Effect of support morphology of Ni3Fe/CeO2 on catalytic performance for dry reforming of methane
-
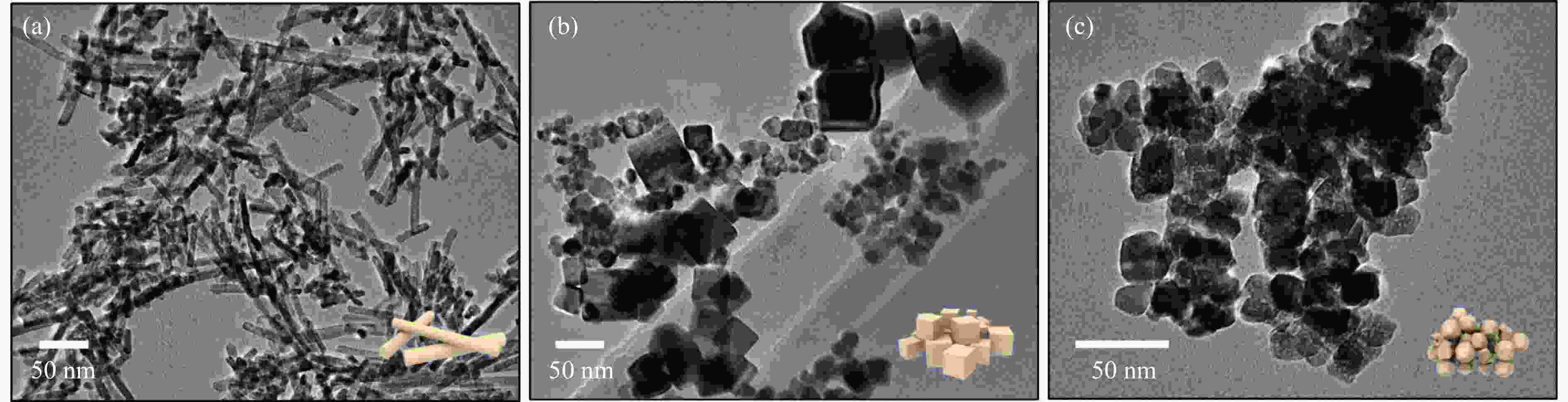
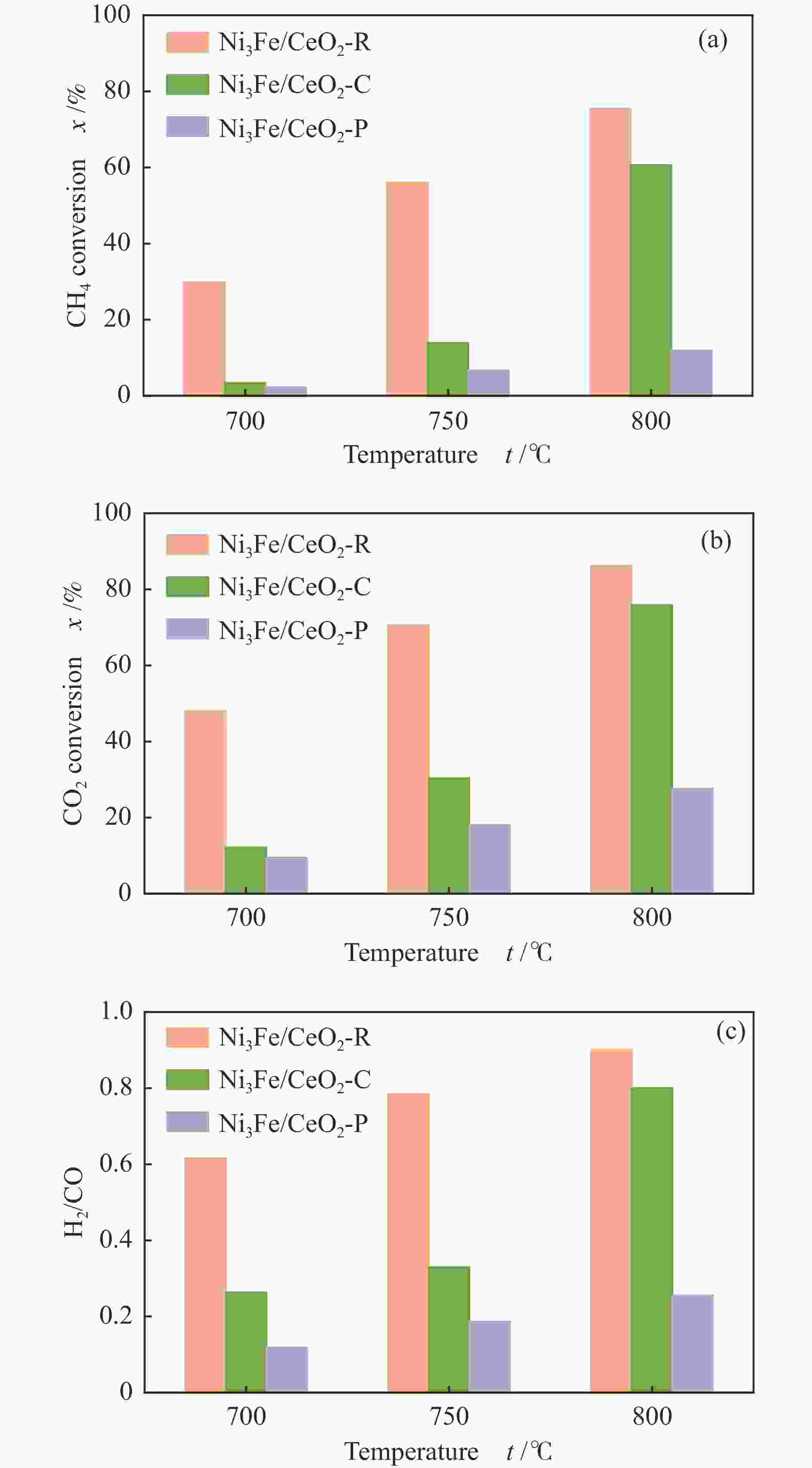
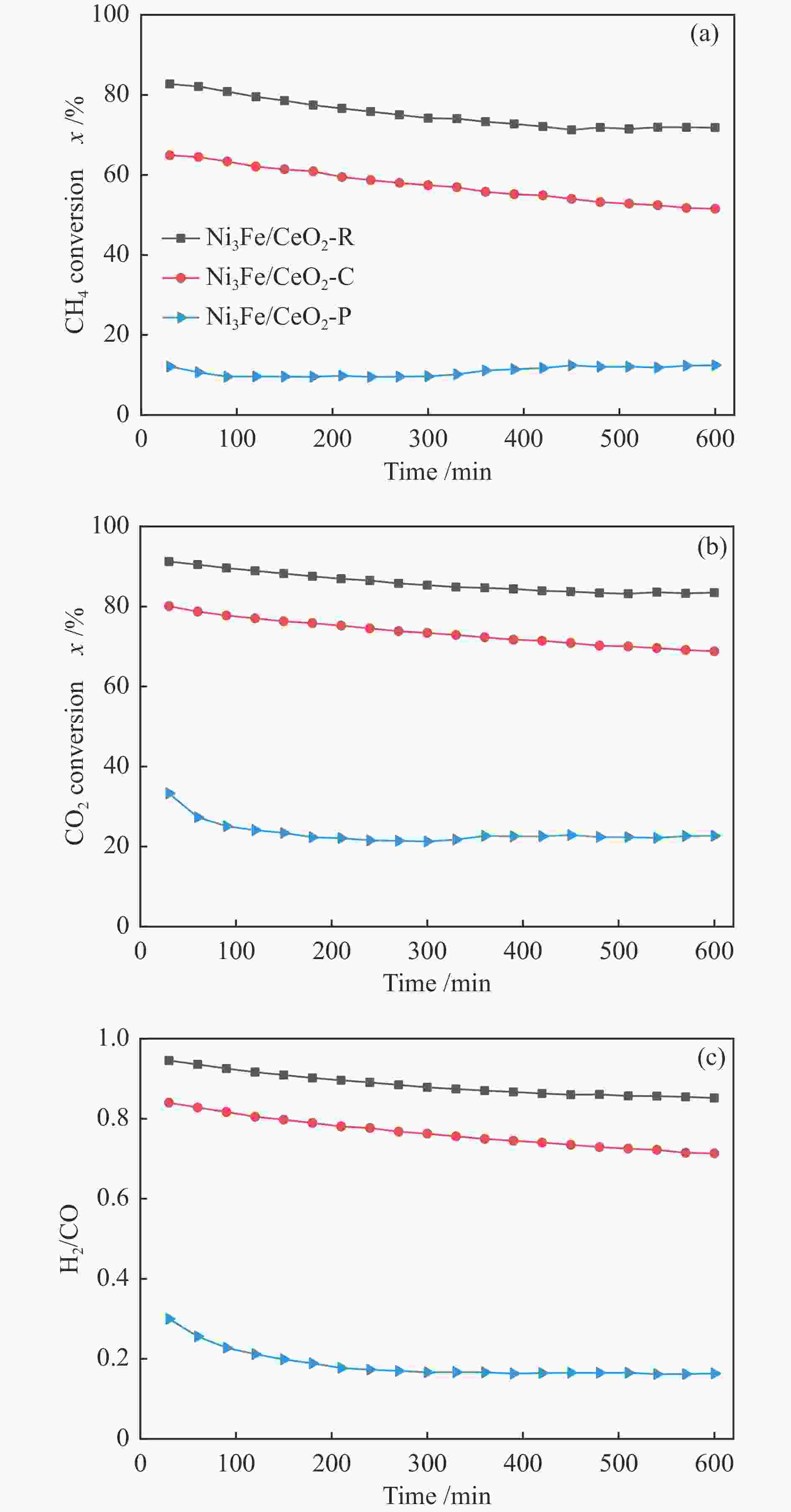
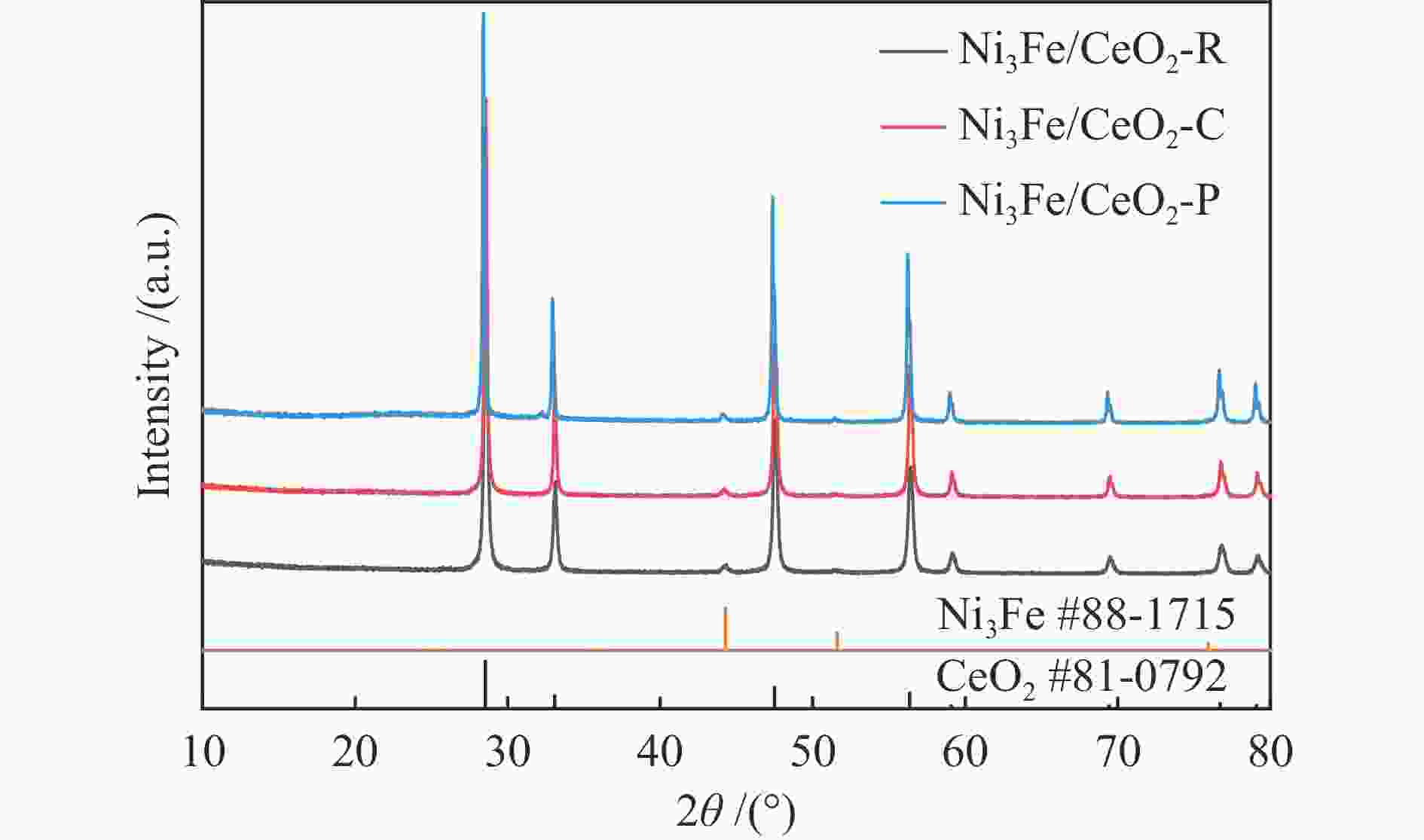
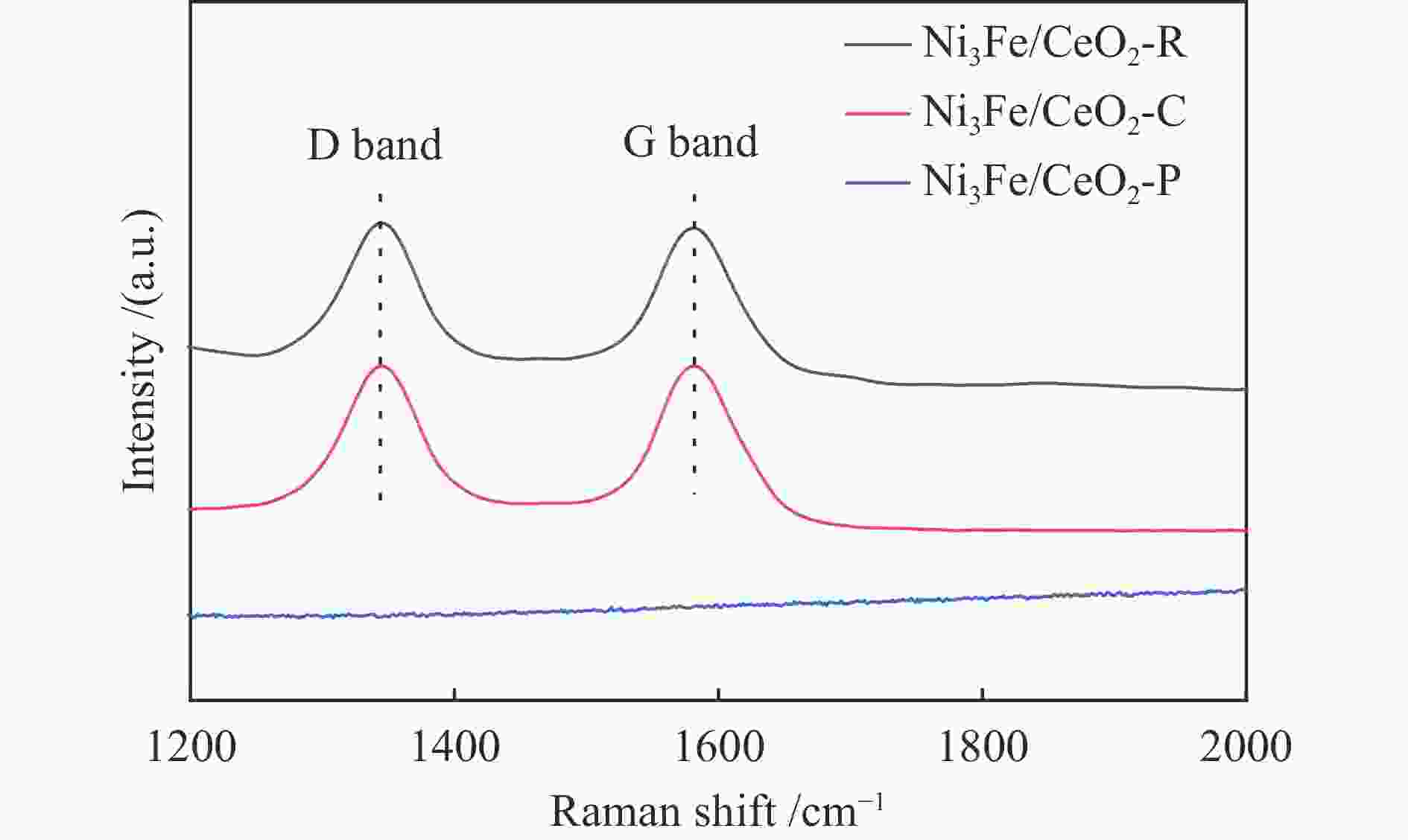
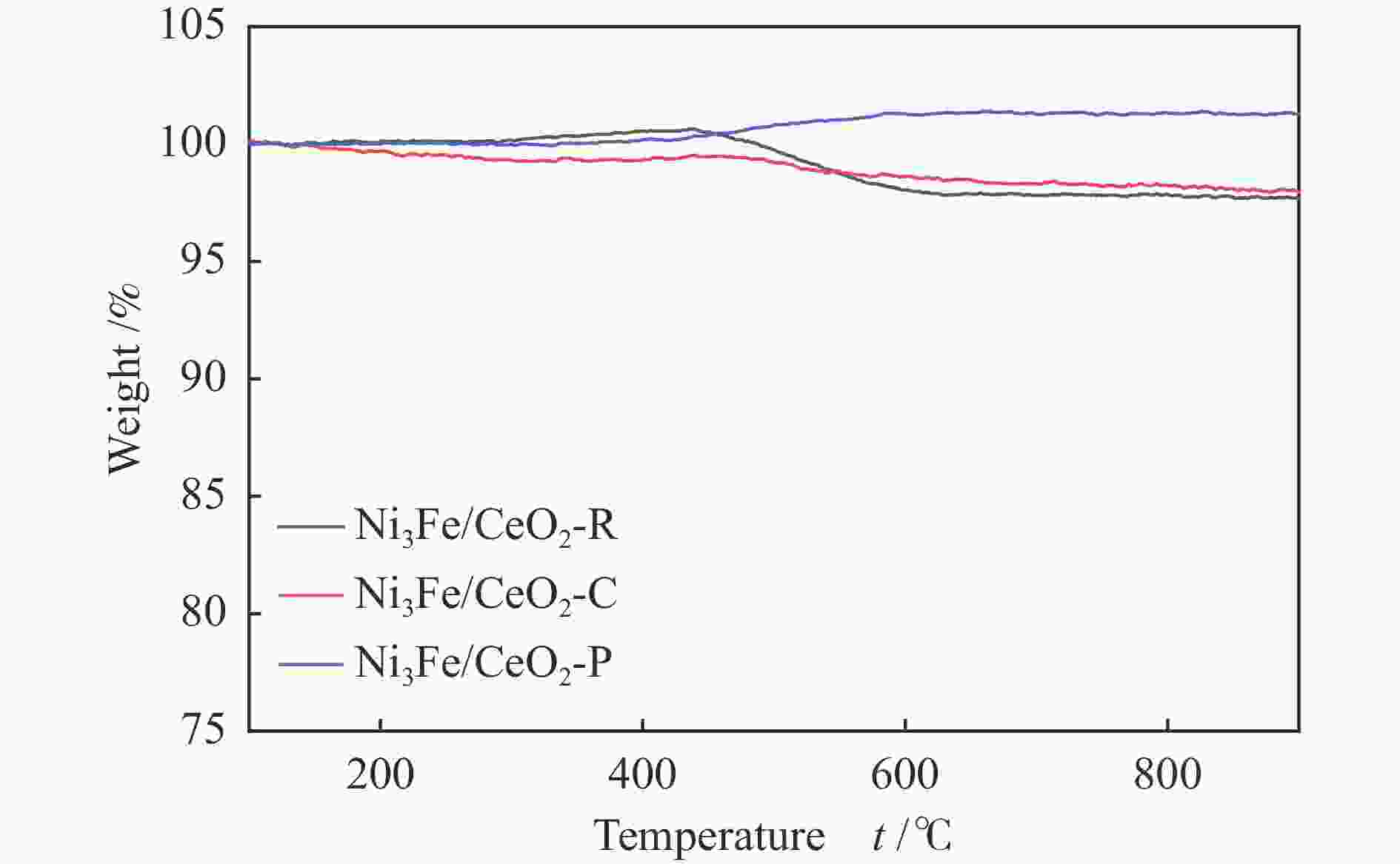
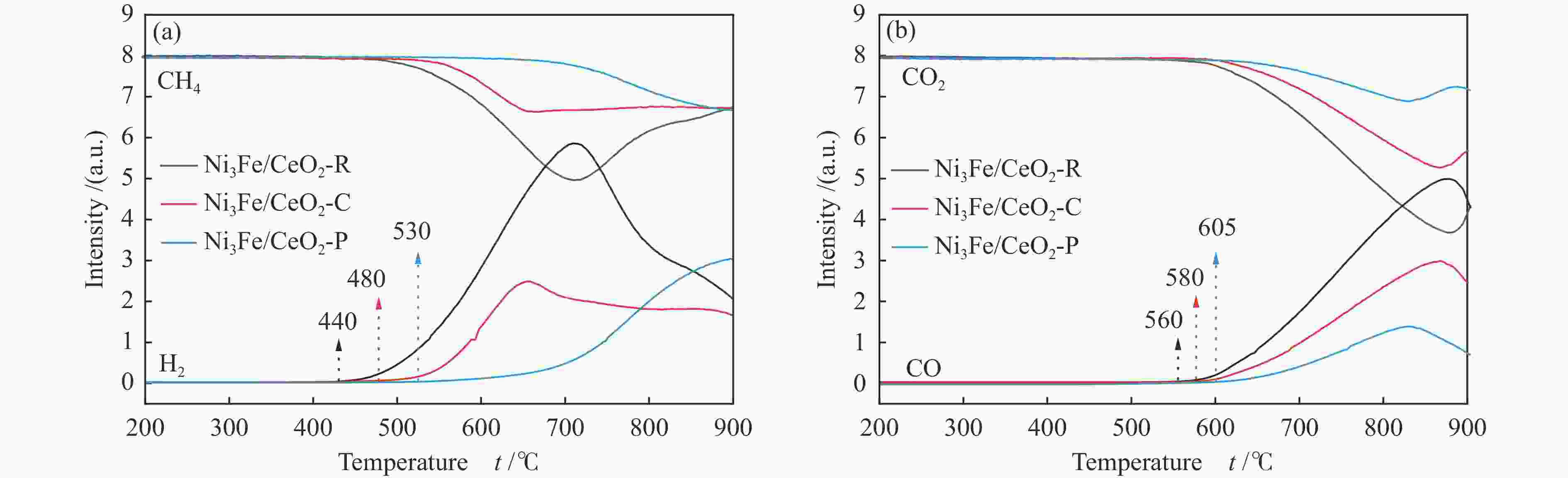
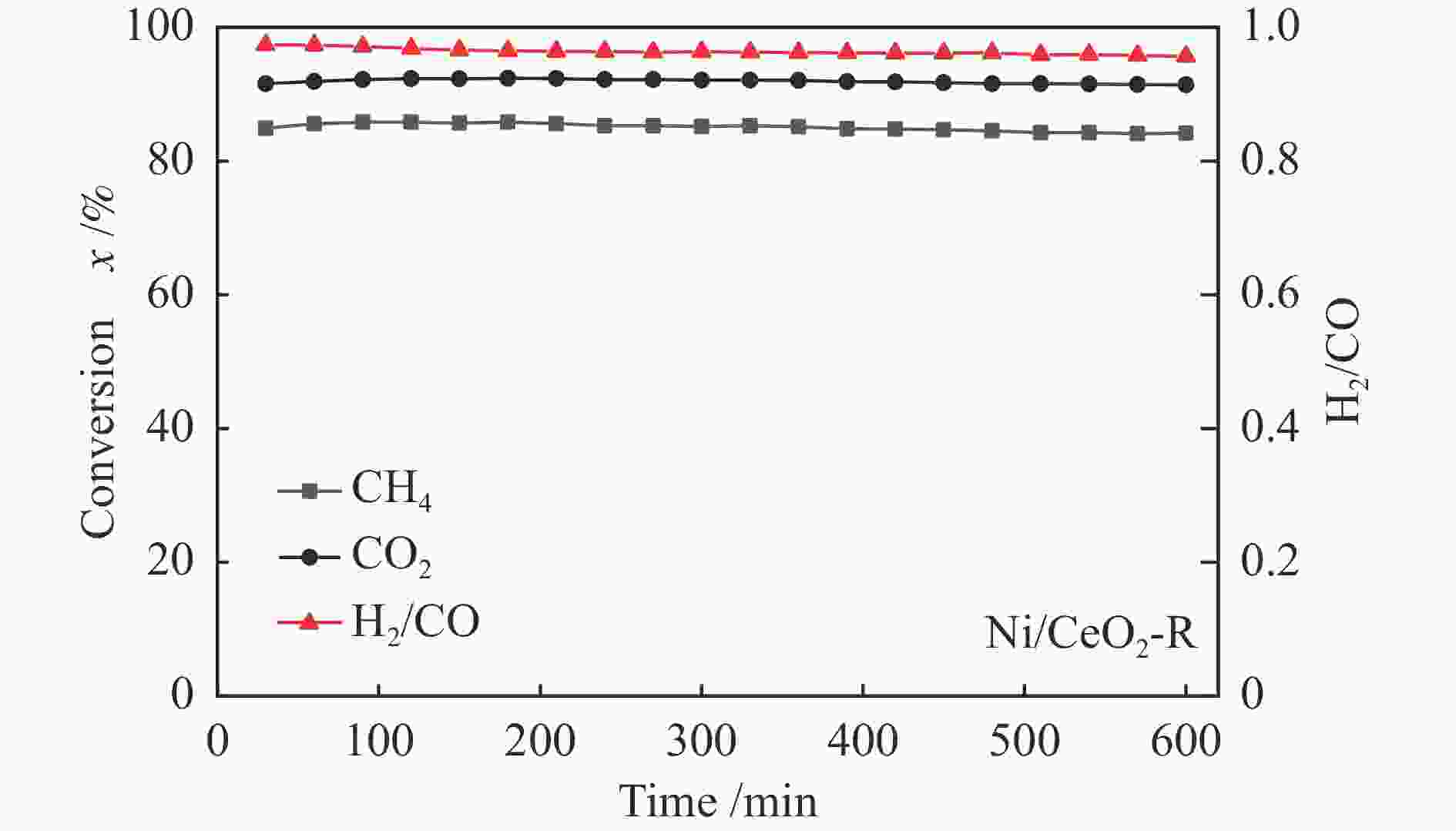
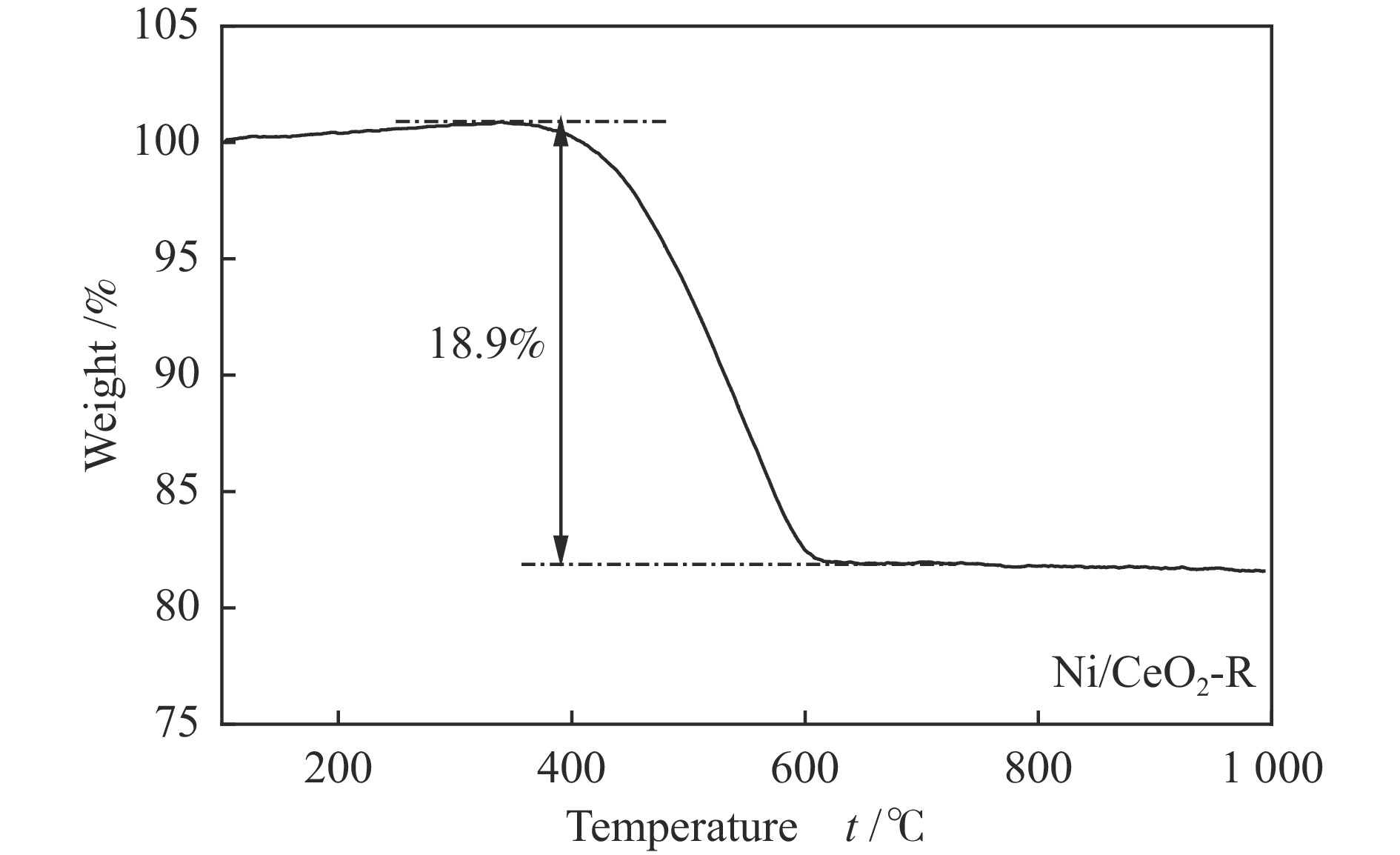
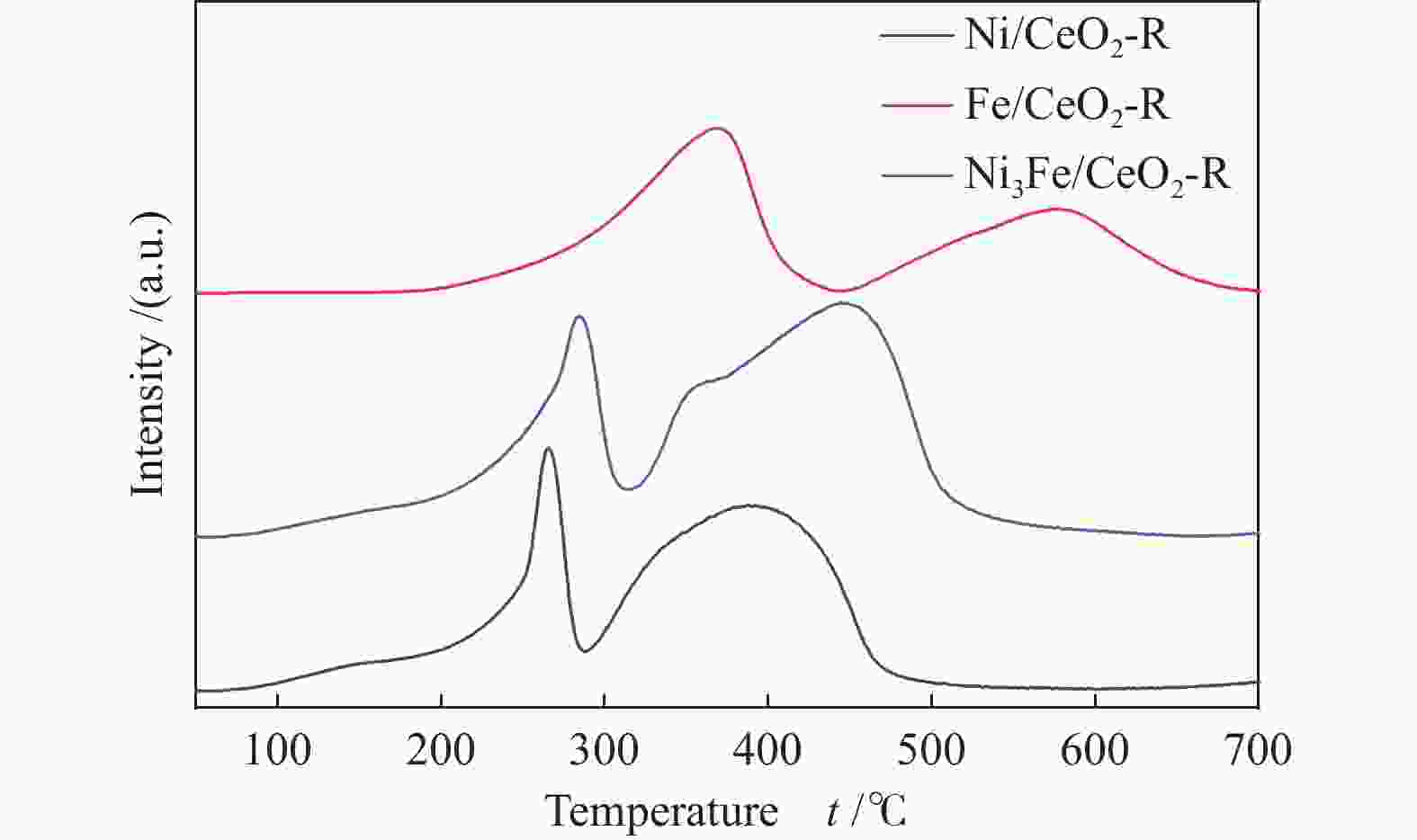
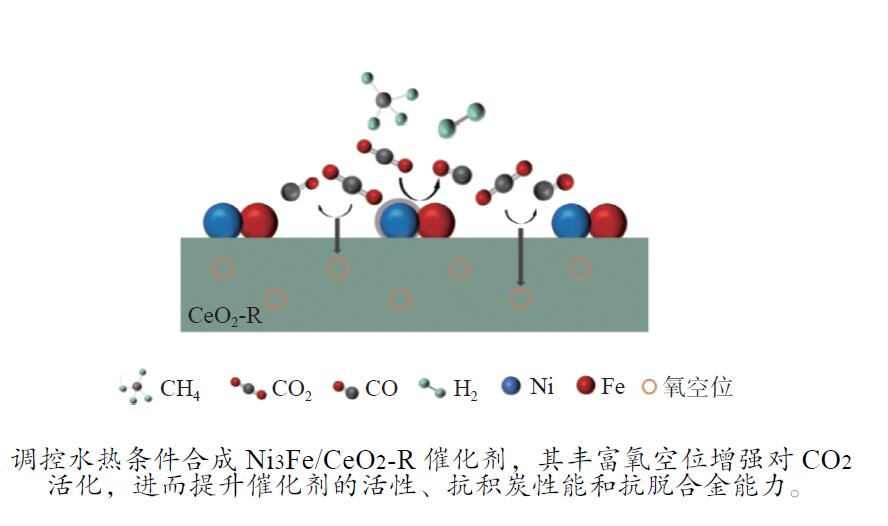
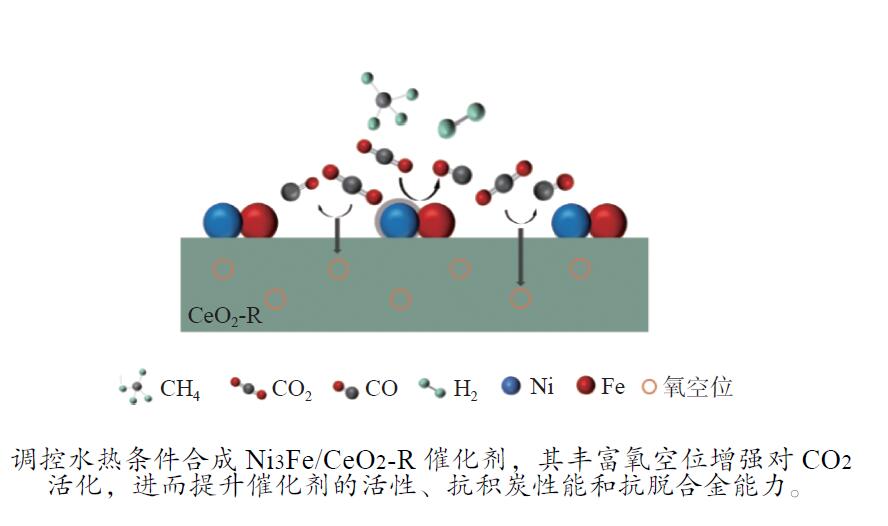
摘要: 通过改变水热法条件合成了不同形貌CeO2载体(棒状CeO2-R、立方体CeO2-C和多面体CeO2-P),并用浸渍法制备了Ni3Fe/CeO2催化剂,继而研究了不同载体形貌Ni3Fe/CeO2催化剂对其甲烷干重整反应性能的影响。采用X射线衍射、N2吸附-脱附、透射电镜、拉曼光谱、X射线光电子能谱、热重等对反应前后催化剂结构进行表征。结果表明,Ni3Fe/CeO2-R具有较大比表面积和较高的氧空位浓度,在甲烷干重整反应中表现出了优异的催化反应活性。800 ℃时,CH4和CO2的转化率分别为82%和91%,且反应10 h性能稳定并且其积炭石墨化程度较低。同时,通过CeO2-R载体氧空位对CO2活化,有效抑制了对亲氧性Fe物种的过度氧化行为,反应前后催化剂Ni3Fe合金结构保持稳定,具有良好的抗脱合金能力。
-
关键词:
- 甲烷干重整 /
- 形貌 /
- Ni3Fe/CeO2 /
- 氧空位 /
- 合金
Abstract: Different morphologies of CeO2 supports (including CeO2-R rod, CeO2-C cube, and CeO2-P polyhedron) were synthesized by hydrothermal method and were used to develop Ni3Fe/CeO2 catalysts by impregnation method for dry reforming of methane (DRM). The structures of the resultant catalysts before and after DRM were characterized by X-ray diffraction, N2 adsorption-desorption, transmission electron microscopy, Raman spectroscopy, X-ray photoelectron spectroscopy and thermogravimetry analysis. The results showed that Ni3Fe/CeO2-R exhibited good catalytic activity in DRM, where CH4 as well as CO2 conversion reached to 82% and 91%, respectively, at 800 ℃, owing to its large specific surface area and high oxygen vacancy concentration. The catalytic performance of Ni3Fe/CeO2-R was relatively stable and graphitic degree of coke deposition was low after 10 h. Meanwhile, the oxidative resistance of Ni3Fe/CeO2-R was improved as confirmed by the existence of stable Ni3Fe alloy after reaction, which mitigated the facile oxidation of more oxyphilic Fe species on the alloy by the promotion of CO2 activation on the vacancies of CeO2-R.-
Key words:
- dry reforming of methane /
- morphology /
- Ni3Fe/CeO2 /
- oxygen vacancy /
- alloy
-
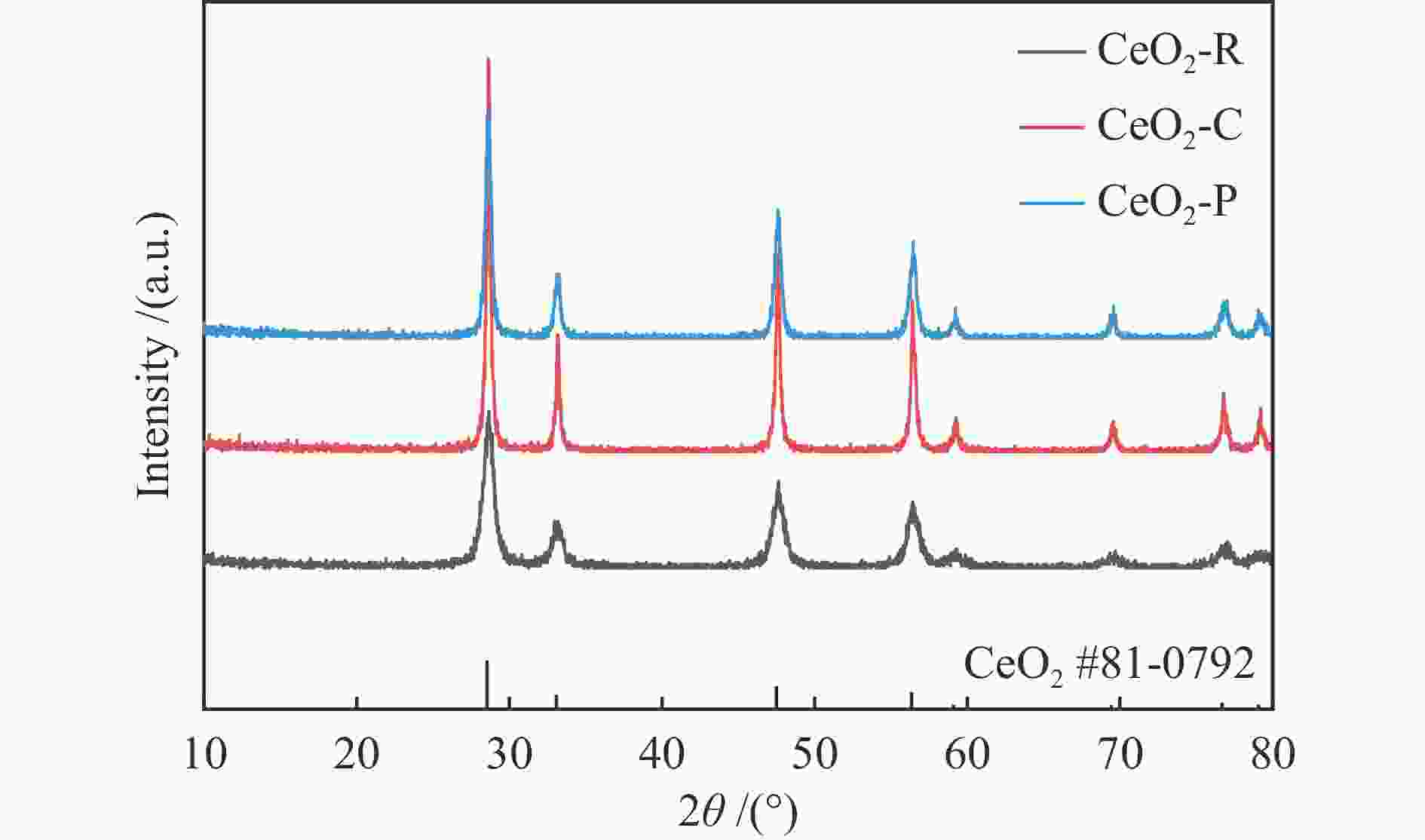
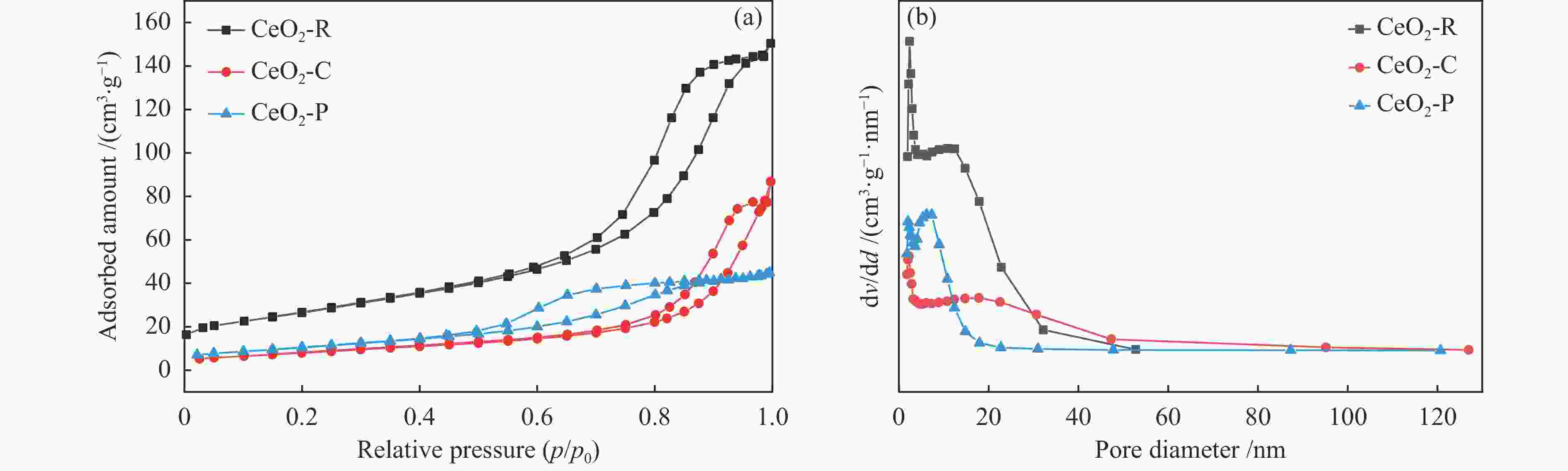
表 1 不同形貌CeO2的物理结构特性参数
Table 1 Physical structural characteristics of CeO2 with different morphologies
Sample SBET /(m2·g−1) Pore volume /(cm3·g−1) Pore size /nm CeO2-R 95 0.23 8 CeO2-C 30 0.13 13 CeO2-P 39 0.07 6 表 2 Ni3Fe/CeO2催化剂表面物种的定量分析
Table 2 Quantitative XPS analysis results of the Ni3Fe/CeO2 catalysts
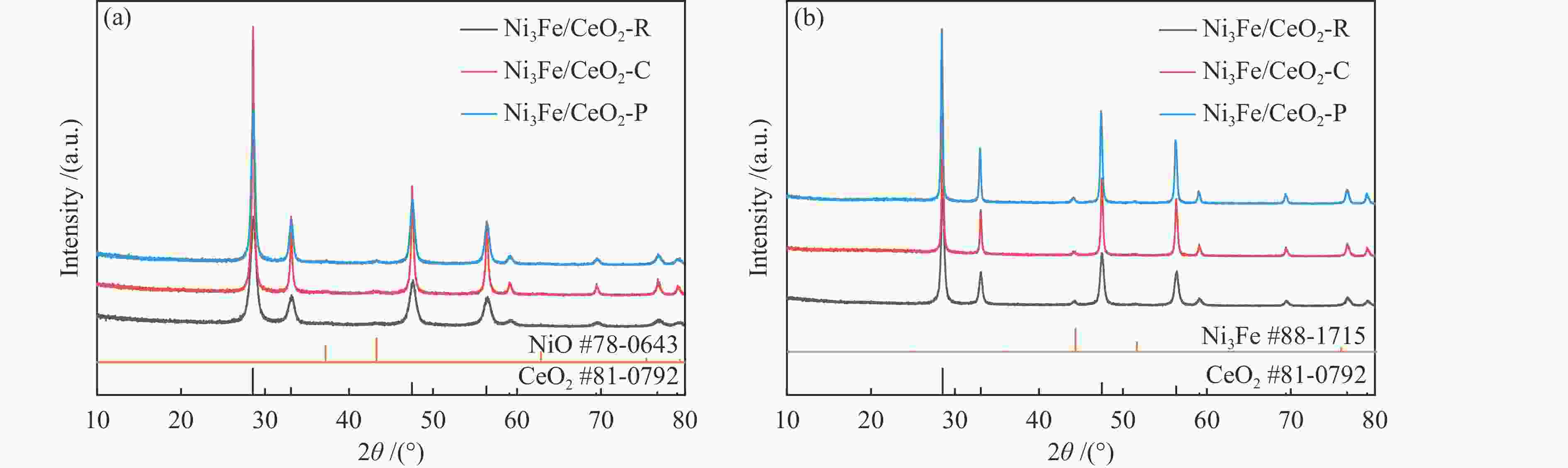
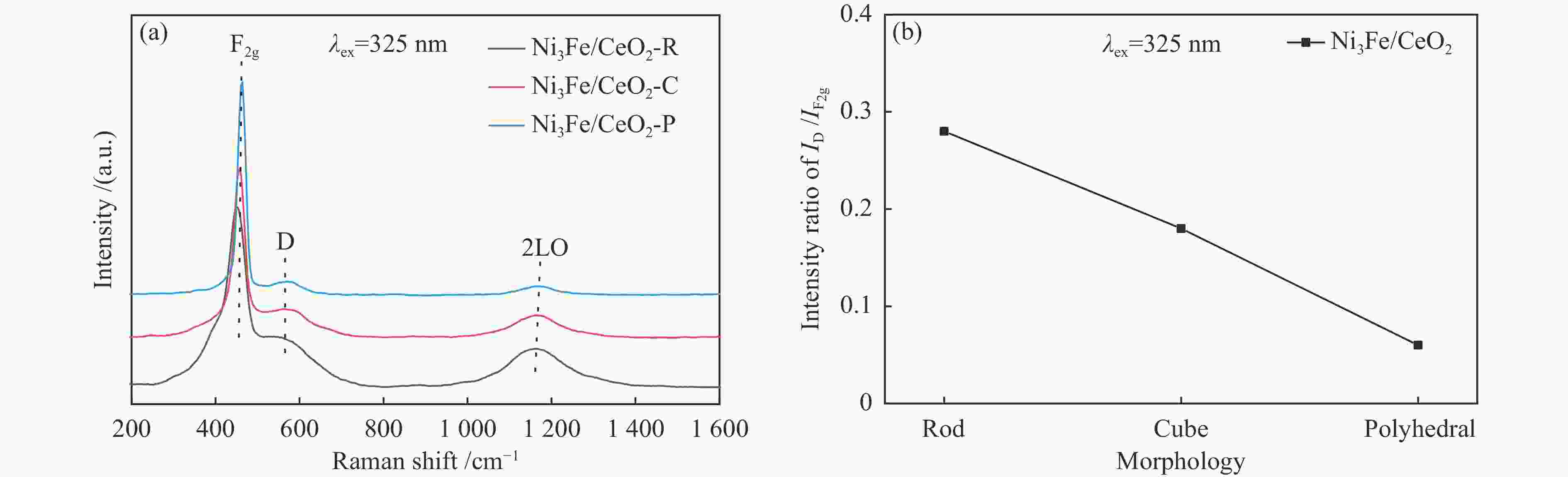
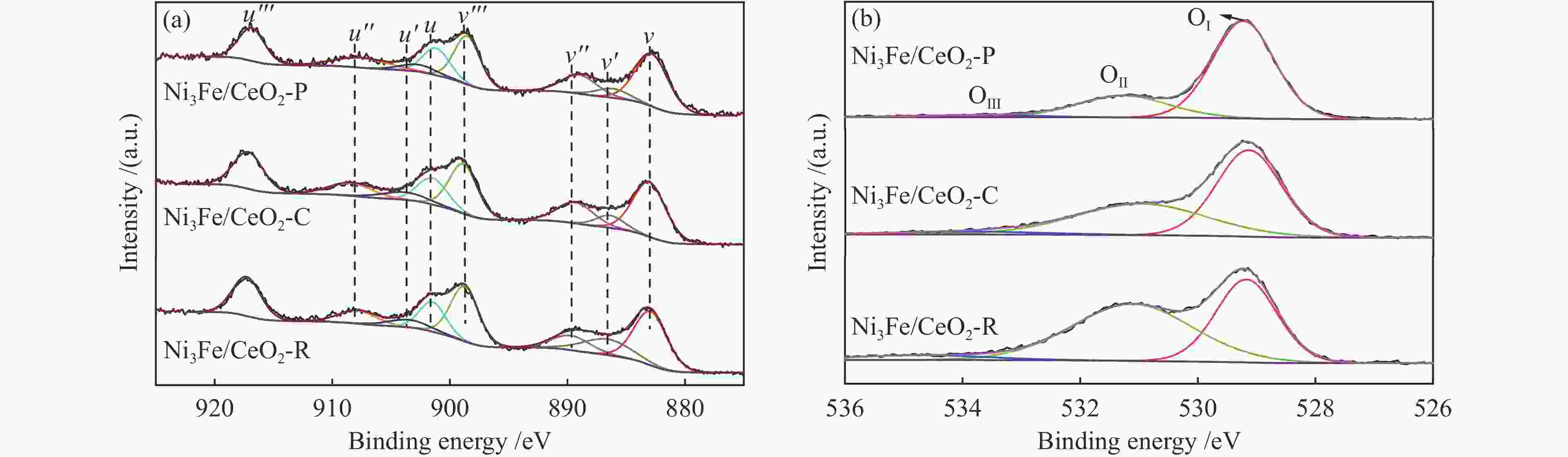
Sample Ce3 + /(Ce3 + + Ce4 + ) ΟⅡ/(ΟⅠ + ΟⅡ + ΟⅢ) Ni3Fe/CeO2-R 0.147 0.545 Ni3Fe/CeO2-C 0.097 0.401 Ni3Fe/CeO2-P 0.086 0.239 -
[1] WANG Q Q, WANG W, CAO M, LI S, WANG P F, HE J Q, LI R F, YAN X L. Effect of interstitial carbon atoms in core-shell Ni3ZnC0.7/Al2O3 catalyst for high-performance dry reforming of methane[J]. Appl Catal B: Environ,2022,317:121806. doi: 10.1016/j.apcatb.2022.121806 [2] ZHANG M, ZHANG J F, ZHOU Z L, ZHANG Q D, TAN Y S, HAN Y Z. Effects of calcination atmosphere on the performance of the co-precipitated Ni/ZrO2 catalyst in dry reforming of methane[J]. Can J Chem Eng,2021,100:S172−S183. [3] 付彧, 孙予罕. CH4-CO2重整技术的挑战与展望[J]. 中国科学(化学),2020,50(7):816−831. doi: 10.1360/SSC-2019-0160FU Yu, SUN Yu-han. CH4-CO2 reforming: Challenges and outlook[J]. Sci Sin Chim,2020,50(7):816−831. doi: 10.1360/SSC-2019-0160 [4] SONG Y, OZDEMIR E, RAMESH S, ADISHEV A, SUBRAMANIAN S, HARALE A, ALBUALI M, FADHEL B A, JAMAL A, MOON D, CHOI S H, YAVUZ C T. Dry reforming of methane by stable Ni-Mo nanocatalysts on single-crystalline MgO[J]. Science,2020,367(6479):777−781. doi: 10.1126/science.aav2412 [5] KIM K Y, LEE J H, LEE H, NOH W Y, KIM E H, RA E C, KIM S K, AN K, LEE J S. Layered double hydroxide-derived intermetallic Ni3GaC0.25 catalysts for dry reforming of methane[J]. ACS Catal,2021,11(17):11091−11102. doi: 10.1021/acscatal.1c02200 [6] YAN X L, HU T, LIU P, LI S, ZHAO B R, ZHANG Q, JIAO W Y, CHEN S, WANG P F, LU J J, FAN L M, DENG X N, PAN Y X. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2[J]. Appl Catal B: Environ,2019,246:221−231. doi: 10.1016/j.apcatb.2019.01.070 [7] DENG J, BU K K, SHEN Y J, ZHANG X Y, ZHANG J P, FAUNGNAWAKIJ K, ZHANG D S. Cooperatively enhanced coking resistance via boron nitride coating over Ni-based catalysts for dry reforming of methane[J]. Appl Catal B: Environ,2021,302:120859. [8] WANG D D, LITTLEWOOD P, MARKS T J, STAIR P C, WEITZ E. Coking can enhance product yields in the dry reforming of methane[J]. ACS Catal,2022,12(14):8352−8362. doi: 10.1021/acscatal.2c02045 [9] 周则龄, 张萌, 张俊峰, 宋法恩, 张清德, 谭猗生, 韩怡卓. 钙钛矿型氧化物负载Ni催化剂上甲烷二氧化碳重整反应研究[J]. 燃料化学学报,2020,48(7):833−841.ZHOU Ze-ling, ZHANG Meng, ZHANG Jun-feng, SONG Fa-en, ZHANG Qing-de, TAN Yi-sheng, HAN Yi-zhuo. Methane reforming with carbon dioxide over the perovskite supported Ni catalysts[J]. J Fuel Chem Technol,2020,48(7):833−841. [10] MARGOSSIAN T, LARMIER K, KIM S M, KRUMEICH F, MULLER C, COPERET C. Supported bimetallic NiFe nanoparticles through colloid synthesis for improved dry reforming performance[J]. ACS Catal,2017,7(10):6942−6948. doi: 10.1021/acscatal.7b02091 [11] PENG R F, CHEN Y M, ZHANG B X, LI Z P, CUI X, GUO C W, ZHAO Y C, ZHANG J Y. Tailoring the stability of Ni-Fe/mayenite in methane-carbon dioxide reforming[J]. Fuel,2021,284:118909. doi: 10.1016/j.fuel.2020.118909 [12] ZHANG T T, LIU Z X, ZHU Y A, LIU Z C, SUI Z J, ZHU K K, ZHOU X G. Dry reforming of methane on Ni-Fe-MgO catalysts: influence of Fe on carbon-resistant property and kinetics[J]. Appl Catal B: Environ,2020,264:118497. doi: 10.1016/j.apcatb.2019.118497 [13] THEOFANIDIS S A, GALVITA V V, POELMAN H, MARIN G B. Enhanced carbon-resistant dry reforming Fe-Ni catalyst: Role of Fe[J]. ACS Catal,2015,5(5):3028−3039. doi: 10.1021/acscatal.5b00357 [14] SONG Z W, WANG Q Q, GUO C, LI S, YAN W J, JIAO W Y, QIU L, YAN X L, LI R F. Improved effect of Fe on the stable NiFe/Al2O3 catalyst in low-temperature dry reforming of methane[J]. Ind Eng Chem Res,2020,59(39):17250−17258. doi: 10.1021/acs.iecr.0c01204 [15] 宋志文. 镍铁合金催化剂的构建及其甲烷干重整抗积炭性能[D]. 太原: 太原理工大学, 2021.SONG Zhi-wen. Preparation of highly coke-resistant nickel-iron alloy catalysts for dry reforming of methane[D]. Taiyuan: Taiyuan University of Technology, 2021. [16] KIM S M, ABDALA P M, MARGOSSIAN T, HOSSEINI D, FOPPA L, ARMUTLULU A, VAN BEEK W, COMAS-VIVES A, COPERET C, MULLER C. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts[J]. J Am Chem Soc,2017,139(5):1937−1949. doi: 10.1021/jacs.6b11487 [17] THEOFANIDIS S A, GALVITA V V, SABBE M, POELMAN H, DETAVERNIER C, MARIN G B. Controlling the stability of a Fe-Ni reforming catalyst: Structural organization of the active components[J]. Appl Catal B: Environ,2017,209:405−416. doi: 10.1016/j.apcatb.2017.03.025 [18] DE COSTER V, SRINATH N V, THEOFANIDIS S A, PIRRO L, VAN ALBOOM A, POELMAN H, SABBE M K, MARIN G B, GALVITA V V. Looking inside a Ni-Fe/MgAl2O4 catalyst for methane dry reforming via mossbauer spectroscopy and in situ QXAS[J]. Appl Catal B: Environ,2022,300:120720. doi: 10.1016/j.apcatb.2021.120720 [19] 李睿杰, 章菊萍, 史健, 李孔斋, 刘慧利, 祝星. Ni/CeO2催化剂的金属-载体界面调控及其低温化学链甲烷干重整性能研究[J]. 燃料化学学报,2022,50(11):1458−1470.LI Rui-jie, ZHANG Ju-ping, SHI Jian, LI Kong-zhai, LIU Hui-li, ZHU Xing. Regulation of metal-support interface of Ni/CeO2 catalyst and the performance of low temperature chemical looping dry reforming of methane[J]. J Fuel Chem Technol,2022,50(11):1458−1470. [20] LOFBERG A, GUERRERO-CABALLERO J, KANE T, RUBBENS A, JALOWIECKI-DUHAMEL L. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production[J]. Appl Catal B: Environ,2017,212:159−174. doi: 10.1016/j.apcatb.2017.04.048 [21] LIU Z Y, GRINTER D C, LUSTEMBERG P G, NGUYEN-PHAN T D, ZHOU Y H, LUO S, WALUYO I, CRUMLIN E J, STACCHIOLA D J, ZHOU J, CARRASCO J, BUSNENGO H F, GANDUGLIA-PIROVANO M V, SENANAYAKE S D, RODRIGUEZ J A. Dry reforming of methane on a highly-active Ni-CeO2 catalyst: Effects of metal-support interactions on C–H bond breaking[J]. Angew Chem Int Ed,2016,55(26):7455−7459. doi: 10.1002/anie.201602489 [22] MAI H X, SUN L D, ZHANG Y W, SI R, FENG W, ZHANG H P, LIU H C, YAN C H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes[J]. J Phys Chem B,2005,109(51):24380−24385. doi: 10.1021/jp055584b [23] 闫宁, 周安宁, 张亚刚, 杨志远, 贺新福, 张亚婷. CeO2的形貌特征对Ni/CeO2催化剂CO甲烷化性能的影响[J]. 燃料化学学报,2020,48(4):466−475.YAN Ning, ZHOU An-ning, ZHANG Ya-gang, YANG Zhi-yuan, HE Xin-fu, ZHANG Ya-ting. Morphologic effect of CeO2 on the catalytic performance of Ni/CeO2 in CO methanation[J]. J Fuel Chem Technol,2020,48(4):466−475. [24] WU Z L, LI M J, HOME J, MEYER H M, OVERBURY S H. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption[J]. Langmuir,2010,26(21):16595−16606. doi: 10.1021/la101723w [25] FRANCISCO M S P, MASTELARO V R, NASCENTE P A P, FLORENTINO A O. Activity and characterization by XPS, HR-TEM, Raman spectroscopy, and BET surface area of CuO/CeO2-TiO2 catalysts[J]. J Phys Chem B,2001,105:10515−10522. doi: 10.1021/jp0109675 [26] LIU W M, YANG S Y, ZHANG Q L, HE T Y, LUO Y W, TAO J X, WU D S, PENG H G. Insights into flower-like Al2O3 spheres with rich unsaturated pentacoordinate Al3 + sites stabilizing Ru-CeOx for propane total oxidation[J]. Appl Catal B: Environ,2021,292:120171. doi: 10.1016/j.apcatb.2021.120171 [27] LI H B, CUI Y Y, LIU Q Q, DAI W L. Insight into the synergism between copper species and surface defects influenced by copper content over copper/ceria catalysts for the hydrogenation of carbonate[J]. ChemCatChem,2018,10(3):619−624. doi: 10.1002/cctc.201701384 [28] JIANG F, WANG S S, LIU B, LIU J, WANG L, XIAO Y, XU Y B, LIU X H. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts[J]. ACS Catal,2020,10(19):11493−11509. doi: 10.1021/acscatal.0c03324 [29] JIANG Z Y, JING M Z, FENG X B, XIONG J C, HE C, DOUTHWAITE M, ZHENG L R, SONG W Y, LIU J, QU Z G. Stabilizing platinum atoms on CeO2 oxygen vacancies by metal-support interaction induced interface distortion: mechanism and application[J]. Appl Catal B-Environ,2021,278:119304. [30] LIANG D F, WANG Y S, CHEN M Q, XIE X L, LI C, WANG J, YUAN L. Dry reforming of methane for syngas production over attapulgite-derived MFI zeolite encapsulated bimetallic Ni-Co catalysts[J]. Appl Catal B: Environ,2023,322:122088. doi: 10.1016/j.apcatb.2022.122088 [31] WANG N, QIAN W Z, CHU W, WEI F. Crystal-plane effect of nanoscale CeO2 on the catalytic performance of Ni/CeO2 catalysts for methane dry reforming[J]. Catal Sci Technol,2016,6(10):3594−3605. doi: 10.1039/C5CY01790D [32] XIE Y, CHEN J J, WU X, WEN J J, ZHAO R, LI Z L, TIAN G C, ZHANG Q L, NING P, HAO J M. Frustrated lewis pairs boosting low-temperature CO2 methanation performance over Ni/CeO2 nanocatalysts[J]. ACS Catal,2022,12(17):10587−10602. doi: 10.1021/acscatal.2c02535 [33] LI Y B, WANG Q Q, CAO M, LI S, SONG Z W, QIU L, YU F, LI R F, YAN X L. Structural evolution of robust Ni3Fe1 alloy on Al2O3 in dry reforming of methane: effect of iron-surplus strategy from Ni1Fe1 to Ni3Fe1[J]. Appl Catal B: Environ,2023,331:122669. doi: 10.1016/j.apcatb.2023.122669 -




 下载:
下载: