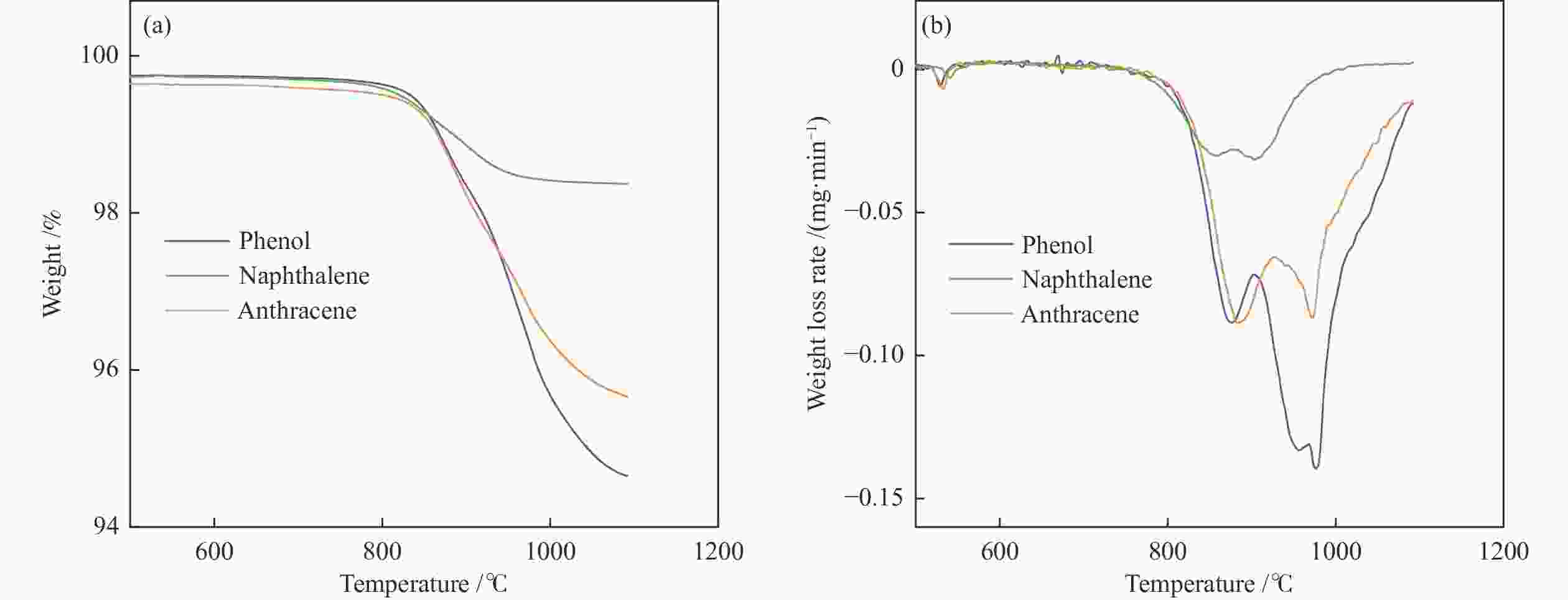
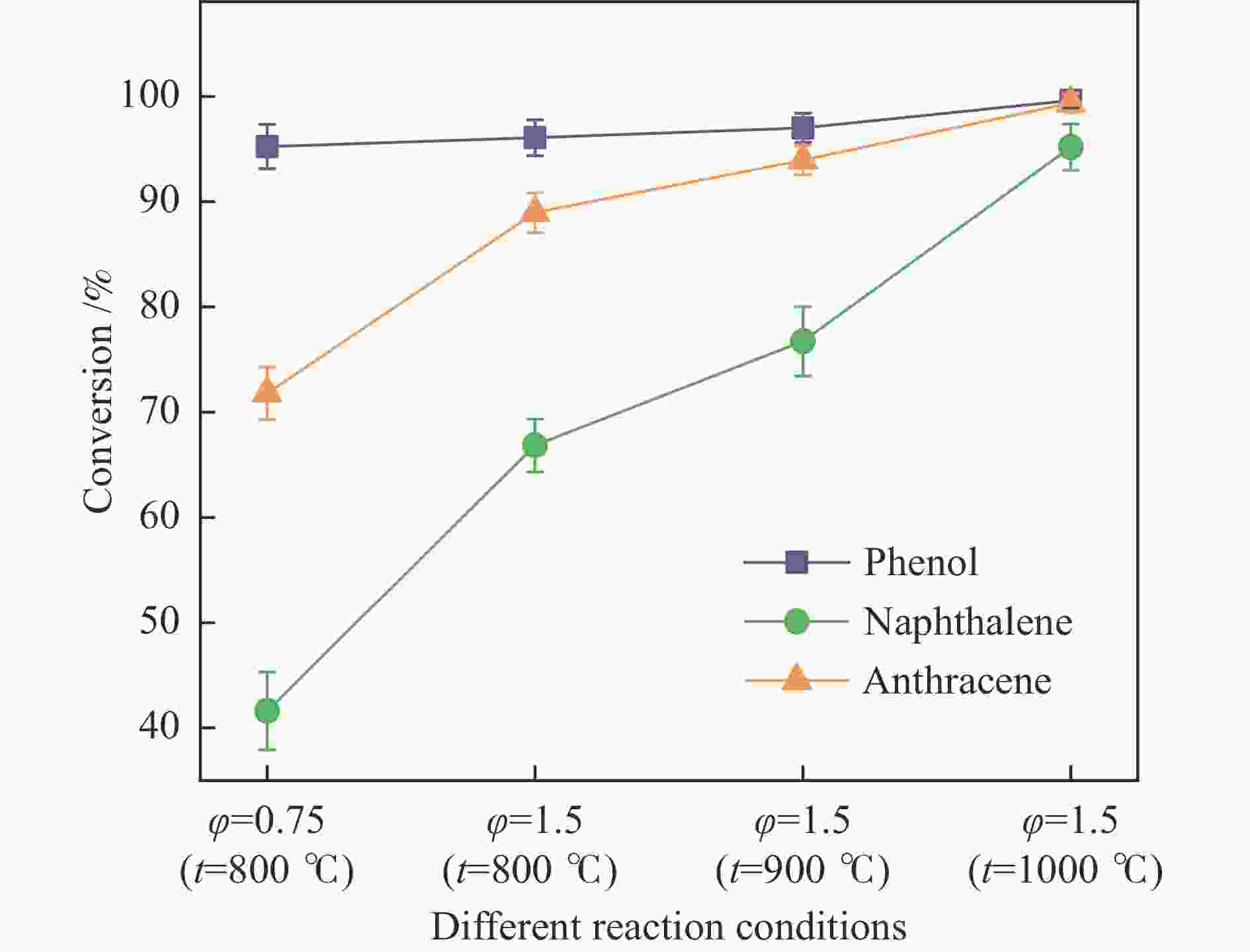
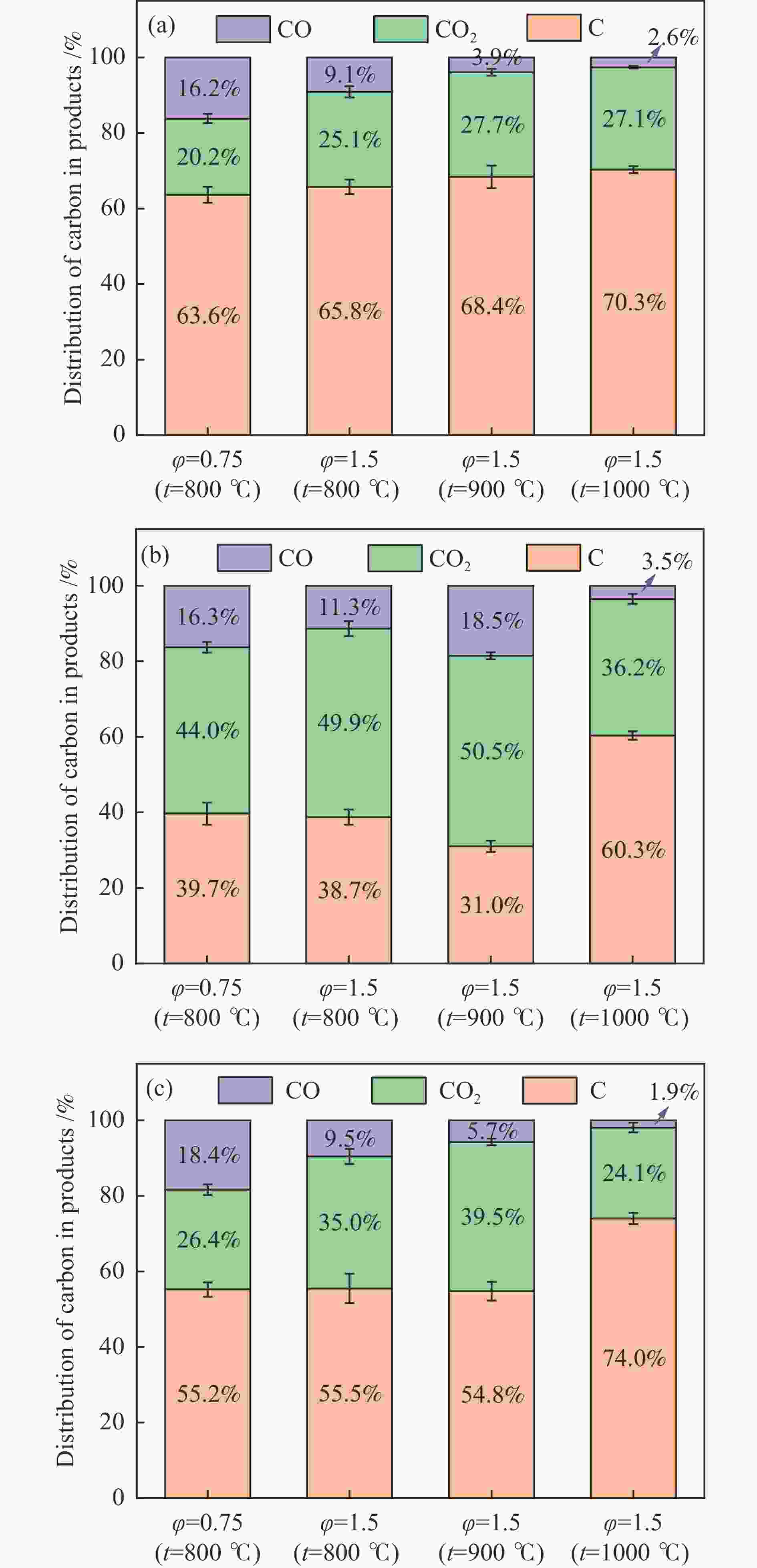
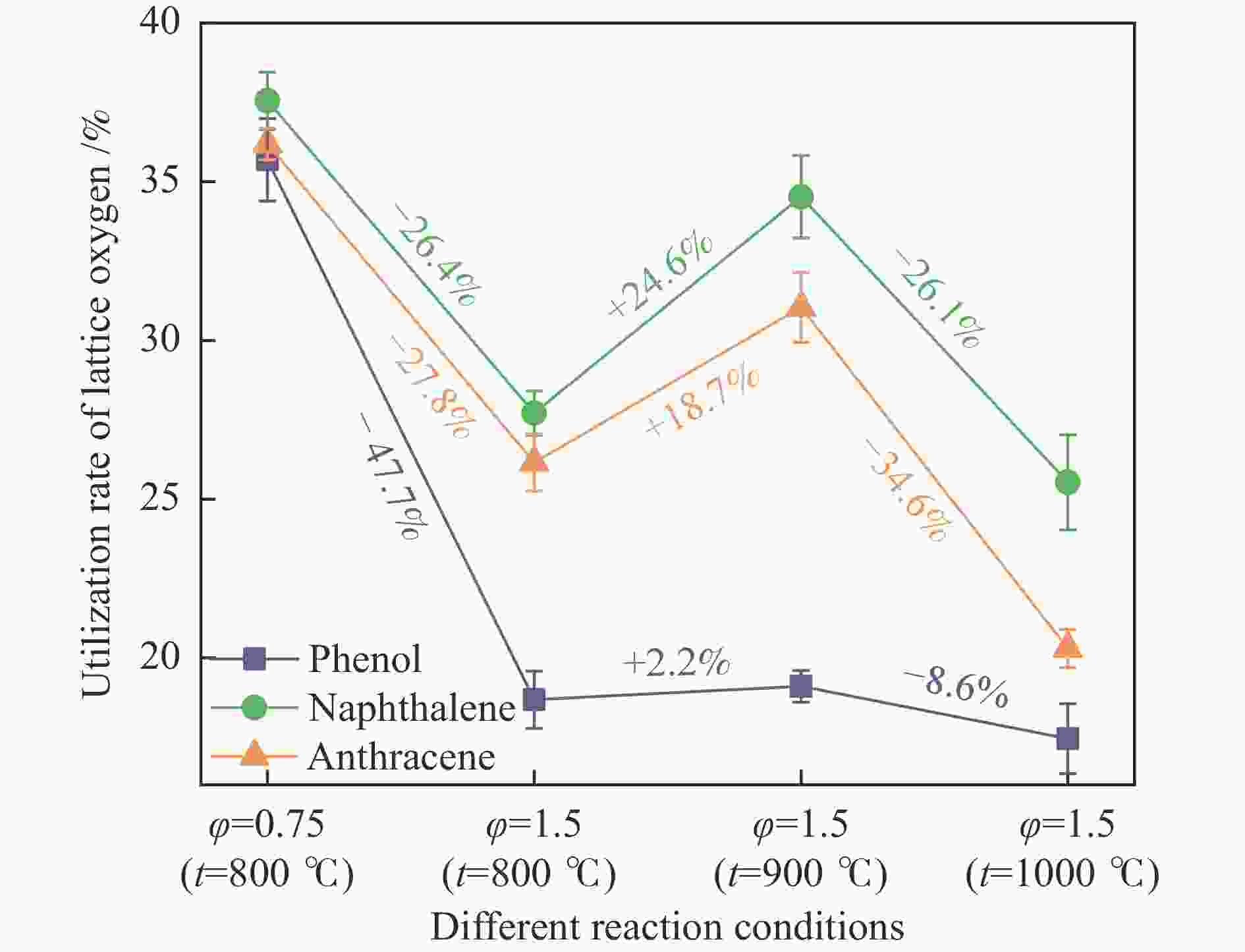
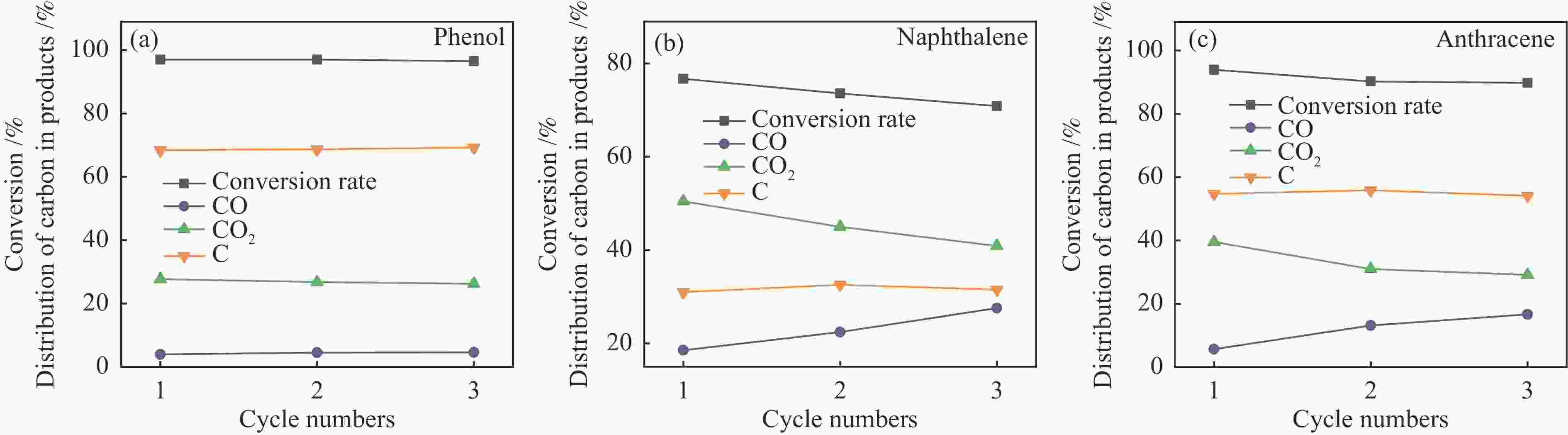
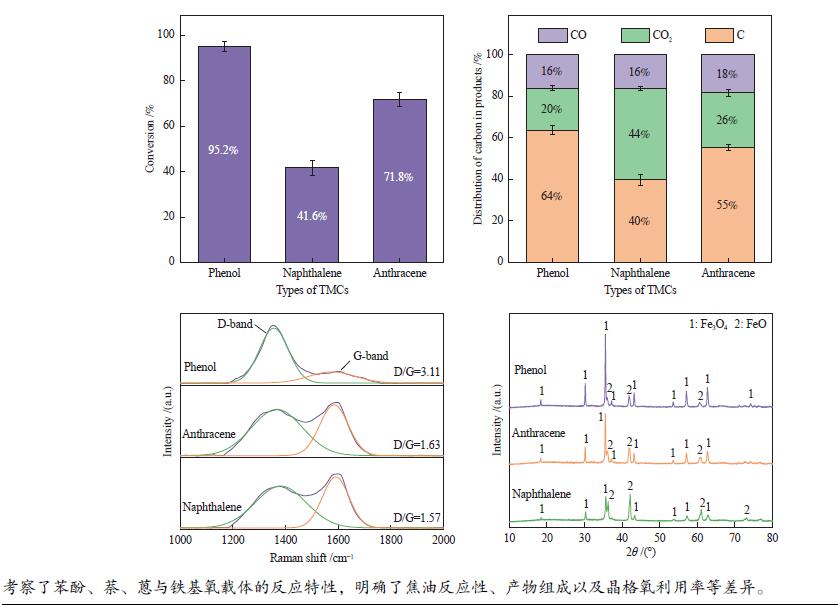
摘要: 本研究以生物质/煤的焦油模型化合物(TMCs)为研究对象,在两阶段固定床实验上探究了铁基氧载体(70%Fe2O3/30%Al2O3)对TMCs的转化特性,考察了不同TMCs的反应性及其转化的影响因素。研究发现,TMCs与氧载体的反应活性为:苯酚>蒽>萘,且苯酚转化生成积炭的比例最多(64%),而萘转化生成积炭的比例最少(40%);氧载体与萘的反应程度相对较高,但容易导致氧载体的烧结。此外,积炭表征显示萘生成的积炭在三种TMCs中具有最高的稳定性。增加氧载体的用量和提高反应温度不仅有利于萘和蒽的进一步转化,而且能够增加气相产物中CO2的分率。由于苯酚分子具有较高的反应活性及较强的裂解效果导致其转化率随氧载体用量和反应温度的增加变化较小,然而,较高的反应温度(1000 ℃)导致焦油发生严重的裂解现象并产生大量积炭。三次循环实验结果表明与萘反应的氧载体失活最为严重。Abstract: This work investigated the conversion characteristics of iron-based oxygen carrier (70%Fe2O3/30% Al2O3) with TMCs in a two-stage fixed-bed reactor using tar model compounds (TMCs) of biomass/coal, and evaluated the reactivity of different TMCs and the factors affecting their conversion. It was found that the reaction degree of TMCs with oxygen carrier was phenol>anthracene>naphthalene, and the conversion of phenol to carbon deposition was the highest (64%), while the conversion of naphthalene to carbon deposition was the lowest (40%); the degree of reaction between oxygen carriers and naphthalene was relatively high, but easily led to the sintering of oxygen carrier. Besides, activity characterization of the carbon deposition showed that the carbon deposition generated from naphthalene had the highest stability among the three TMCs. Increasing the amount of oxygen carrier and reaction temperature was beneficial to the further conversion of naphthalene and anthracene, and could also increase the fraction of CO2 in gaseous products. The high reaction activity and strong cracking effect of phenol led to a small change in the conversion rate with increasing the amount of oxygen carrier and reaction temperature. However, high reaction temperature (1000 ℃) could lead to severe cracking of the tar to generate a large amount of carbon deposition. The results of the cycle experiment showed that the oxygen carrier reacting with naphthalene was most severely deactivated.
-
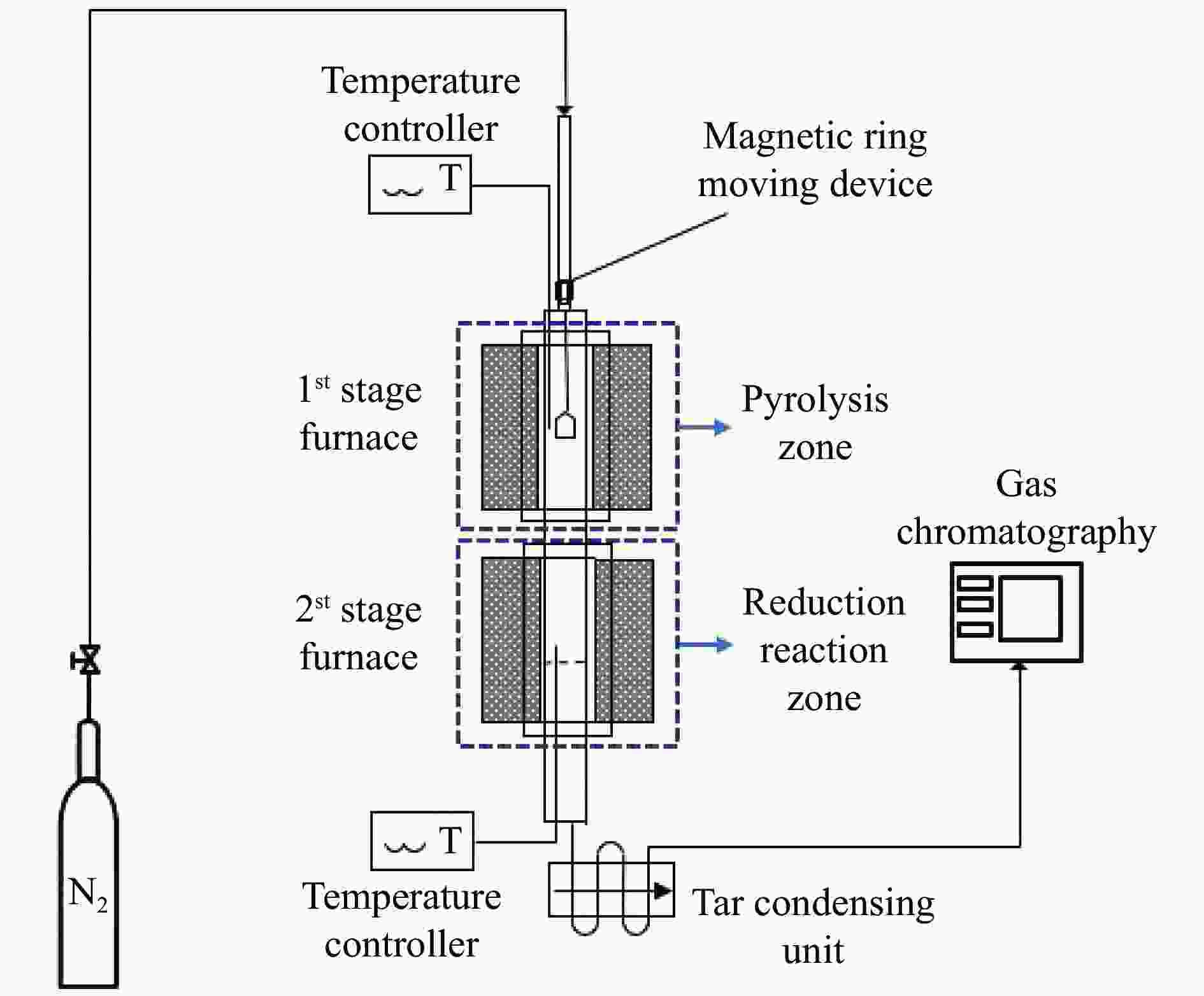
表 1 在两阶段固定床反应器中进行的实验操作参数
Table 1 Summary of experimental operating parameters carried out in the 2-stage fixed bed reactor
Operating condition 1st stage 2st stage Feedstock TMCs OC: 70% Fe2O3/30% Al2O3
(0.3−0.8 mm)Temperature /℃ 200 800, 900, 1000 Oxygen/carbon ratio (φ) φ= 0.75, 1.5
(Corresponding weight of OC = 1.25, 2.5 g)
(Corresponding bed height of OC = 0.25, 0.5 cm)Superficial velocity
Weight hourly space velocity (WHSV)0.007 m/s
0.047–0.093 h−1表 2 与相关TMCs反应前后氧载体的比表面积及孔分布
Table 2 Specific surface area and pore distribution of oxygen carriers before and after reaction with relevant TMCs
Sample Specific surface area
/(m2·g−1)Total pore volume
/(cm3·g−1)Pore size distribution micropore
(<2 nm,%)mesopore
(2–50 nm,%)macropore
(>50 nm,%)Fresh oxygen carrier 11.3 0.062 0.75 74.71 24.54 Reacted with phenol 12.5 0.055 1.34 75.38 23.28 Reacted with naphthalene 9.7 0.050 0.65 75.28 24.07 Reacted with anthracene 10.4 0.058 0.70 73.86 25.44 表 3 热重实验前后氧载体样品中碳的定量分析
Table 3 Quantitative analysis of carbon in oxygen carrier before and after thermogravimetric test
Phenol w/% Naphthalene w/% Anthracene w/% Before reaction 2.37 0.79 1.89 After reaction 0.37 0.22 0.29 表 4 TMCs在不同反应条件下的焦油产物
Table 4 Tar products of TMCs under different reaction conditions
TMCs Reaction products Peak area of various tar component ( × 106) φ = 0.75 (t=800 ℃) φ = 1.5 (t=800 ℃) φ = 1.5 (t=900 ℃) φ = 1.5 (t=1000 ℃) Phenol benzene 14.4 5.01 0.23 0.11 toluene 0.15 0.09 − − styrene 0.14 0.10 − − naphthalene 2.95 2.48 1.23 − Naphthalene benzene 7.79 7.76 3.31 0.26 toluene 0.15 0.09 − − styrene − 0.21 0.45 − naphthalene 258 224 121 0.03 1,1'-binaphthalene 3.33 5.58 3.34 − Anthracene benzene 3.08 3.96 4.65 − toluene 0.17 0.64 0.93 − styrene 0.12 0.41 0.52 − naphthalene 2.97 3.67 4.21 − anthracene 67.7 46.5 17.4 − -
[1] DANESHVAR E, WICKER R J, SHOW P L, BHATNAGAR A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization-A review[J]. Chem Eng J,2022,427:15. [2] TAN Y T, NOOKUEA W, LI H L, THORIN E, YAN J Y. Property impacts on Carbon Capture and Storage (CCS) processes: A review[J]. Energy Convers Manag,2016,118:204−222. doi: 10.1016/j.enconman.2016.03.079 [3] ADANEZ J, ABAD A. Chemical-looping combustion: Status and research needs[J]. Proc Combust Inst,2019,37(4):4303−4317. doi: 10.1016/j.proci.2018.09.002 [4] ADANEZ J, ABAD A, MENDIARA T, GAYAN P, DE DIEGO L F, GARCIA-LABIANO F. Chemical looping combustion of solid fuels[J]. Progr Energy Combust Sci,2018,65:6−66. doi: 10.1016/j.pecs.2017.07.005 [5] JIANG S C, DING W X, ZHAO K, HUANG Z, WEI G Q, FENG Y Y, LV Y J, HE F. Enhanced chemical looping oxidative coupling of methane by Na-doped LaMnO3 redox catalysts[J]. Fuel,2021,299:12. [6] WANG B W, ZHAO H B, ZHENG Y, LIU Z H, YAN R, ZHENG C G. Chemical looping combustion of a Chinese anthracite with Fe2O3-based and CuO-based oxygen carriers[J]. Fuel Process Technol,2012,96:104−115. doi: 10.1016/j.fuproc.2011.12.030 [7] SAHA C, BHATTACHARYA S. Comparison of CuO and NiO as oxygen carrier in chemical looping combustion of a Victorian brown coal[J]. Int J Hydrogen Energy,2011,36(18):12048−12057. doi: 10.1016/j.ijhydene.2011.06.065 [8] HUANG Z, DENG Z B, HE F, CHEN D Z, WEI G Q, ZHAO K, ZHENG A Q, ZHAO Z L, LI H B. Reactivity investigation on chemical looping gasification of biomass char using nickel ferrite oxygen carrier[J]. Int J Hydrogen Energy,2017,42(21):14458−14470. doi: 10.1016/j.ijhydene.2017.04.246 [9] KOBAYASHI N, FAN L S. Biomass direct chemical looping process: A perspective[J]. Biomass Bioenerg,2011,35(3):1252−1262. [10] JOSHI R K, SHAH V, FAN L S. Acetic acid production using calcium ferrite-assisted chemical looping gasification of petroleum coke with in situ sulfur capture[J]. Energy Fuels,2020,34(12):16560−16571. doi: 10.1021/acs.energyfuels.0c03408 [11] HU D H, HUANG J J, ZHAO J T, WANG Z Q, YU Z L, XIAO H C, BAI H C, LI C Y, FANG Y T. Reaction characteristics of Shenmu coal pyrolysis volatiles with an iron-based oxygen carrier in a two-stage fixed-bed reactor[J]. Fuel Process Technol,2022,235:9. [12] MENDIARA T, JOHANSEN J M, UTRILLA R, GERALDO P, JENSEN A D, GLARBORG P. Evaluation of different oxygen carriers for biomass tar reforming (I): Carbon deposition in experiments with toluene[J]. Fuel,2011,90(3):1049−1060. doi: 10.1016/j.fuel.2010.11.028 [13] CORBELLA B M, DE DIEGO L F, GARCIA-LABIANO F, ADANEZ J, PALACIOS J M. Characterization study and five-cycle tests in a fixed-bed reactor of titania-supported nickel oxide as oxygen carriers for the chemical-looping combustion of methane[J]. Environ Sci Technol,2005,39(15):5796−5803. doi: 10.1021/es048015a [14] ZHAO X, ZHOU H, SIKARWAR V S, ZHAO M, PARK A H A, FENNELL P S, SHEN L H, FAN L S. Biomass-based chemical looping technologies: the good, the bad and the future[J]. Energy Environ Sci,2017,10(9):1885−1910. doi: 10.1039/C6EE03718F [15] BOOT-HANDFORD M E, FLORIN N, FENNELL P S. Investigations into the effects of volatile biomass tar on the performance of Fe-based CLC oxygen carrier materials[J]. Environ Res Lett,2016,11(11):10. [16] WANG C P, YAN H, YU Y B, LIANG W Z, YUAN S R, CUI W W, WANG F Y, BAI H C, HU X D. Chemical looping reforming of coal Tar vapor on the surface of CaO-modified Fe-based oxygen carrier[J]. Energy Fuels,2020,34(7):8534−8542. doi: 10.1021/acs.energyfuels.0c00839 [17] WEI G Q, HUANG J, FAN Y Y, HUANG Z, ZHENG A Q, HE F, MENG J G, ZHANG D Y, ZHAO K, ZHAO Z L, LI H B. Chemical looping reforming of biomass based pyrolysis gas coupling with chemical looping hydrogen by using Fe/Ni/Al oxygen carriers derived from LDH precursors[J]. Energy Convers Manag,2019,179:304−313. doi: 10.1016/j.enconman.2018.10.065 [18] NAM H, WANG Z H, SHANMUGAM S R, ADHIKARI S, ABDOULMOUMINE N. Chemical looping dry reforming of benzene as a gasification tar model compound with Ni- and Fe-based oxygen carriers in a fluidized bed reactor[J]. Int J Hydrogen Energy,2018,43(41):18790−18800. doi: 10.1016/j.ijhydene.2018.08.103 [19] HUANG Z, DENG Z B, FEN Y H, CHEN T J, CHEN D Z, ZHENG A Q, WEI G Q, HE F, ZHAO Z L, WU J H, LI H B. Exploring the conversion mechanisms of toluene as a biomass tar model compound on NiFe2O4 oxygen carrier[J]. ACS Sustainable Chem Eng,2019,7(19):16539−16548. doi: 10.1021/acssuschemeng.9b03831 [20] HUANG Z, ZHENG A Q, DENG Z B, WEI G Q, ZHAO K, CHEN D Z, HE F, ZHAO Z L, LI H B, LI F X. In-situ removal of toluene as a biomass tar model compound using NiFe2O4 for application in chemical looping gasification oxygen carrier[J]. Energy,2020,190:12. [21] ZENG J M, HU J W, QIU Y, ZHANG S, ZENG D W, XIAO R. Multi-function of oxygen carrier for in-situ tar removal in chemical looping gasification: Naphthalene as a model compound[J]. Appl Energy,2019,253:8. [22] GREWAL A, ABBEY L, GUNUPURU L R. Production, prospects and potential application of pyroligneous acid in agriculture[J]. Anal Appl Pyrolysis,2018,135:152−159. doi: 10.1016/j.jaap.2018.09.008 [23] LIN Y, WANG H T, HUANG Z, LIU M, WEI G Q, ZHAO Z L, LI H B, FANG Y T. Chemical looping gasification coupled with steam reforming of biomass using NiFe2O4: Kinetic analysis of DAEM-TI, thermodynamic simulation of OC redox, and a loop test[J]. Chem Eng J,2020,395:12. [24] LIU T, YU Z L, LI G, GUO S, SHAN J, LI C Y, FANG Y T. Performance of potassium-modified Fe2O3/Al2O3 oxygen carrier in coal-direct chemical looping hydrogen generation[J]. Int Hydrogen Energy,2018,43(42):19384−19395. doi: 10.1016/j.ijhydene.2018.08.089 [25] LIND F, SEEMANN M, THUNMANI H. Continuous catalytic tar reforming of biomass derived raw gas with simultaneous catalyst regeneration[J]. Ind Eng Chem Res,2011,50(20):11553−11562. doi: 10.1021/ie200645s [26] MENG J Q, ZHAO Z L, WANG X B, CHEN J, ZHENG A Q, HUANG Z, WEI G Q, LI H B. Steam reforming and carbon deposition evaluation of phenol and naphthalene used as tar model compounds over Ni and Fe olivine-supported catalysts[J]. J Energy Inst,2019,92(6):1765−1778. doi: 10.1016/j.joei.2018.12.004 [27] CHEN B, SHI Z M, JIANG S J, TIAN H. Catalytic cracking mechanisms of tar model compounds[J]. J Central South Univ,2016,23(12):3100−3107. doi: 10.1007/s11771-016-3375-7 [28] LI Q W, YAN H, ZHANG J, LIU Z F. Effect of hydrocarbons precursors on the formation of carbon nanotubes in chemical vapor deposition[J]. Carbon,2004,42(4):829−835. doi: 10.1016/j.carbon.2004.01.070 [29] MA S W, CHEN S Y, SOOMRO A, XIANG W G. Effects of supports on hydrogen production and carbon deposition of Fe-based oxygen carriers in chemical looping hydrogen generation[J]. Int J Hydrogen Energy,2017,42(16):11006−11016. doi: 10.1016/j.ijhydene.2017.02.132 [30] ROBERTSON J. Diamond-like amorphous carbon[J]. Mater Sci Eng: R-Rep,2022,37(4/6):129−281. [31] HUANG X, WANG X J, FAN M H, WANG Y G, ADIDHARMA H, GASEM K A M, RADOSZ M. A cost-effective approach to reducing carbon deposition and resulting deactivation of oxygen carriers for improvement of energy efficiency and CO2 capture during methane chemical-looping combustion[J]. Appl Energy,2017,193:381−392. doi: 10.1016/j.apenergy.2017.02.059 [32] SPRAGG J, MAHMUD T, DUPONT V. Hydrogen production from bio-oil: A thermodynamic analysis of sorption-enhanced chemical looping steam reforming[J]. Int J Hydrogen Energy,2018,43(49):22032−22045. doi: 10.1016/j.ijhydene.2018.10.068 [33] ZHU J, WANG W, LIAN S J, HUA X N, XIA Z. Stepwise reduction kinetics of iron-based oxygen carriers by CO/CO2 mixture gases for chemical looping hydrogen generation[J]. J Mater Cycles Waste Manag,2017,19(1):453−462. doi: 10.1007/s10163-015-0443-2 [34] WANG S, SONG T, YIN S Y, HARTGE E U, DYMALA T, SHEN L H, HEINRICH S, WERTHER J. Syngas, tar and char behavior in chemical looping gasification of sawdust pellet in fluidized bed[J]. Fuel,2020,270:11. [35] LINDERHOLM C, MATTISSON T, LYNGFELT A. Long-term integrity testing of spray-dried particles in a 10-kW chemical-looping combustor using natural gas as fuel[J]. Fuel,2009,88(11):2083−2096. doi: 10.1016/j.fuel.2008.12.018 [36] SONG H B, WANG W, SUN J C, WANG X H, ZHANG X H, CHEN S, PEI C L, ZHAO Z J. Chemical looping oxidative propane dehydrogenation controlled by oxygen bulk diffusion over FeVO4 oxygen carrier pellets[J]. Chin J Chem Eng,2023,53:409−420. doi: 10.1016/j.cjche.2022.10.006 [37] MIN Z H, YIMSIRI P, ASADULLAH M, ZHANG S, LI C Z. Catalytic reforming of tar during gasification. Part II. Char as a catalyst or as a catalyst support for tar reforming[J]. Fuel,2011,90(7):2545−2552. doi: 10.1016/j.fuel.2011.03.027 [38] HUANG Z, HE F, ZHENG A Q, ZHAO K, CHANG S, ZHAO Z L, LI H B. Synthesis gas production from biomass gasification using steam coupling with natural hematite as oxygen carrier[J]. Energy,2013,53:244−251. doi: 10.1016/j.energy.2013.02.068 [39] LI C S, SUZUKI K. Tar property, analysis, reforming mechanism and model for biomass gasification-An overview[J]. Renewable Sustainable Energy Rev,2009,13(3):594−604. doi: 10.1016/j.rser.2008.01.009 [40] XU R Y, ZHU F S, ZHANG H, RUYA P M, KONG X Z, LI L, LI X D. Simultaneous removal of toluene, naphthalene, and phenol as tar surrogates in a rotating gliding arc discharge reactor[J]. Energy Fuels,2020,34(2):2045−2054. doi: 10.1021/acs.energyfuels.9b03529 [41] SUN Z ZHANG X H, LI H F, LIU T, SANG S E, CHEN S Y, DUAN L B, ZENG L, XIANG W G, GONG J L. Chemical looping oxidative steam reforming of methanol: A new pathway for auto-thermal conversion[J]. Appl Catal B: Environ,2020,269:12. [42] KOIKE M, LI D, WATANABE H, NAKAGAWA Y, TOMISHIGE K. Comparative study on steam reforming of model aromatic compounds of biomass tar over Ni and Ni-Fe alloy nanoparticles[J]. Appl Catal A: Gen,2015,506:151−162. doi: 10.1016/j.apcata.2015.09.007 -




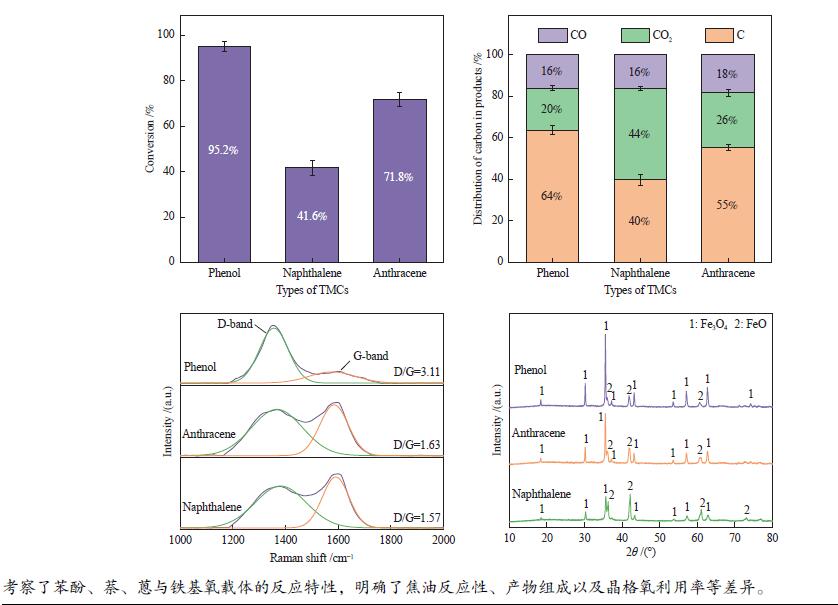
 下载:
下载: