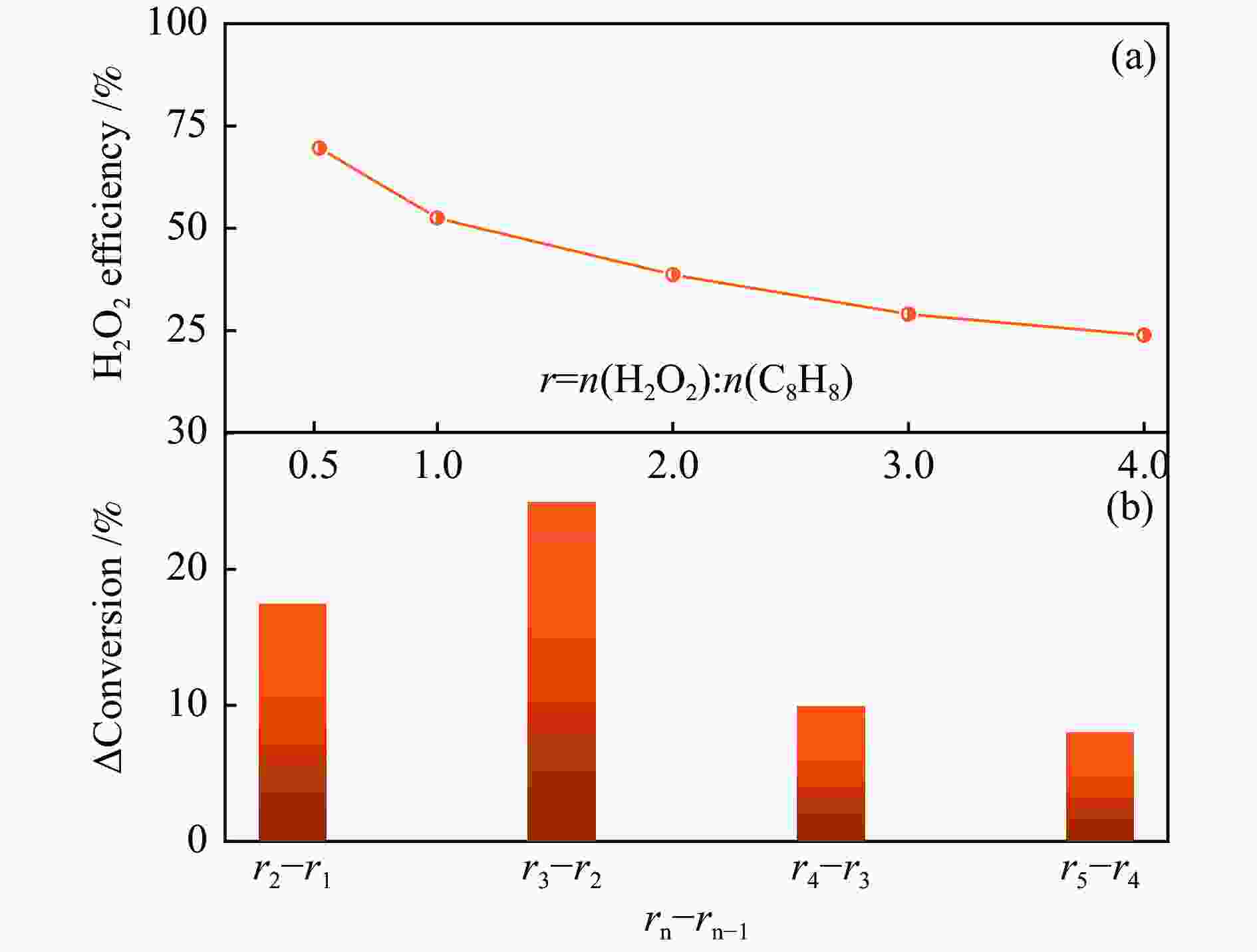
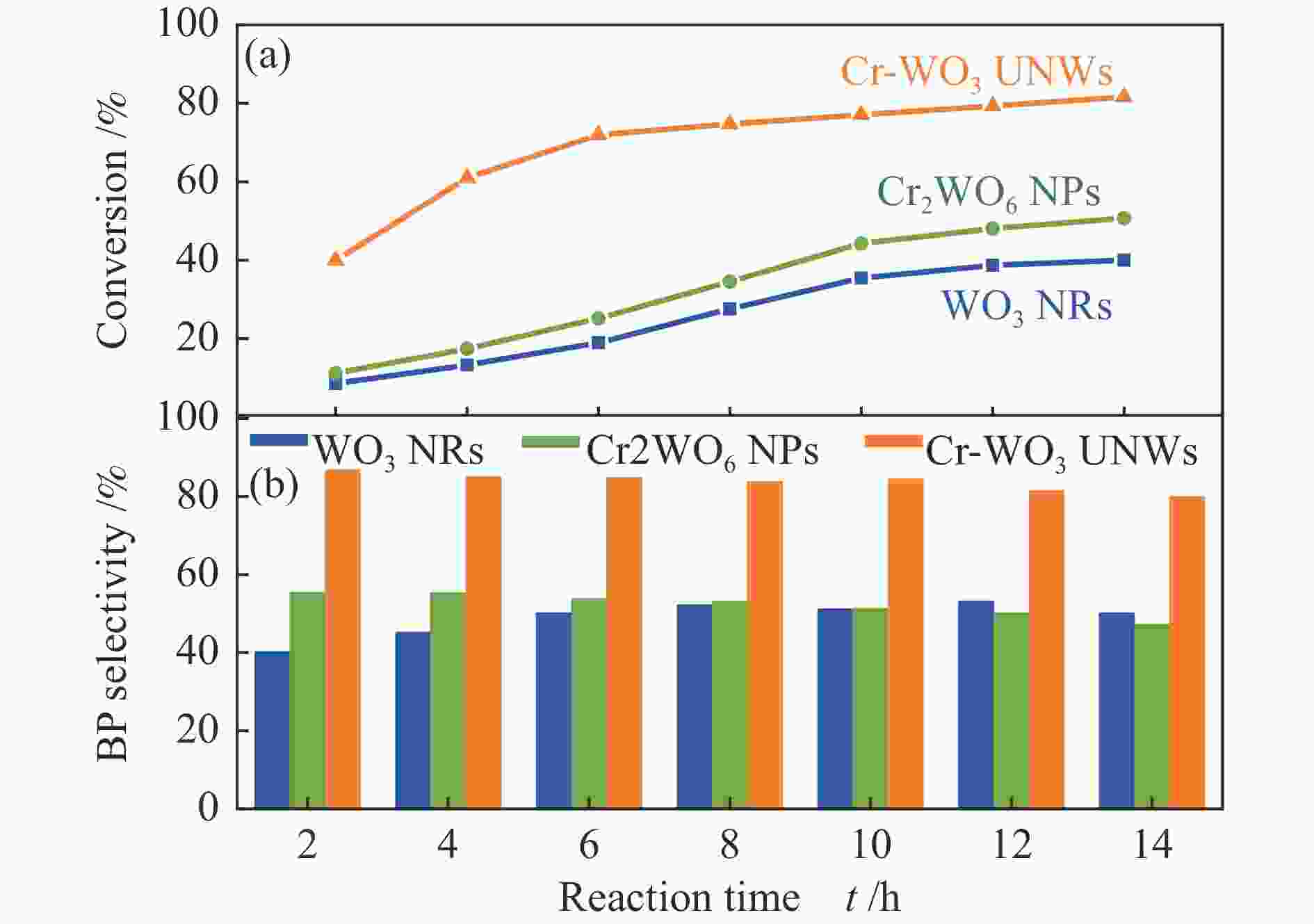
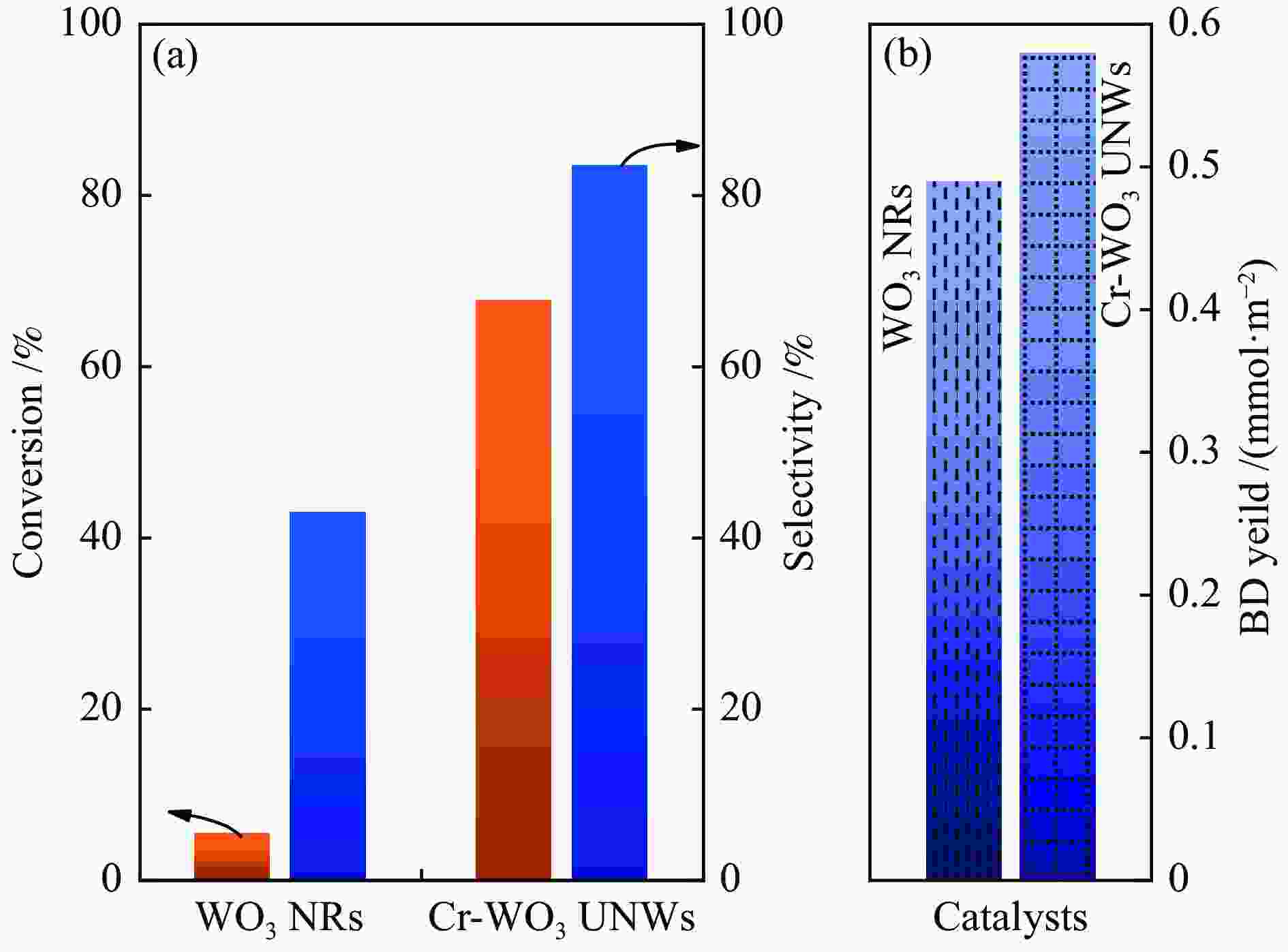
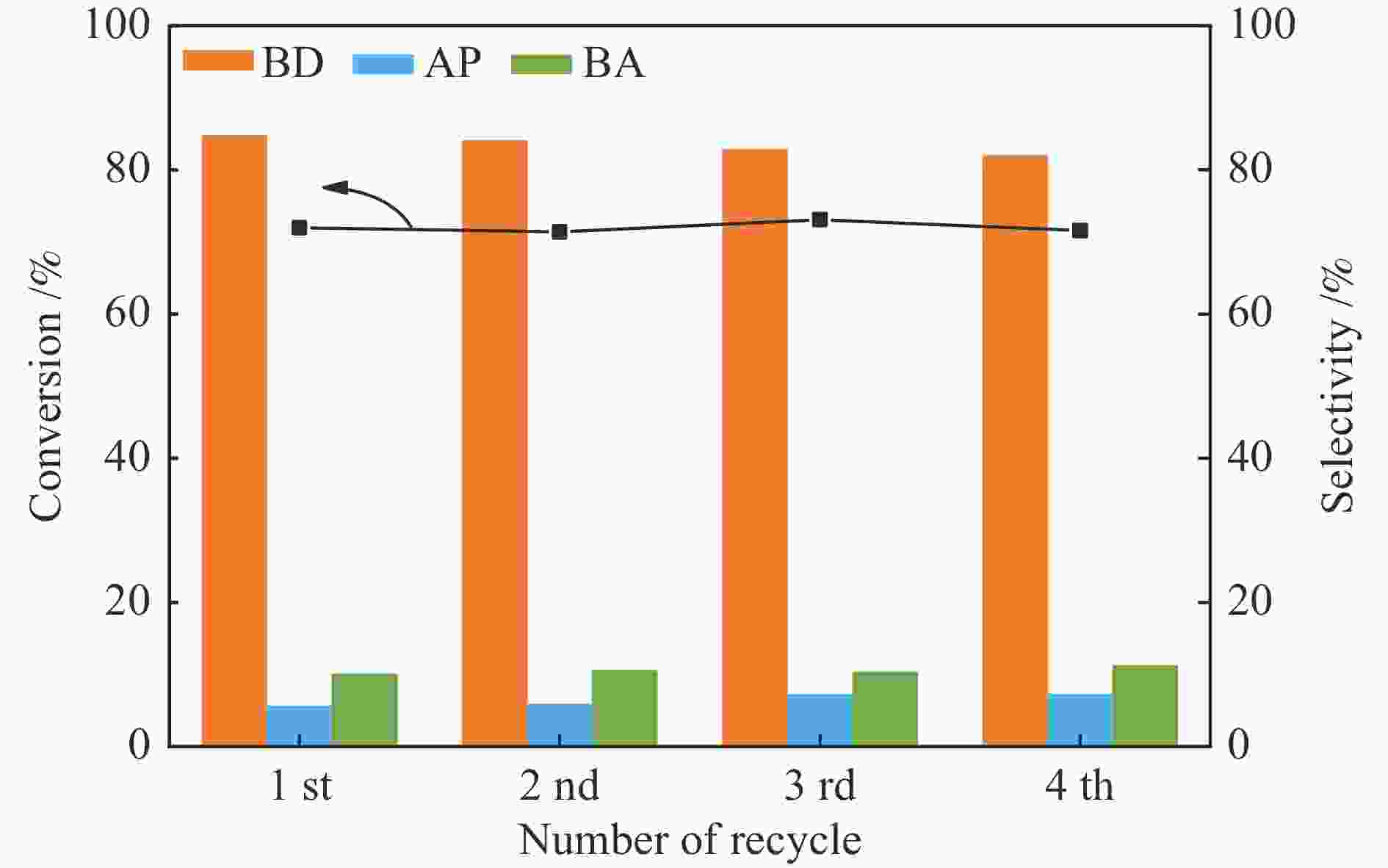
| [1] |
ANDAS J, EKHBAL S H, ALI T H. MCM-41 modified heterogeneous catalysts from rice husk for selective oxidation of styrene into benzaldehyde[J]. Environ Technol Innovation, 2021, 21: 101308.
|
| [2] |
FLORES J G, DÍAZ-GARCÍA M, IBARRA I A, AGUILAR-PLIEGO J, SANCHEZ-SANCHEZ M. Sustainable M-MOF-74 (M=Cu, Co, Zn) prepared in methanol as heterogeneous catalysts in the synthesis of benzaldehyde from styrene oxidation[J]. J Solid State Chem, 2021, 298: 122151 (p8).
|
| [3] |
王彬, 房克功, 陈建刚, 孙予罕. VOx/SBA-15催化剂上甲苯气相部分氧化[J]. 燃料化学学报,2008,36(1):94−98.WANG Shan, FANG Ke-gong, CHEN Jian-gang, SUN Yu-han. Partial oxidation of toluene over VOx/SBA-15 catalyst[J]. J Fuel Chem Technol,2008,36(1):94−98.
|
| [4] |
GUAN X, DUAN C D, WANG H X, LU B, ZHAO J X, CAI Q H. Tuneable oxidation of styrene to benzaldehyde and benzoic acid over Co/ZSM-5[J]. New J Chem,2021,45(38):18192−18201. doi: 10.1039/D1NJ03145G
|
| [5] |
张旭, 张利雄, 徐南平. 苯乙烯氧化合成环氧苯乙烷和苯甲醛催化剂的研究进展[J]. 石油化工,2009,38(2):215−220. doi: 10.3321/j.issn:1000-8144.2009.02.020ZHANG Xu, ZHANG Li-xiong, XU Nan-ping. Advances in development of catalysts for selective oxidation of styrene to styrene oxide and benzaldehyde[J]. Petrochem Technol,2009,38(2):215−220. doi: 10.3321/j.issn:1000-8144.2009.02.020
|
| [6] |
ANDRADE M A, MARTINS L M D R S. Selective styrene oxidation to benzaldehyde over recently developed heterogeneous catalysts[J]. Molecules, 2021, 26(6): 1680 (p38).
|
| [7] |
YANG X J, WANG H X, LI S N, LU B, ZHAO J X, CAI Q H. Fe3O4/g-C3N4-CeOx fabricated by in situ-reduction towards solvent-free oxidation of styrene to benzaldehyde[J]. Colloids Surf A, 2021, 616: 126309.
|
| [8] |
GUIN D, BARUWATI B, MANORAMA S V. A simple chemical synthesis of nanocrystalline AFe2O4 (A=Fe, Ni, Zn): An efficient catalyst for selective oxidation of styrene[J]. J Mol Catal A: Chem, 2005, 242(1/2): 26–31.
|
| [9] |
PARDESHI S K, PAWAR R Y. Optimization of reaction conditions in selective oxidation of styrene over fine crystallite spinel-type CaFe2O4 complex oxide catalyst[J]. Mater Res Bull,2010,45(5):609−615. doi: 10.1016/j.materresbull.2010.01.011
|
| [10] |
PARDESHI S K, PAWAR R Y. SrFe2O4 complex oxide an effective and environmentally benign catalyst for selective oxidation of styrene[J]. J Mol Catal A: Chem, 2011, 334(1/2): 35–43.
|
| [11] |
CONG S, GENG F X, ZHAO Z G. Tungsten oxide materials for optoelectronic applications[J]. Adv Mater,2016,28(47):10518−10528. doi: 10.1002/adma.201601109
|
| [12] |
LONG H W, ZENG W, ZHANG H. Synthesis of WO3 and its gas sensing: A review[J]. J Mater Sci: Mater in Electron,2015,26(7):4698−4707. doi: 10.1007/s10854-015-2896-4
|
| [13] |
LWIN S, WACHS I E. Olefin metathesis by supported metal oxide catalysts[J]. ACS Catal,2014,4(8):2505−2520. doi: 10.1021/cs500528h
|
| [14] |
GOYAL R, SARKAR B, SAMEER S, BAG A, BORDOLOI A. Ag and WOx nanoparticles embedded in mesoporous SiO2 for cyclohexane oxidation[J]. ACS Appl Nano Mater,2019,2(9):5989−5999. doi: 10.1021/acsanm.9b01430
|
| [15] |
YAN W J, ZHANG G Y, YAN H, LIU Y B, CHEN X B, FENG X, JIN X, YANG C H. Liquid-phase epoxidation of light olefins over W and Nb nanocatalysts[J]. ACS Sustainable Chem Eng,2018,6(4):4423−4452. doi: 10.1021/acssuschemeng.7b03101
|
| [16] |
李伟, 迟克彬, 马怀军, 刘浩, 曲炜, 田志坚. 载体对Pt/WO3-ZrO2催化临氢异构反应性能的影响[J]. 燃料化学学报,2017,45(3):329−336.LI Wei, CHI Ke-bin, MA Huai-jun, LIU Hao, QU Wei, TIAN Zhi-jian. Effect of supports on the catalytic performance of Pt/WO3-ZrO2 catalysts for hydroisomerization[J]. J Fuel Chem Technol,2017,45(3):329−336.
|
| [17] |
KANAN S M, LU Z, COX J K, BERNHARDT G, TRIPP C P. Identification of surface sites on monoclinic WO3 powders by infrared spectroscopy[J]. Langmuir,2002,18(5):1707−1712. doi: 10.1021/la011428u
|
| [18] |
XING X Y, WANG H X, SHI J, LI P H, REN J Z, WANG L C, ZHANG J L, LIU Z, LV B L. Heterogeneous catalyst with oxygen vacancies and deficient Brönsted acid for epoxidation of 1-hexene[J]. Catal Lett,2022,153:1180−1192.
|
| [19] |
余勇, 刘士军, 李洁, 陈启元. 氧化钨介孔材料的制备与表征[J]. 物理化学学报,2009,25(9):1890−1894. doi: 10.3866/PKU.WHXB20090914YU Yong, LIU Shi-jun, LI Jie, CHEN Qi-yuan. Preparation and characterization of mesoporous tungsten oxides[J]. Acta Phys-Chim Sin,2009,25(9):1890−1894. doi: 10.3866/PKU.WHXB20090914
|
| [20] |
LI P H, GAO J H, SHI J, WANG H X, XING X Y, REN J Z, MENG Y, WANG L C, LV B L. Insights into the effect of oxygen vacancies on the epoxidation of 1-hexene with hydrogen peroxide over WO3-x/SBA-15[J]. Catal Sci Technol,2022,12(22):6827−6837. doi: 10.1039/D2CY01123A
|
| [21] |
邢向英, 王会香, 王连成, 吕宝亮. Co2 + 调控WOx表面Brönsted 酸和空位含量用于提高1-己烯环氧化性能[J]. 燃料化学学报,2022,50(11):1480−1490.XING Xiang-ying, WANG Hui-xiang, WANG Lian-cheng, LÜ Bao-liang. Regulation of Co2 + cations on the content of Brönsted acid site and oxygen vacancy of WOx to improve the epoxidation performance of 1-hexene[J]. J Fuel Chem Technol,2022,50(11):1480−1490.
|
| [22] |
SHI J, XING X Y, WANG H X, GE L, SUN H Z LV B L. Oxygen vacancy enriched Cu-WO3 hierarchical structures for the thermal decomposition of ammonium perchlorate[J]. Inorg Chem Front,2022,9(1):136−145. doi: 10.1039/D1QI01027A
|
| [23] |
大连理工大学无机化学教研室. 无机化学第五版[M]. 北京: 高等教育出版社, 2006: 564–570.Department of Inorganic Chemistry, Dalian University of Technology. Inorganic Chemistry with Fifth Edition[M]. Beijing: Higher Education Press, 2006: 564–570.
|
| [24] |
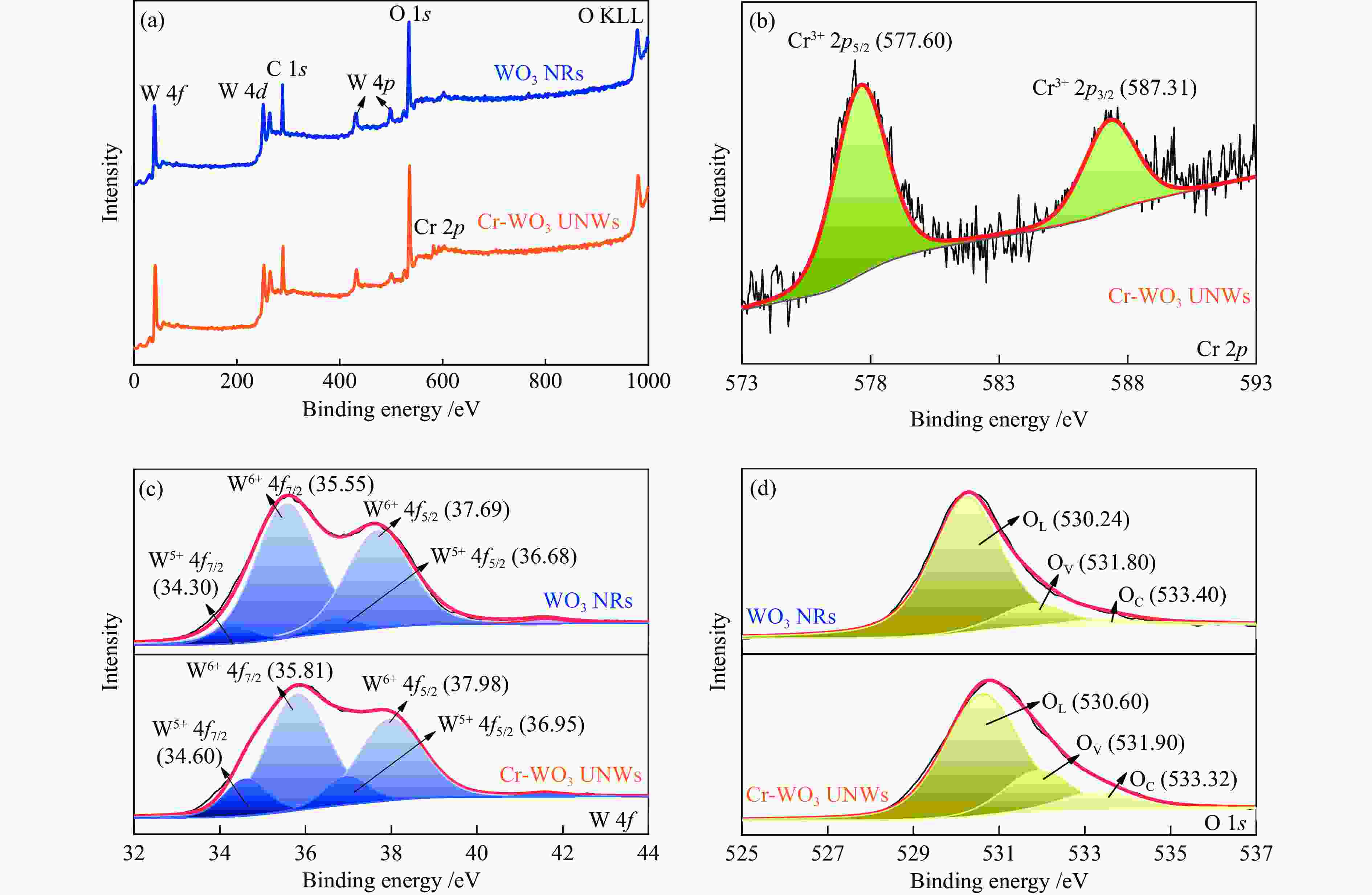
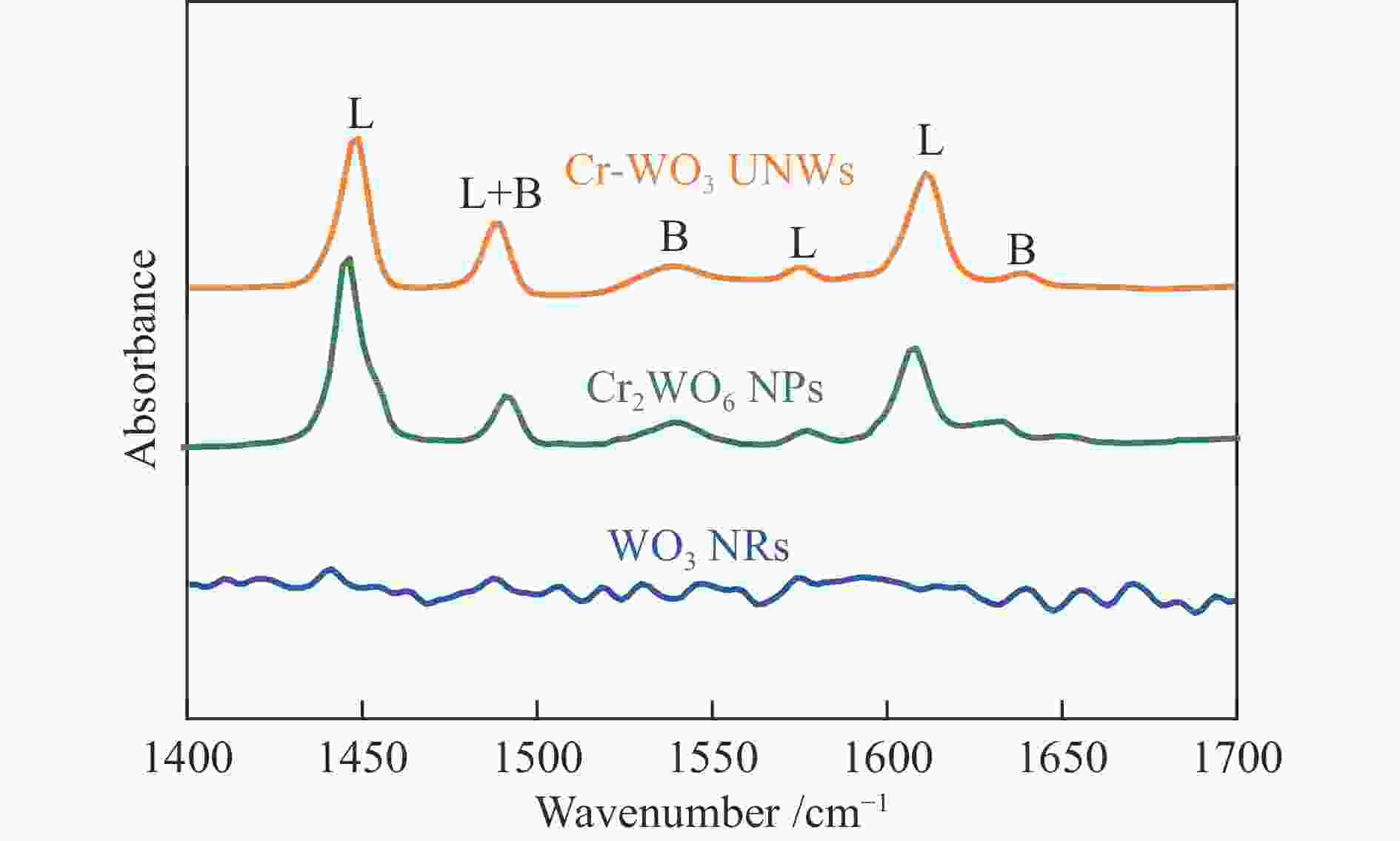
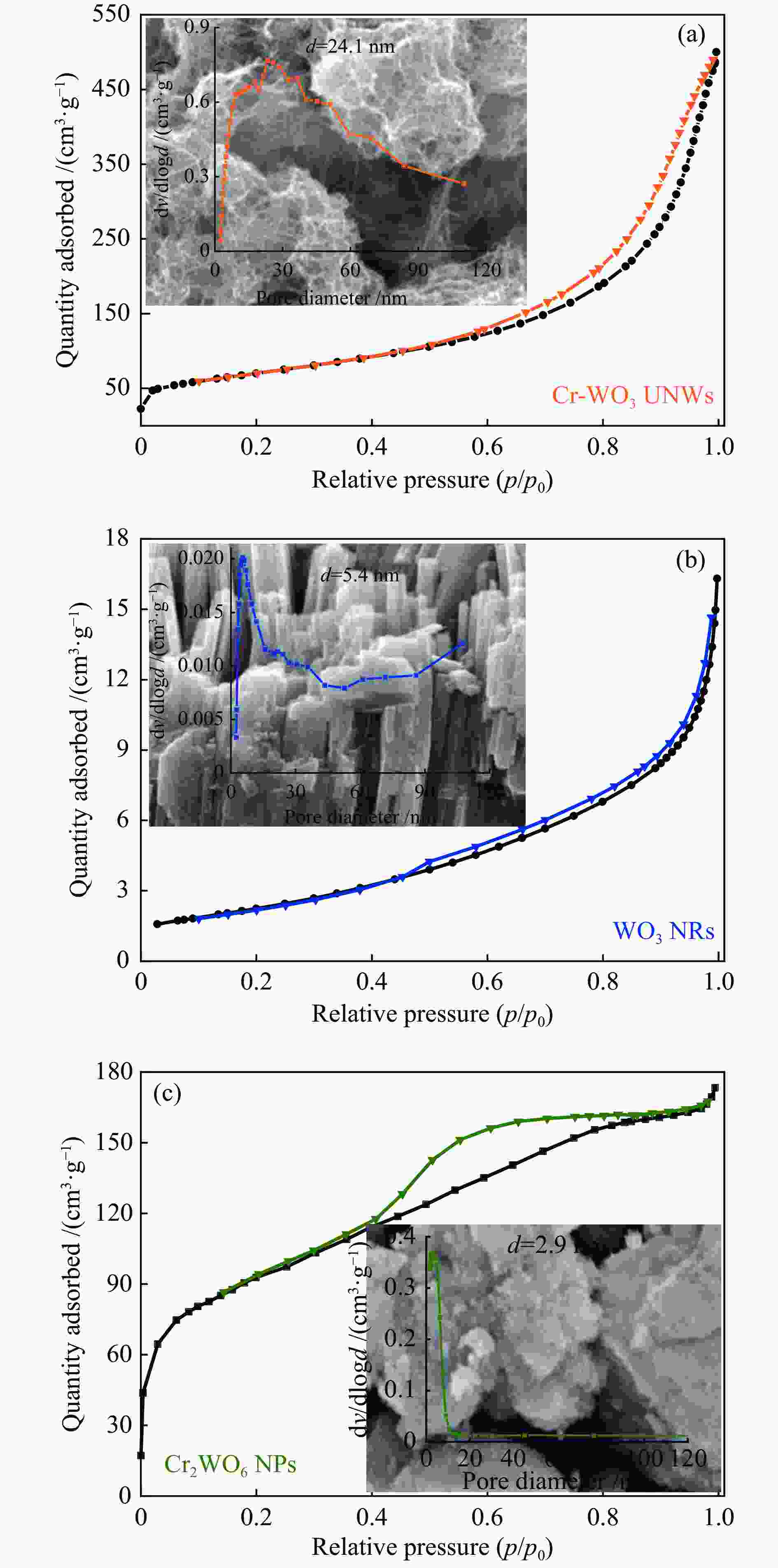
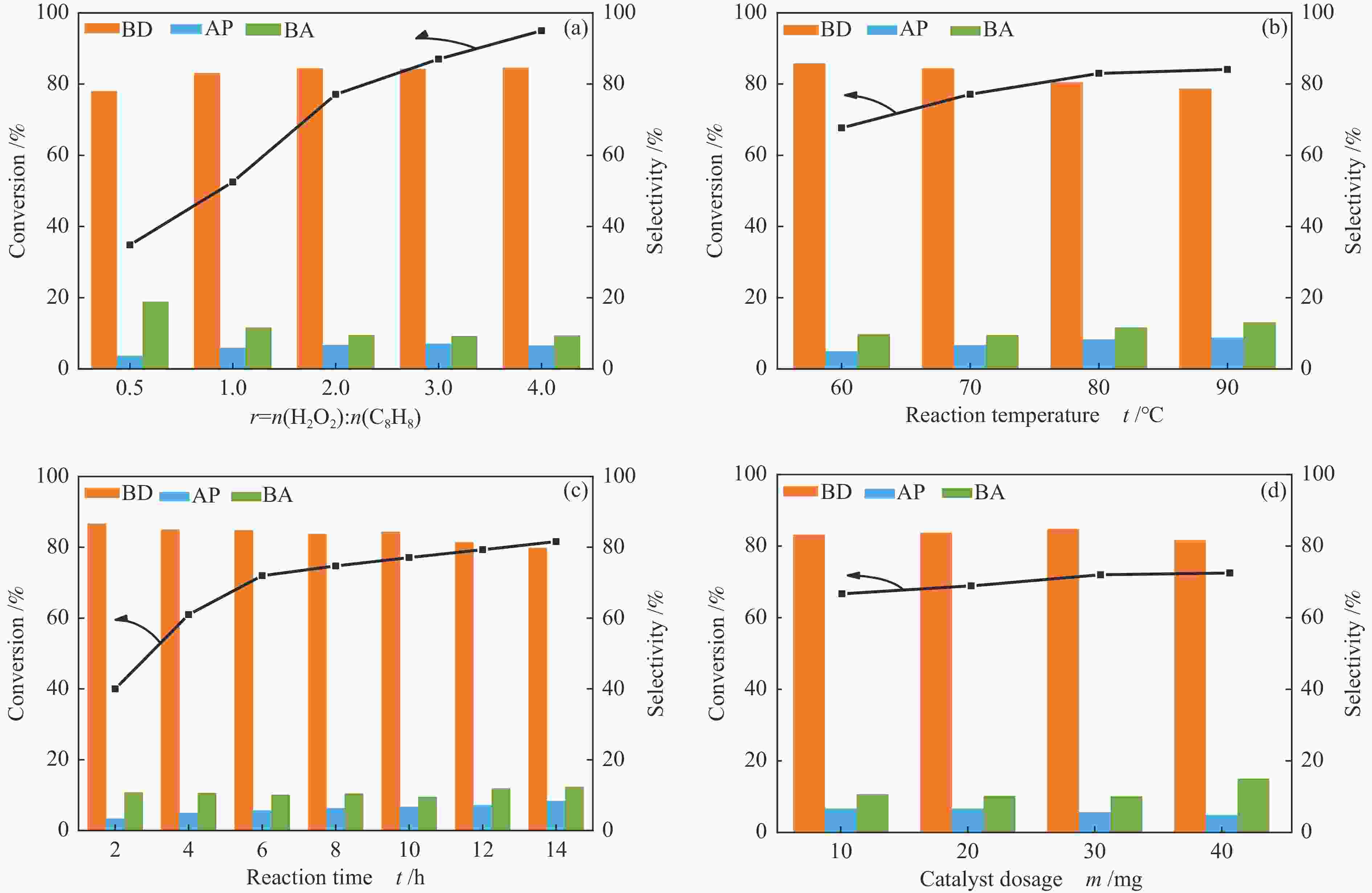
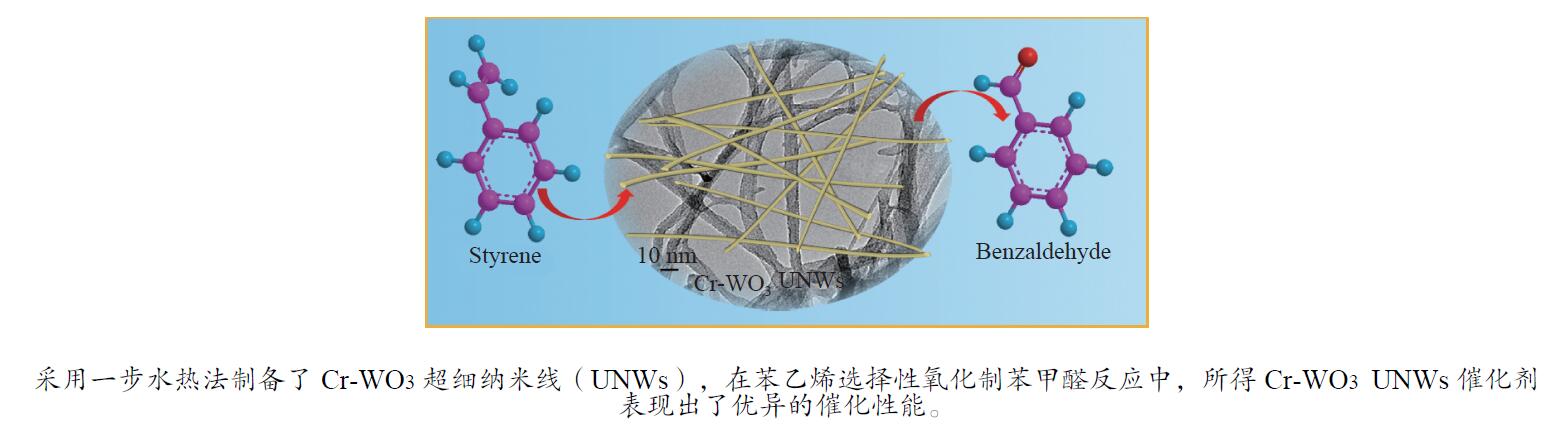
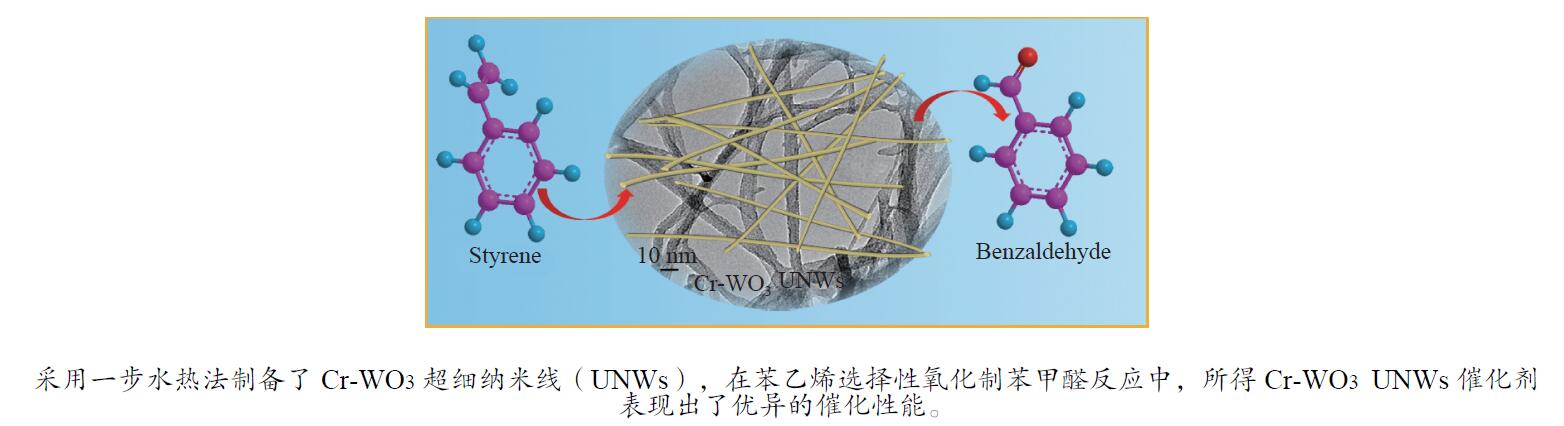
WANG H X, TANG M X, SHI F L, DING R M, WANG L C, WU J B, LI X K, LIU Z, LV B L. Amorphous Cr2WO6-modified WO3 nanowires with a large specific surface area and rich Lewis acid sites: A highly efficient catalyst for oxidative desulfurization[J]. ACS Appl Mater Interfaces,2020,12(34):38140−38152. doi: 10.1021/acsami.0c10118
|
| [25] |
HA J, MURALIDHARAN P, KIM D K. Hydrothermal synthesis and characterization of self-assembled h-WO3 nanowires/nanorods using EDTA salts[J]. J Alloys Compd, 2009, 475(1/2): 446–451.
|
| [26] |
MU W J, LI M, LI X L, MA Z P, ZHANG R, YU Q H LV K, XIE X, HE J H, WEI H Y, JIAN Y. Guanidine sulfate-assisted synthesis of hexagonal WO3 nanoparticles with enhanced adsorption properties[J]. Dalton Trans,2015,44(16):7419−7427. doi: 10.1039/C5DT00103J
|
| [27] |
JIMÉNEZ I, CENTENO M A, SCOTTI R, MORAZZONI F, ARBIOL J, CORNET A, MORANTE J R. NH3 interaction with chromium-doped WO3 nanocrystalline powders for gas sensing applications[J]. J Mater Chem,2004,14(15):2412−2420. doi: 10.1039/B400872C
|
| [28] |
WANG Y R, LIU B, XIAO S H, WANG X H, SUN L M, LI H, XIE W Y, LI Q H, ZHANG Q, WANG T H. Low-temperature H2S detection with hierarchical Cr-doped WO3 microspheres[J]. ACS Appl Mater Interfaces,2016,8(15):9674−9683. doi: 10.1021/acsami.5b12857
|
| [29] |
XIA H J, WANG Y, KONG F H, WANG S R, ZHU B L, GUO X Z, ZHANG J, WANG Y M, WU S H. Au-doped WO3-based sensor for NO2 detection at low operating temperature[J]. Sens Actuators B,2008,134(1):133−139. doi: 10.1016/j.snb.2008.04.018
|
| [30] |
SUN J H, GUO J, YE J Y, SONG B J, ZHANG K W, BAI S L, LUO R X, LI D Q, CHEN A F. Synthesis of Sb doping hierarchical WO3 microspheres and mechanism of enhancing sensing properties to NO2[J]. J Alloys Compd,2017,692:876−884. doi: 10.1016/j.jallcom.2016.09.061
|
| [31] |
OU G, XU Y S, WEN B, LIN R, GE B H, TANG Y, LIANG Y W, YANG C, HUANG K, ZU D, YU R, CHEN W X, LI J, WU H, LIU L, LI Y D. Tuning defects in oxides at room temperature by lithium reduction[J]. Nat Commun,2018,9(1):1302−1310. doi: 10.1038/s41467-018-03765-0
|
| [32] |
LI P X, QU L M, ZHANG C H, REN X B, WANG H X, ZHANG J L, MU Y W, LV B L. Probing into the crystal plane effect on the reduction of α-Fe2O3 in CO by Operando Raman spectroscopy[J]. J Fuel Chem Technol,2021,49(10):1558−1566. doi: 10.1016/S1872-5813(21)60154-8
|
| [33] |
KUZNETSOV D A, NAEEM M A, KUMAR P V, ABDALA P M, FEDOROV A, MÜLLER C R. Tailoring lattice oxygen binding in ruthenium pyrochlores to enhance oxygen evolution activity[J]. J Am Chem Soc,2020,142(17):7883−7888. doi: 10.1021/jacs.0c01135
|
| [34] |
WANG C Z, YANG S J, CHANG H Z, PENG Y, LI J H. Dispersion of tungsten oxide on SCR performance of V2O5-WO3/TiO2: Acidity, surface species and catalytic activity[J]. Chem Eng J,2013,225:520−527. doi: 10.1016/j.cej.2013.04.005
|
| [35] |
FENG B, HOU Z S, WANG X R, HU Y, LI H, QIAO Y X. Selective aerobic oxidation of styrene to benzaldehyde catalyzed by water-soluble palladium(II) complex in water[J]. Green Chem,2009,11(9):1446−1452. doi: 10.1039/b900807a
|
| [36] |
HU J L, LI K X, LI W, MA F Y, GUO Y H. Selective oxidation of styrene to benzaldehyde catalyzed by Schiff base-modified ordered mesoporous silica materials impregnated with the transition metal-monosubstituted Keggin-type polyoxometalates[J]. Appl Catal A: Gen, 2009, 364(1/2): 211–220.
|
| [37] |
LIU J Y, WANG Z, JIAN P M, JIAN R Q. Highly selective oxidation of styrene to benzaldehyde over a tailor-made cobalt oxide encapsulated zeolite catalyst[J]. J Colloid Interface Sci,2018,517:144−154. doi: 10.1016/j.jcis.2018.01.113
|
| [38] |
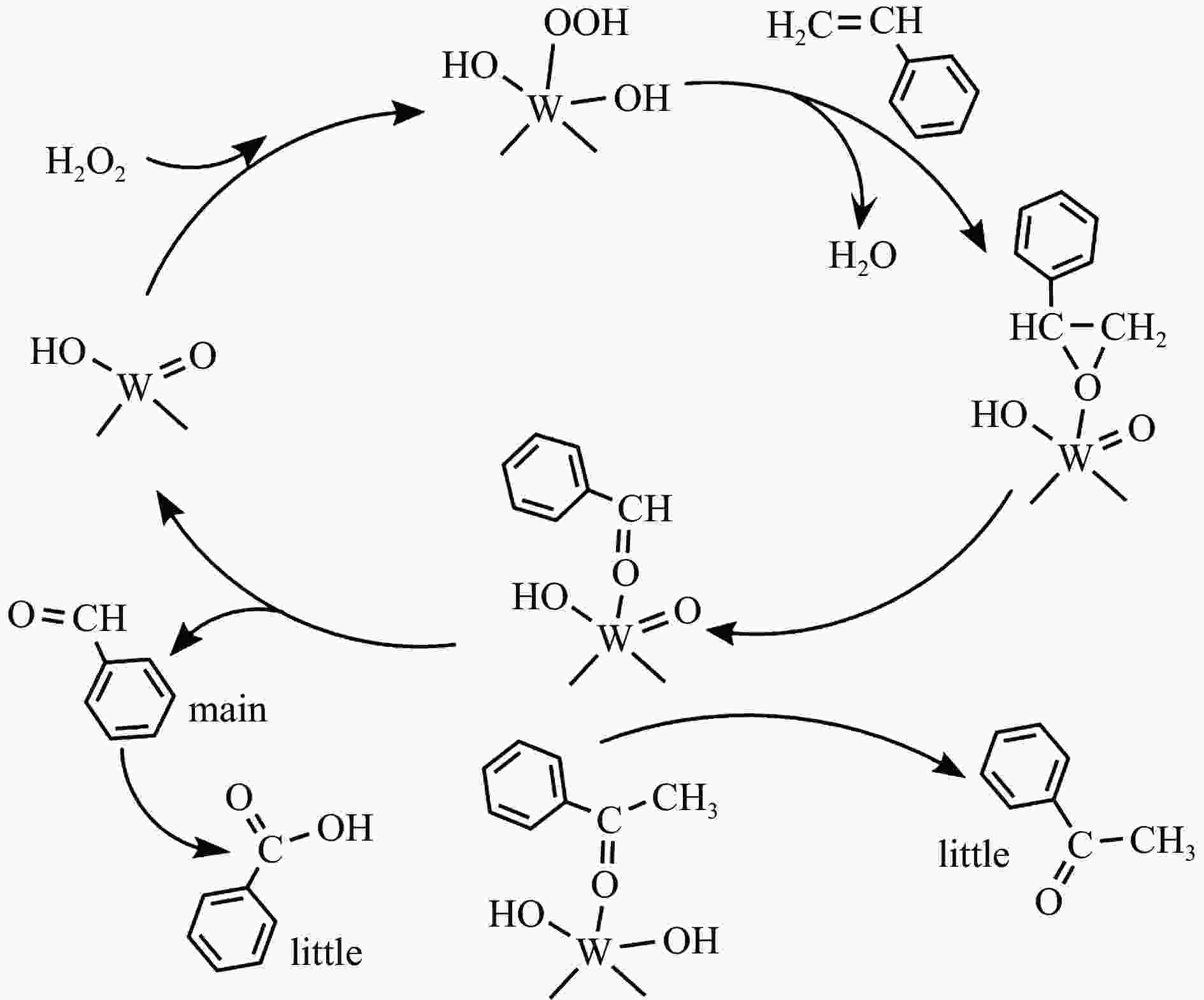
ZHANGM R, SINGH V, HU X F, MA X Y, LU J K, ZHANG C, WANG J P, NIU J Y. Efficient olefins epoxidation on ultrafine H2O-WOx nanoparticles with spectroscopic evidence of intermediate species[J]. ACS Catal,2019,9(9):7641−7650. doi: 10.1021/acscatal.9b01226
|
| [39] |
FRAILE J M, GARCÍA N, MAYORAL J A, SANTOMAURO F G, GUIDOTTI M. Multifunctional catalysis promoted by solvent effects: Ti-MCM41 for a one-pot, four-step, epoxidation-rearrangement-oxidation-decarboxylation reaction sequence on stilbenes and styrenes[J]. ACS Catal,2015,5(6):3552−3561. doi: 10.1021/cs501671a
|




 下载:
下载: