Highly efficient Co-Al2O3 catalysts for oxidative dehydrogenation of ethylbenzene to styrene with CO2
-
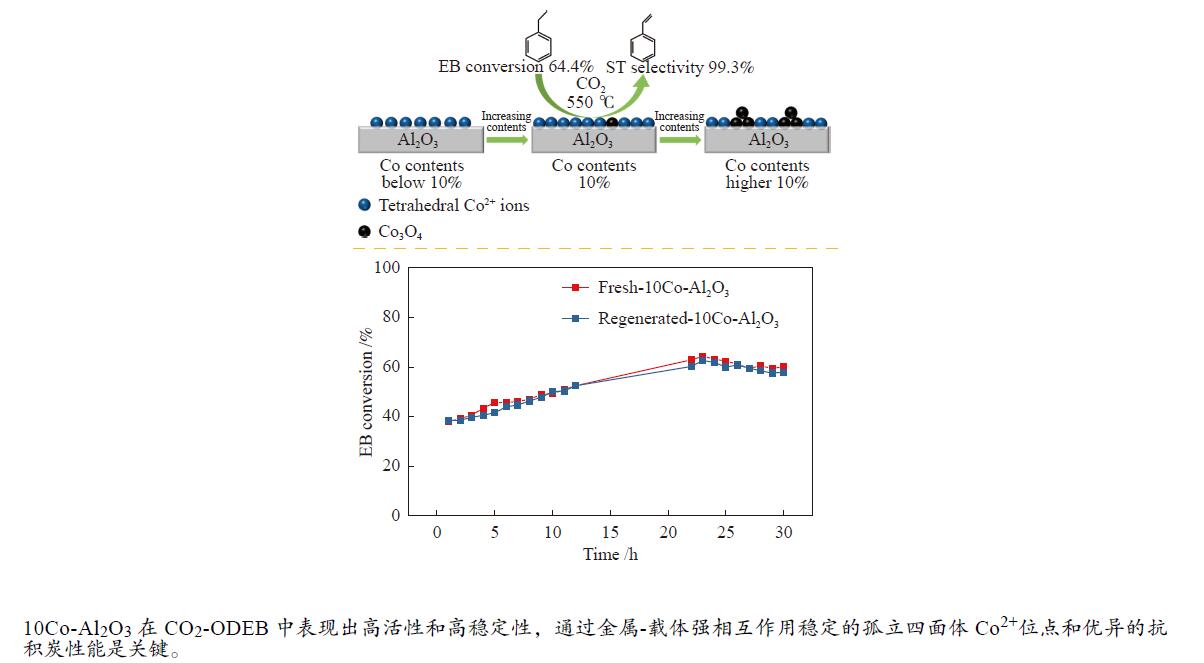
摘要: 采用溶胶-凝胶法制备了不同Co含量的nCo-Al2O3催化剂(n = 2%、5%、10%、15%、20%),研究了Co含量对催化剂结构和CO2氧化乙苯脱氢性能的影响。结果发现,nCo-Al2O3催化剂上孤立的Co2 + 离子与催化活性具有良好的对应关系,表明孤立的四面体Co2 + 物种是其活性位点。Co-Al2O3催化剂上的Co物种结构和催化性能与Co含量相关。Co含量较低(≤10%)时,催化剂上优先形成孤立的四面体Co2 + 物种;随着Co含量的增加,孤立的Co2 + 位点增加,催化剂活性随之提高。Co含量较高(>10%)时,催化剂上形成Co3O4晶体颗粒,导致孤立的Co2 + 位点减少,催化剂活性降低。10Co-Al2O3表现出最佳催化性能,550 ℃下乙苯转化率达64.4%,苯乙烯选择性为99.3%,反应30 h后,催化剂仍无明显失活,表明孤立的Co2 + 活性位点具有良好的结构稳定性和优异的抗积炭性能。Abstract: nCo-Al2O3 catalysts with different Co contents (n=2%, 5%, 10%, 15%, 20%) were prepared by a sol-gel approach. The effect of Co content on the nCo-Al2O3 catalyst structure and performance in the oxidative dehydrogenation of ethylbenzene to styrene by CO2 was investigated. The results showed that the isolated Co2 + ions on the nCo-Al2O3 catalysts had a positive influence on the catalytic activity, where the isolated tetrahedral Co2 + species were considered as the active sites. Co contents on the Co-Al2O3 catalyst greatly affected the structure of Co species and the catalytic performance. The isolated tetrahedral Co2 + species are preferentially generated on the resultant nCo-Al2O3 catalyst when the content of Co (n) is less than 10%; as a result, an increase of Co content here leads to the formation of more isolated Co2 + sites and then improves the catalytic activity of nCo-Al2O3 in the dehydrogenation of ethylbenzene. When Co content exceeded 10%, crystalline Co3O4 particles were obtained on the formed catalyst, which resulted in the decline of the isolated Co2 + sites and catalytic activity. Among various nCo-Al2O3 catalysts, 10Co-Al2O3 exhibited the best catalytic performance, with 64.4% conversion rate for ethylbenzene and 99.3% selectivity for styrene at 550 ℃. This catalyst remained stable without obvious deactivation for 30 h of reaction, which suggests that the isolated Co2 + species as active sites presented excellent structural stability and excellent anti-coke deposition.
-
Key words:
- Co-based catalyst /
- ethylbenzene /
- oxidative dehydrogenation /
- CO2 /
- styrene
1) #These authors contributed equally to this work and should be considered co-first authors. -
表 1 nCo-Al2O3催化剂的织构性质
Table 1 Textural properties of nCo-Al2O3 catalysts
Sample SBET /(m2·g−1)a vp /(cm3·g−1)b dp /nmc Al2O3(HSA) 386 0.79 7.81 2Co-Al2O3 376 0.73 7.64 5Co-Al2O3 350 0.68 6.95 10Co-Al2O3 329 0.62 5.72 15Co-Al2O3 260 0.56 5.25 20Co-Al2O3 237 0.43 4.84 a BET surface areas were obtained from N2 adsorption isotherms; b pore volume based on the Multi-point BET method; c average pore diameter based on the BJH method 表 2 nCo-Al2O3催化剂的XPS表征
Table 2 XPS results of nCo- Al2O3 catalysts
Sample Binding energy /eV ΔE /eV Co2 + /Co3 + Co 2p3/2 Co 2p1/2 2Co-Al2O3 781.8 797.6 15.8 100∶0 5Co-Al2O3 781.8 797.5 15.7 95∶5 10Co-Al2O3 781.6 797.1 15.5 89∶11 15Co-Al2O3 781.1 796.3 15.2 63∶37 20Co-Al2O3 780.7 795.8 15.1 45∶55 表 3 550 ℃下nCo-Al2O3催化CO2氧化乙苯脱氢活性
Table 3 Activity of nCo-Al2O3 catalysts for CO2-ODEB at 550 ℃
Sample EB conversion /% ST selectivity /% ST yield /% 1 h 12 h 1 h 12 h 1 h 12 h 2Co-Al2O3 17.1 17.5 98.4 98.7 16.8 17.3 5Co-Al2O3 27.5 37.6 98.5 98.6 27.1 37.1 10Co-Al2O3 39.5 55.6 98.4 98.8 38.9 54.9 10Co-Al2O3a 45.2 33.1 87.6 88.3 39.6 29.2 15Co-Al2O3 32.5 40.8 98.3 98.7 31.9 40.3 20Co-Al2O3 20.8 19.2 98.3 98.6 20.4 18.9 a: Under an inert He atmosphere -
[1] LEE E H. iron oxide catalysts for dehydrogenation of ethylbenzene in the presence of steam iron oxide catalysts for dehydrogenation of ethylbenzene in the presence of steam[J]. Catal Rev,2006,8(1):285−305. [2] RAHMAN S T, CHOI J R, LEE J H, PARK S J. The role of CO2 as a mild oxidant in oxidation and dehydrogenation over catalysts: A Review[J]. Catalysts,2020,10(9):1075−1100. doi: 10.3390/catal10091075 [3] 王光耀. 重油性质对油煤浆表观黏度的影响[J]. 煤炭转化,2022,45(2):74−83. doi: 10.19726/j.cnki.ebcc.202202009WANG Guang-yao. Effect of heavy oil properties on apparent viscosity of oil-coal slurry[J]. Coal Convers,2022,45(2):74−83. doi: 10.19726/j.cnki.ebcc.202202009 [4] MIMURA N, TAKAHARA I, SAITO M, HATTORI T, ANDO M. Dehydrogenation of ethylbenzene over iron oxide-based catalyst in the presence of carbon dioxide[J]. Catal Today,1998,45(1):61−64. [5] MUKHERJEE D, PARK S E, REDDY B M. CO2 as a soft oxidant for oxidative dehydrogenation reaction: An eco benign process for industry[J]. J CO2 Util,2016,16:301−312. doi: 10.1016/j.jcou.2016.08.005 [6] ANSARI M B, PARK S E. Carbon dioxide utilization as a soft oxidant and promoter in catalysis[J]. Energy Environ sci,2012,5:9419−9437. doi: 10.1039/c2ee22409g [7] WANG T, QI L, LU H, JI M. Flower-like Al2O3-supported iron oxides as an efficient catalyst for oxidative dehydrogenation of ethlybenzene with CO2[J]. J CO2 Util,2017,17:162−169. doi: 10.1016/j.jcou.2016.12.005 [8] 张淑娇, 李文英, 李晓红. 制备方法对Fe2O3/Al2O3-ZrO2在乙苯脱氢反应中催化性能的影响[J]. 燃料化学学报,2015,43(4):437−441. doi: 10.1016/S1872-5813(15)30012-8ZHANG Shu-jiao, LI Wen-ying, LI Xiao-hong. Effect of preparation methods on the catalytic properties of Fe2O3/Al2O3-ZrO2 for ethylbenzene dehydrogenation[J]. J Fuel Chem Technol,2015,43(4):437−441. doi: 10.1016/S1872-5813(15)30012-8 [9] CHEN S W, XU Z Q, TAN D C, PAN D H, LI R F. Oxidative dehydrogenation of ethylbenzene to styrene with CO2 over Al-MCM-41-supported vanadia catalysts: Al-MCM-41-supported vanadia catalysts for CO2-ODEB[J]. Appl Organomet Chem,2019,34(2):1−10. [10] YANG G Q, WANG H, GONG T, SONG Y H, FENG H, GE H Q, GE H B, LIU Z T, LIU Z W. Understanding the active-site nature of vanadia-based catalysts for oxidative dehydrogenation of ethylbenzene with CO2 via atomic layer deposited VOx on γ-Al2O3[J]. J Catal,2019,380(5):195−203. [11] BETIHA M A, RABIE A M, ELFADLY A M, YEHIA F Z. Microwave assisted synthesis of a VOx-modified disordered mesoporous silica for ethylbenzene dehydrogenation in presence of CO2[J]. Microporous Mesoporous Mater,2016,222(1):44−54. [12] 张海新, 陈树伟, 崔杏雨, 潘大海, 秦张峰, 王建国. Ce助剂对V/SiO2催化CO2氧化乙苯脱氢性能的影响[J]. 物理化学学报,2014,30(2):351−358. doi: 10.3866/PKU.WHXB201312021ZHANG Hai-xin, CHEN Shu-wei, CUI Xin-yu, PAN Da-hai, QING Zhang-feng, WANG Jan-guo. Effect of Ce promoter on catalytic performance of V/SiO2 in oxidative dehydrogenation of ethylbenzene with carbon dioxide[J]. Acta Phys-Chim Sin,2014,30(2):351−358. doi: 10.3866/PKU.WHXB201312021 [13] 李春光, 缪长喜, 聂颖颖, 乐英红, 顾松园, 杨为民, 华伟明, 高滋. CO2气氛下MCF负载氧化钒催化剂上乙苯脱氢反应(英文)[J]. 催化学报,2010,31(8):993−998.LI Chun-guang, LIAO Chang-xi, NIE Yin-yin, LE Yin-hong, GU Song-yuan, YANG Wei-ming, HUA Wei-ming, GAO Zi. Ethylbenzene dehydrogenation in the presence of CO2 over MCF-supported vanadium oxide catalysts[J]. J Catal,2010,31(8):993−998. [14] FAN H X, FENG J, LI W Y. Promotional effect of oxygen storage capacity on oxy-dehydrogenation of ethylbenzene with CO2 over κ-Ce2Zr2O8 (111)[J]. Appl Surf Sci,2019,486:411−419. [15] ZHANG L, WU Z, NELSON N C, SADOW A D, SLOWING I I, OVERBURY S H. Role of CO2 as a soft oxidant for dehydrogenation of ethylbenzene to styrene over a high-surface-area ceria catalyst[J]. ACS Catal,2015,5(11):6426−6435. doi: 10.1021/acscatal.5b01519 [16] DIAO J, HU M, LIAN Z, LI Z, LIU H. Ti3C2Tx MXene catalyzed ethylbenzene dehydrogenation: Active sites and mechanism exploration from both experimental and theoretical aspects[J]. ACS Catal,2018,8(11):10051−10057. doi: 10.1021/acscatal.8b02002 [17] PAN D H, RU Y, LIU T L, WANG Y J, YU F, CHEN S W, YAN X L, FAN B B, LI R F. Highly efficient and stable ordered mesoporous Ti-Al composite oxide catalyst for oxidative dehydrogenation of ethylbenzene to styrene with CO2[J]. Chem Eng Sci,2022,250:117388. doi: 10.1016/j.ces.2021.117388 [18] LI Y F, SU J J, YU F, YAN X L, PAN D H, LI R F, YANG Y X. Dehydrogenation of ethylbenzene with CO2 over porous Co/Al2O3-ZrO2 catalyst[J]. Mater Chem Phys,2021,257:123773. doi: 10.1016/j.matchemphys.2020.123773 [19] MADDULURI V R, RAO K S R. Correction to: Advantage of Co embedded γ-Al2O3 catalysts over MgO and SiO2 solid oxides in the selective production of styrene monomer[J]. Catal Lett,2019,149(11):3253−3253. doi: 10.1007/s10562-019-02926-0 [20] POCHAMONI R, NARANI A, VARKOLU M, GUDIMELLA M D, POTHARAJU S SAI P, BURRI D R, R. K S R. Studies on ethylbenzene dehydrogenation with CO2 as soft oxidant over Co3O4/COK-12 catalysts[J]. J Chem Sci,2015,127(4):701−709. doi: 10.1007/s12039-015-0826-x [21] CHEN C, AHN W S. CO2 capture using mesoporous alumina prepared by a sol-gel process[J]. Chem Eng J,2011,166(2):646−651. doi: 10.1016/j.cej.2010.11.038 [22] SUN Y, WU Y, SHAN H, LI C. Studies on the nature of active cobalt species for the production of methane and propylene in catalytic dehydrogenation of propane[J]. Catal Lett,2015,145(7):1413−1419. doi: 10.1007/s10562-015-1533-4 [23] DAI Y H, GU J J, TIAN S Y, WU Y, CHEN J C, LI F X, DU Y H, PENG L M, DING W P, YANG Y H. γ-Al2O3 sheet-stabilized isolate Co2 + for catalytic propane dehydrogenation[J]. J Catal,2020,381(1):482−492. [24] 高恋, 徐耀, 侯博, 吴东, 孙予罕. 介孔氧化硅球负载钴基催化剂在费托合成中的应用[J]. 化学学报,2008,66(16):1851−1856. doi: 10.3321/j.issn:0567-7351.2008.16.001GAO Lian, XU Yao, HOU Bo, WU Dong, SUN Yu-han. Application of hollow mesoporous silica sphere supported cobalt catalysts in Fischer-Tropsch synthesis[J]. Acta Chim,2008,66(16):1851−1856. doi: 10.3321/j.issn:0567-7351.2008.16.001 [25] LI X Y, WANG P Z, WANG H R, LI C Y. Effects of the state of Co species in Co/Al2O3 catalysts on the catalytic performance of propane dehydrogenation[J]. Appl Surf Sci,2018,441(1):688−693. -





 下载:
下载: