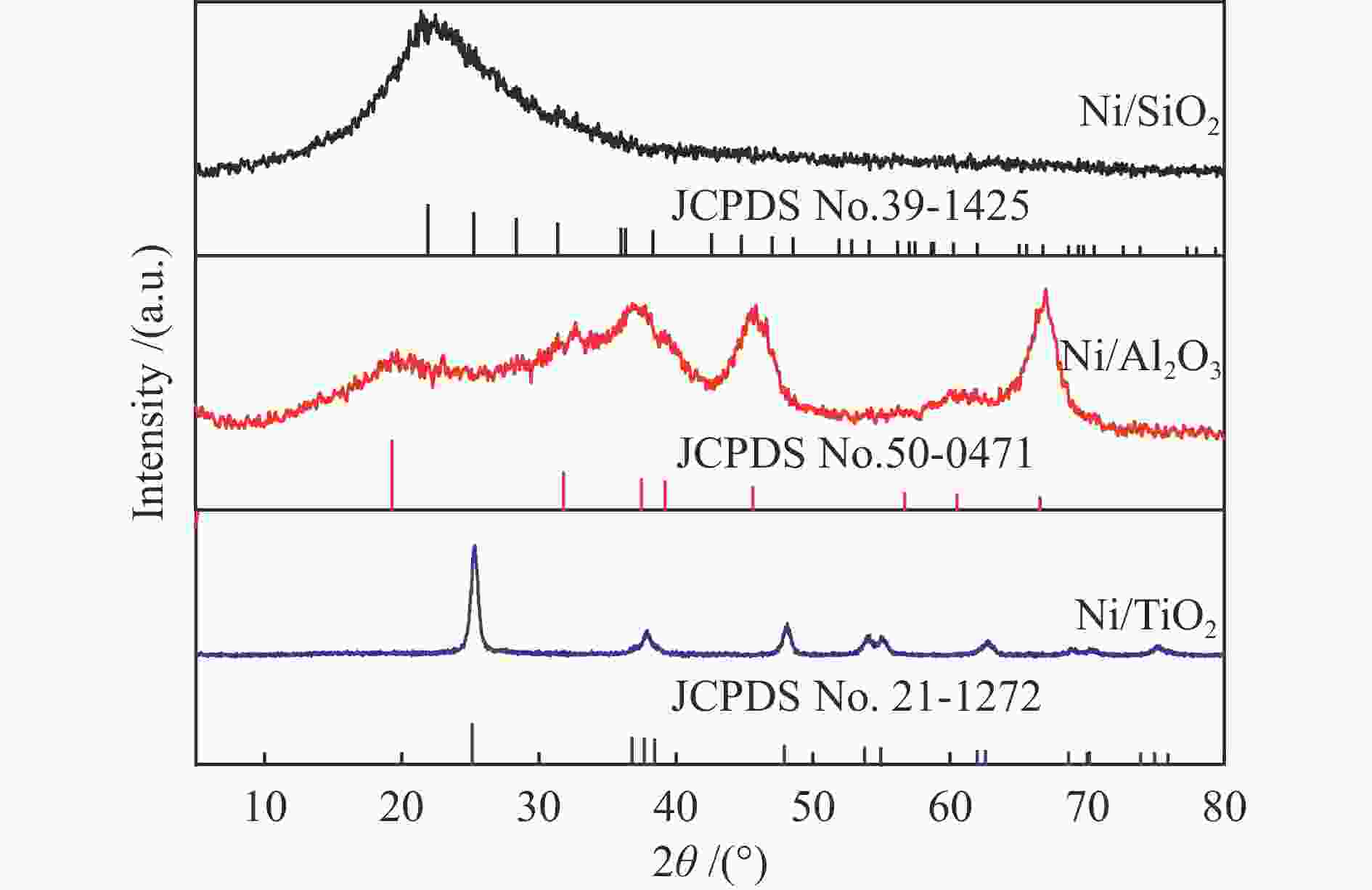
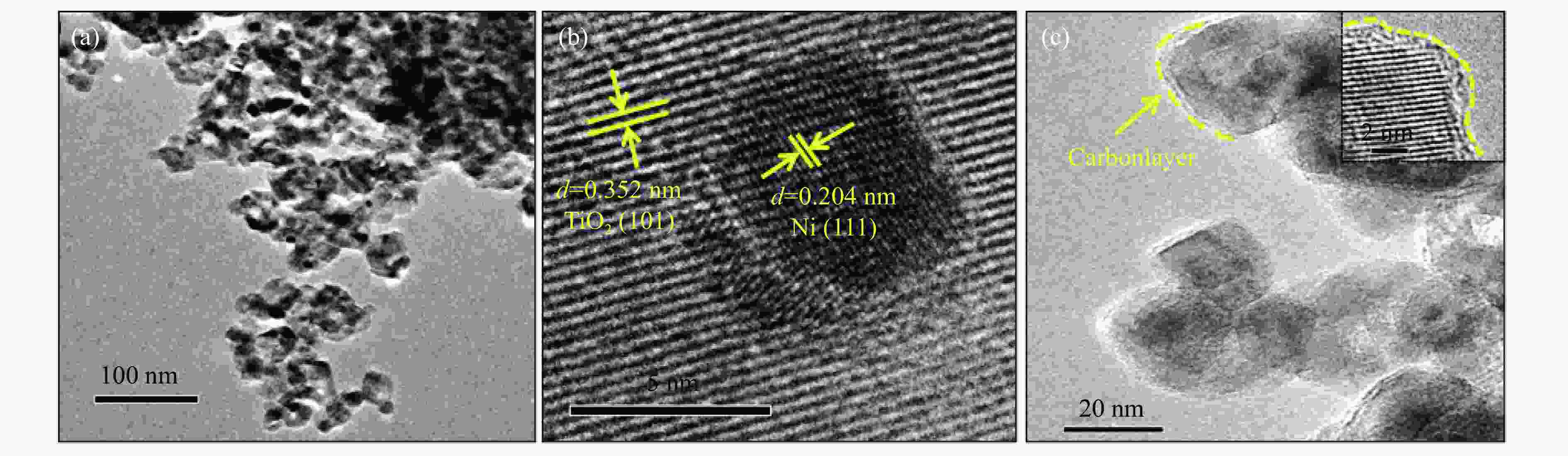
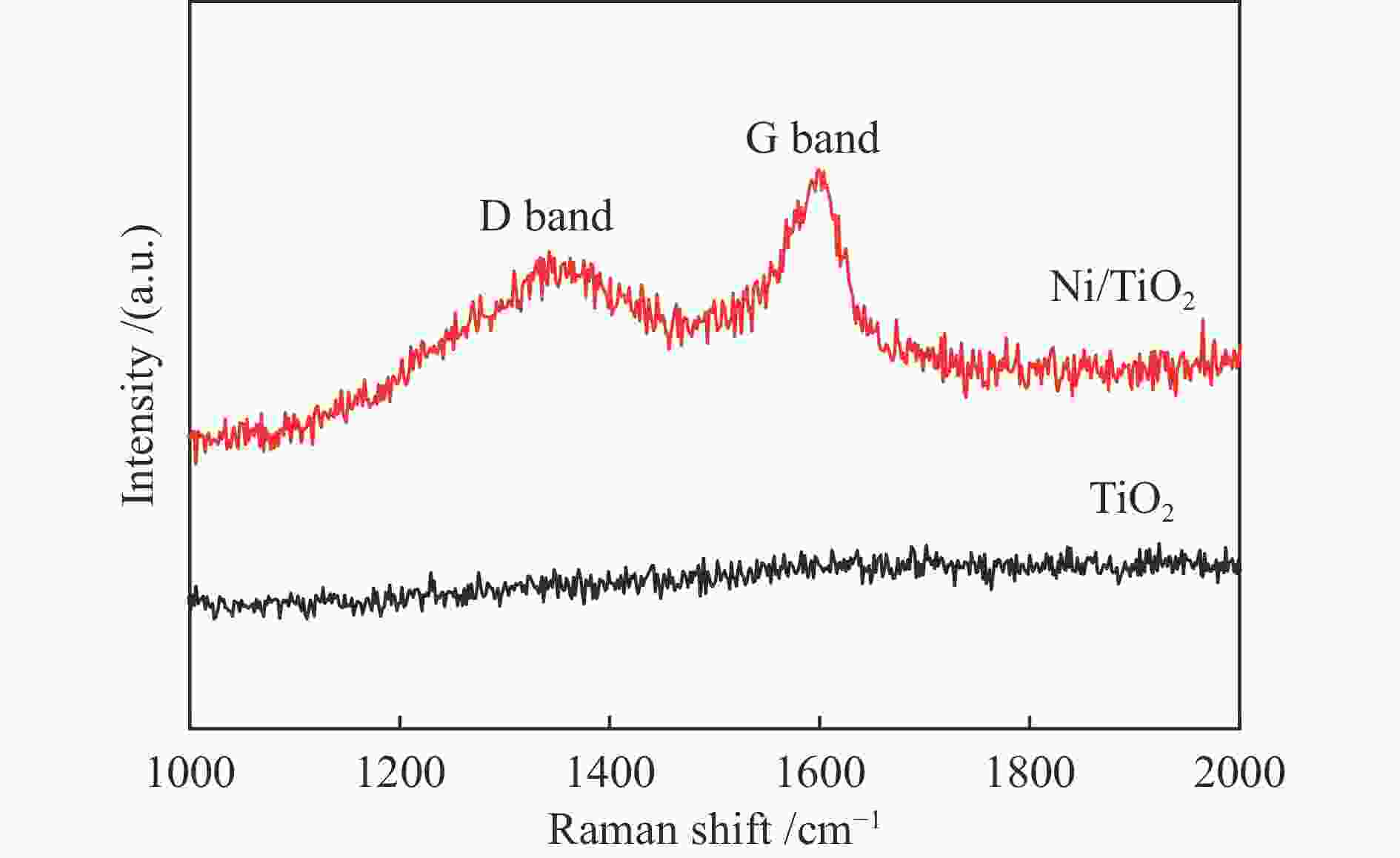
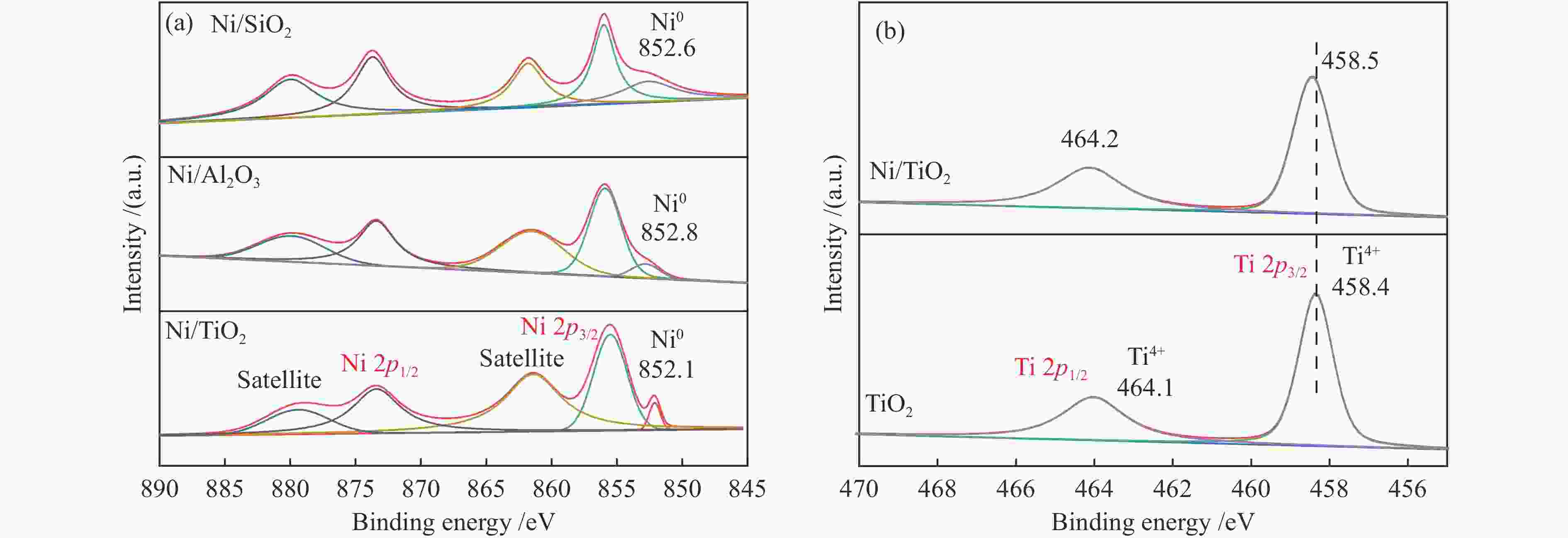
Plasmon-enhanced photocatalytic selective hydrogenation of phenylacetylene over Ni/TiO2 catalysts
-
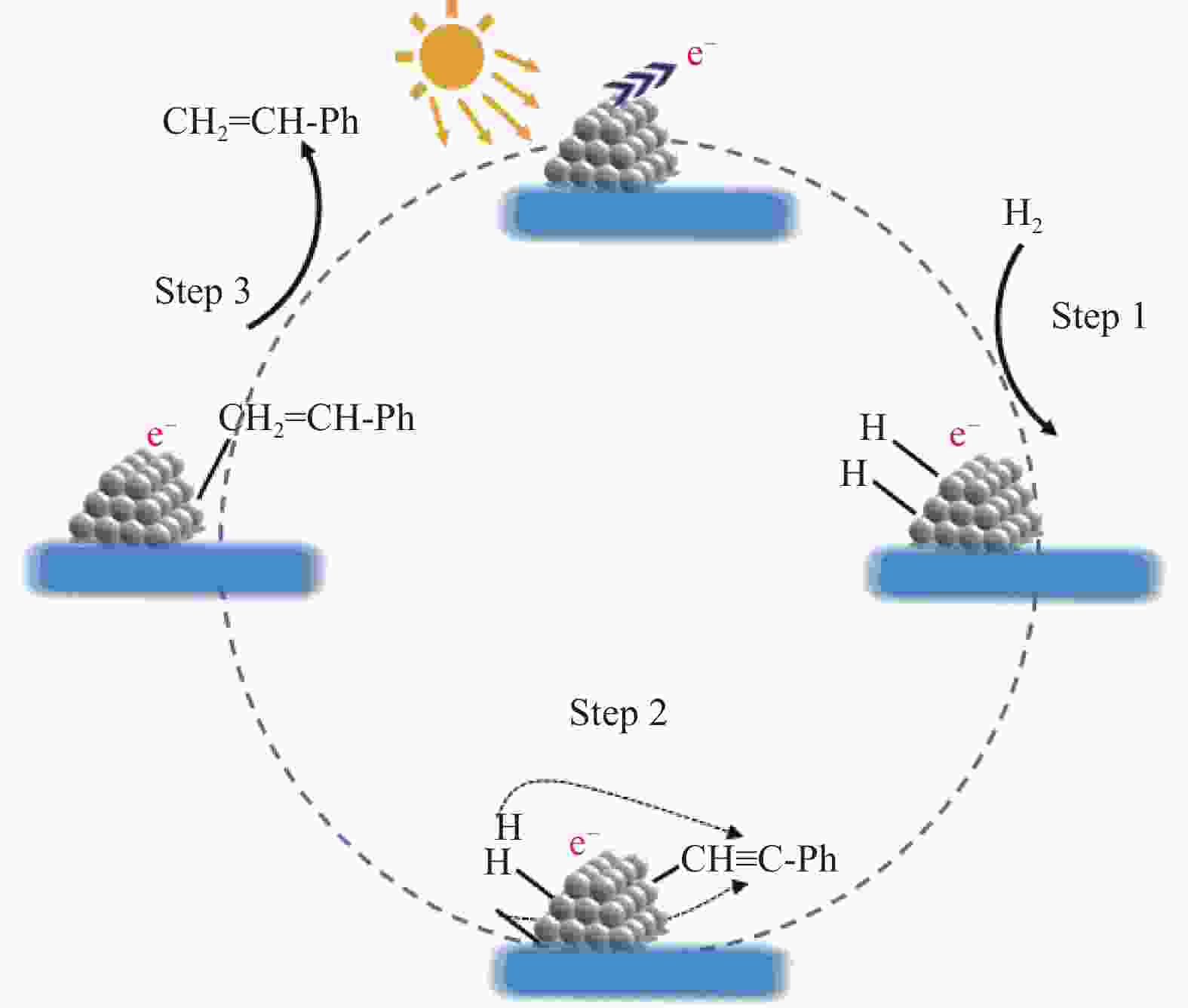
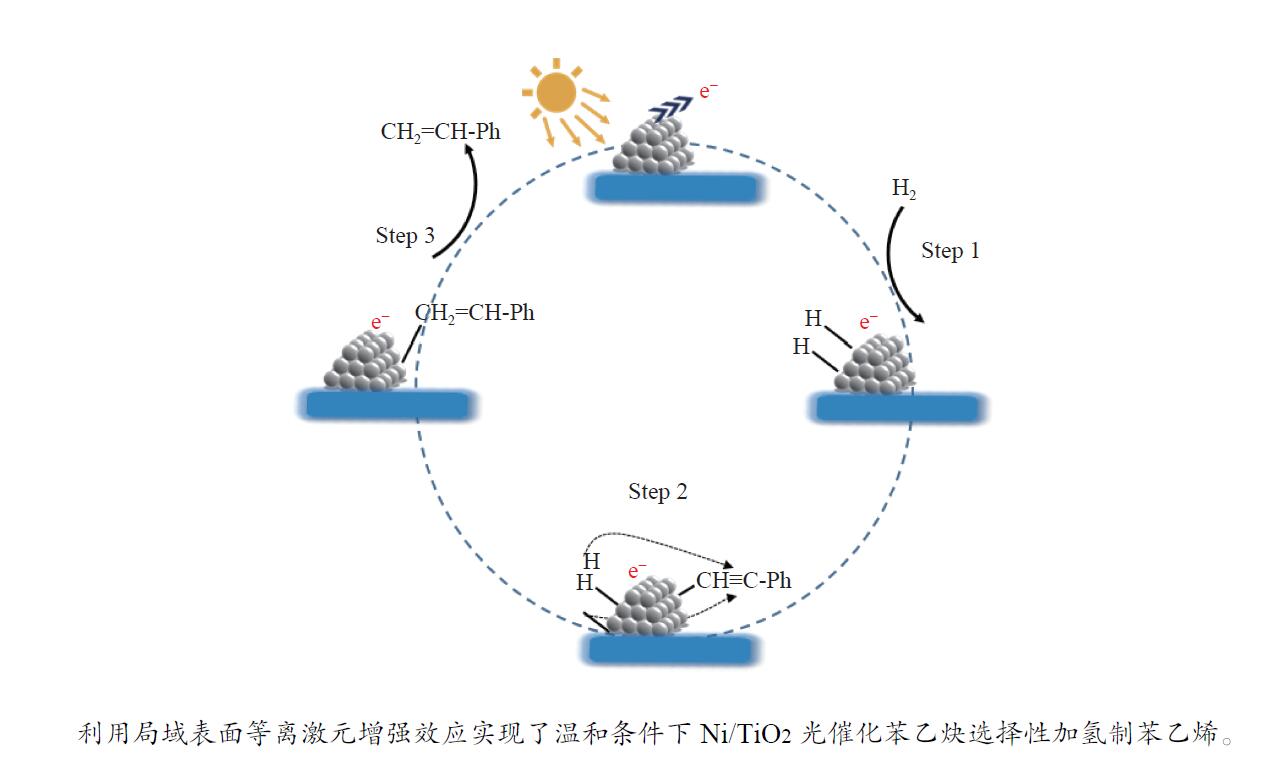
摘要: 研究采用浸渍还原法制备了碳修饰的二氧化钛负载的Ni纳米颗粒催化剂,在苯乙炔选择性加氢反应中表现出良好的光催化性能。Ni纳米颗粒在可见光激发下产生高能“热电子”促进了反应底物的解离和活化。富电子态的Ni纳米颗粒抑制了苯乙烯在Ni/TiO2表面的吸附,提高了苯乙烯的选择性。本工作为光催化苯乙炔选择性加氢反应提供了一种绿色且高效的方法。Abstract: It is a great challenge for the selective hydrogenation of phenylacetylene to styrene over non-noble metal catalyst under mild reaction conditions. Carbon-modified TiO2 supported nickel nanoparticles catalyst was prepared using impregnation-reduction method, which exhibited excellent photocatalytic performance in selective hydrogenation of phenylacetylene under visible light irradiation. The photo-excited hot electrons over Ni nanoparticles promoted the activation of reactants. The electron-rich Ni nanoparticles inhibited the adsorption of phenylethylene on the surface of Ni/TiO2, which increased the selectivity of phenylethylene. This work provides an environmentally-benign and efficient method for photocatalytic hydrogenation of phenylacetylene.
-
Key words:
- Ni nanoparticles /
- phenylacetylene /
- photocatalysis /
- selective hydrogenation /
- hot electrons
-
表 1 不同样品的物理化学参数
Table 1 Physicochemical parameters of varioussamples
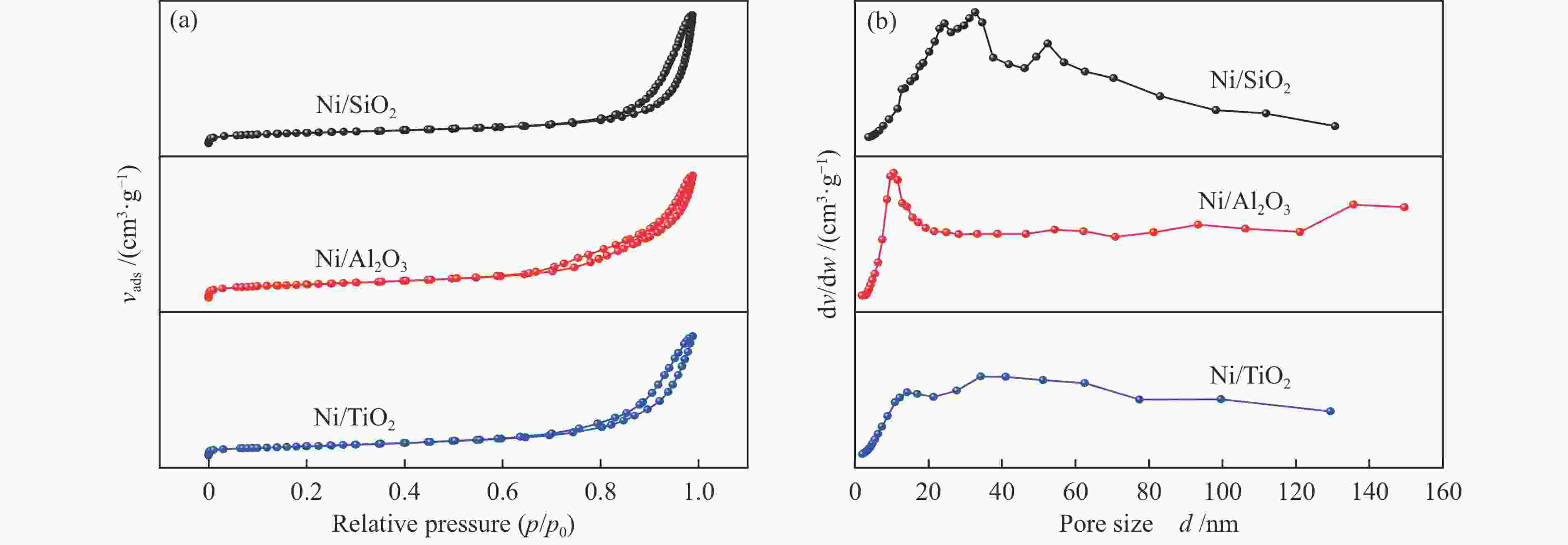
Entry Sample SBET /(m2·g−1) vPore /(cm3·g−1) dPore /nm 1 TiO2 82.30 0.35 15.21 2 Ni/TiO2 62.35 0.32 19.13 3 Al2O3 188.89 0.67 12.86 4 Ni/Al2O3 167.15 0.66 16.91 5 SiO2 170.55 0.71 21.05 6 Ni/SiO2 144.43 0.75 24.95 表 2 光催化苯乙炔选择性加氢活性
Table 2 Comparison of photocatalytic selective hydrogenation of phenylacetylene
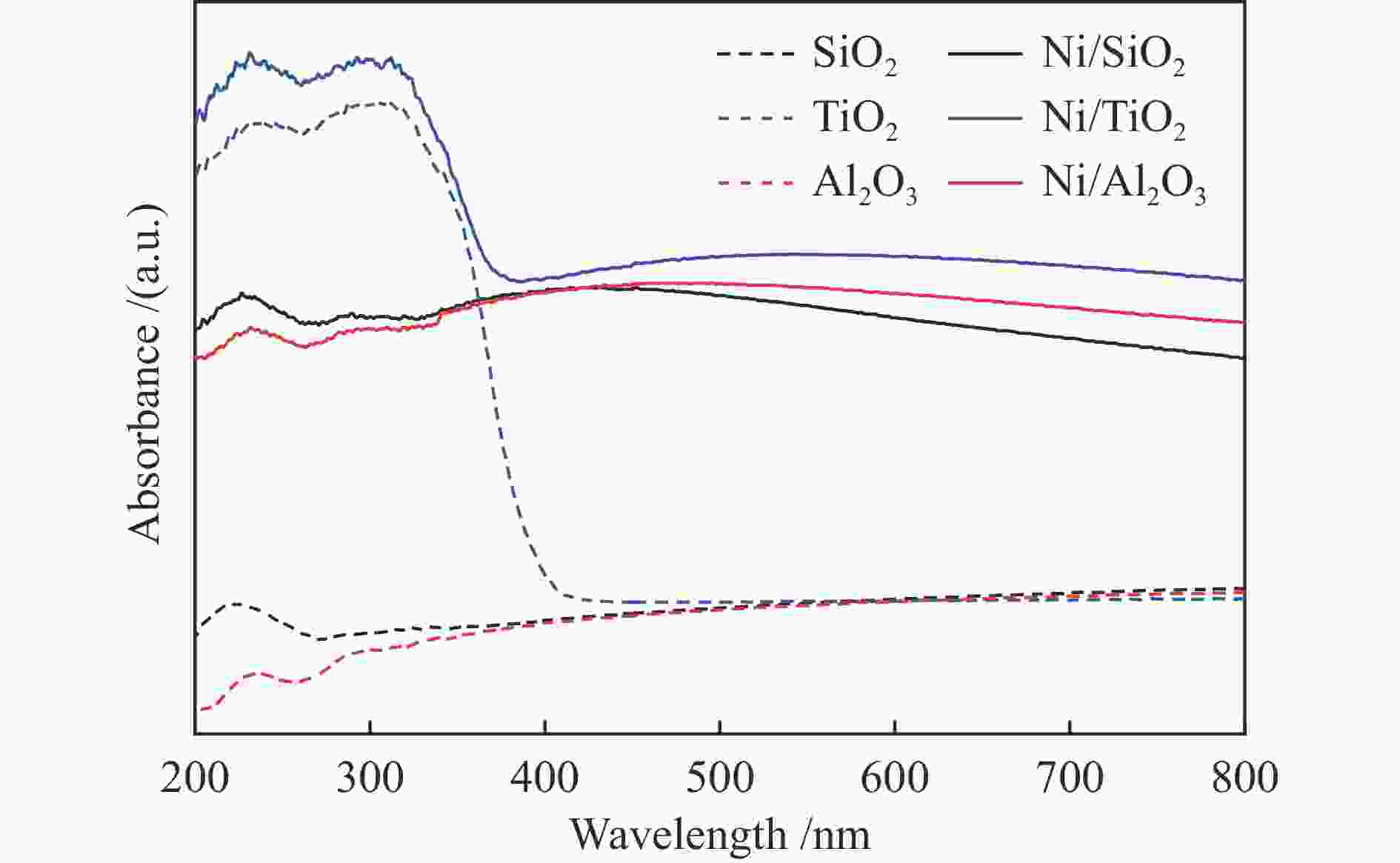
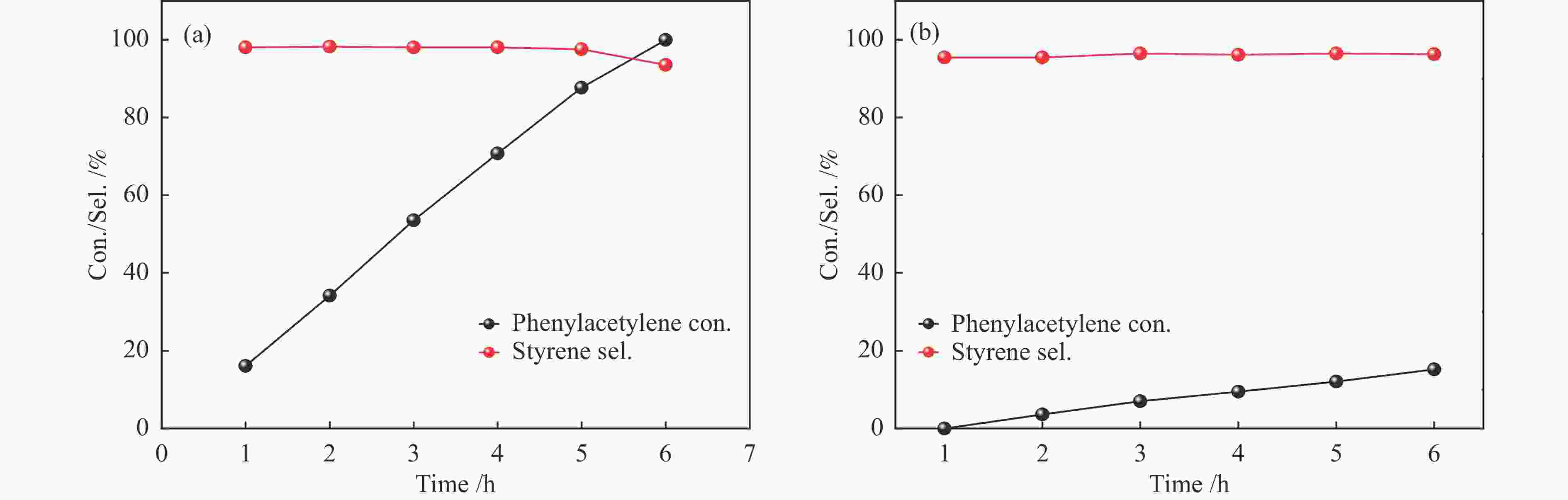
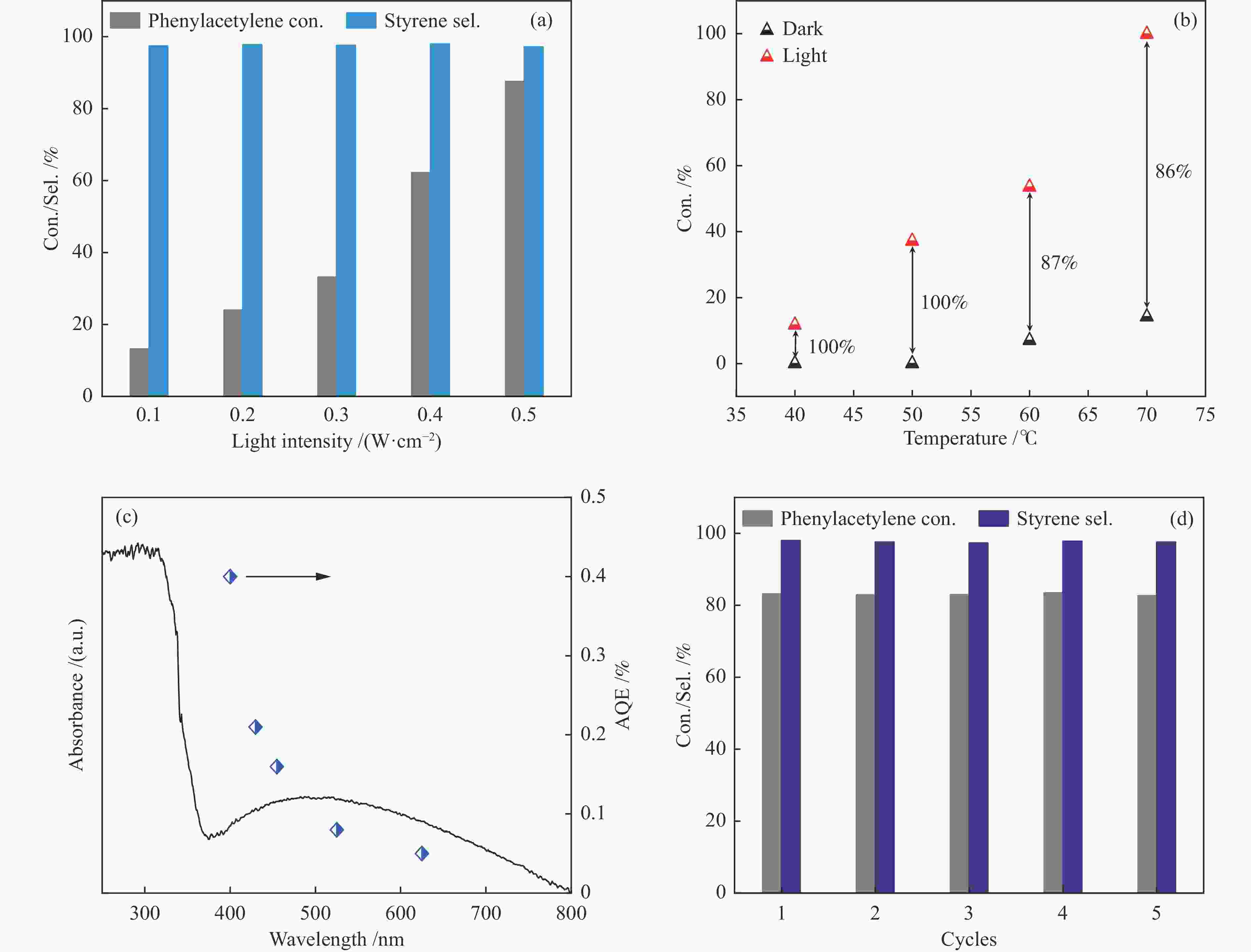
Entry Catalyst Irradiation Time /h Con. /% Sel. /% 1a 1.5% Ni/TiO2 light 5 33.2 97.6 dark 5 − − 2 a 3.0% Ni/TiO2 light 5 70.7 98.0 dark 5 5.3 96.1 3 a 4.5% Ni/TiO2 light 5 87.6 97.5 dark 5 12.1 96.4 4 a 5.5% Ni/TiO2 light 5 62.2 98.4 dark 5 7.1 95.9 5 Ni/Al2O3 light 6 39.7 93.0 dark 6 6.9 95.8 6 Ni/SiO2 light 6 7.9 96.0 dark 6 3.1 99.9 7 a TiO2 light 5 − − 8b no catalyst light 5 − − 9c 4.5% Ni/TiO2 light 5 − − 10d Ni/TiO2 light 4 66.9 98.2 11e 4.5% Ni/TiO2 light 3 81.9 98.6 dark 3 48.7 99.9 a: Reaction conditions: phenylacetylene (0.1 mmol), catalyst (20 mg), isopropanol (2 mL), H2 (1 atm), 60 ℃, 5 h, LED lamp (wavelength 430−720 nm, light intensity 0.5 W/cm2); b: Without catalyst; c: Ar (1 atm); d: Prepared using Ni(OH)2 as precursor; e: A mixture of phenylacetylene (0.05 mmol) and styrene (0.05 mmol) was used as reactant 表 3 Ni基催化剂在苯乙炔选择性加氢中的性能
Table 3 Performance comparison of various Ni based catalysts for the selective hydrogenation of phenylacetylene
Catalyst Solvent Phenylacetylene /mmol t /℃ p /MPa Con. /% Sel. /% Ref Ni NSs ethanol 1.0 50 0.1 98 89 [3] Ni/2D BP tetrahydrofuran/toluene (3∶1) 0.3 80 1.0 93.2 92.8 [26] Ni-fructose@SiO2-800 acetonitrile 1.0 110 1.0 − 88a [27] Ni/C-400-6 ethanol 10 50 1.0 >99 77.3 [28] Ni-CNFs(1)/MS 2-propanol 1.7 80 0.1 90.8 ~ 90 [29] H350-Ni/COF methanol 0.4 100 1.0 >99 85 [30] Ni2P/MZSM-5-2 ethanol 45.6 100 1.0 ~ 99 ~ 85 [31] Ni2P/Al2O3 2-propanol 9.1 100 0.3 98.6 88.2 [32] Ni2Si/SiO2 ethanol 10 80 1.0 79.0 87.7 [33] 450-NiSix ethanol 10 50 0.41 79.9 87.7 [34] NiCo0.09/ SiO2 ethanol 49.0 60 0.5 >99 88 [2] NiZn3/Al2O3 methanol 49.0 60 0.5 >99 92 [35] Pre-NiCu/MMO toluene 5.0 100 0.4 95.8 90.3 [23] Ni3Sn/MgAl2O4 hexane 1.0 40 0.5 >99 89 [36] Ni5Mg4Ga3-700 2-propanol 9.1 40 0.3 95.1 92.2 [37] Ni/TiO2 2-propanol 0.1 60 0.1 >99 93.5 this work -
[1] FAN Q, HE S, HAO L, LIU X, ZHU Y, XU S, ZHANG F. Photodeposited Pd nanoparticles with disordered structure for phenylacetylene semihydrogenation[J]. Sci Rep,2017,7:42172. doi: 10.1038/srep42172 [2] CHEN W, BAO Z, ZHOU Z. Selective hydrogenation of phenylacetylene over non-precious bimetallic Ni-Zn/SiO2 and Ni-Co/SiO2 catalysts prepared by glucose pyrolysis[J]. React Kinet, Mech Catal,2022,135:2533−2550. doi: 10.1007/s11144-022-02276-w [3] YU J W, WANG X Y, YUAN C Y, LI W Z, WANG Y H, ZHANG Y W. Synthesis of ultrathin Ni nanosheets for semihydrogenation of phenylacetylene to styrene under mild conditions[J]. Nanoscale,2018,10(15):6936−6944. doi: 10.1039/C8NR00532J [4] ZHANG L, ZHOU M, WANG A, ZHANG T. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms[J]. Chem Rev,2020,120(2):683−733. doi: 10.1021/acs.chemrev.9b00230 [5] LI Z, HU M, LIU B, LIU J, WANG P, YAO J, ZHANG X, HE M, SONG W. Pd-Zn alloy nanoparticles encapsulated into mesoporous silica with confinement effect for highly selective semi-hydrogenation of phenylacetylene[J]. ChemCatChem,2020,13(3):868−873. [6] YANG F, DING S, SONG H, YAN N. Single-atom Pd dispersed on nanoscale anatase TiO2 for the selective hydrogenation of phenylacetylene[J]. Sci China Mater,2020,63(6):982−992. doi: 10.1007/s40843-020-1271-x [7] HU M, JIN L, DANG Y, SUIB S L, HE J, LIU B. Supported Pt nanoparticles on mesoporous titania for selective hydrogenation of phenylacetylene[J]. Front Chem,2020,8:581512. doi: 10.3389/fchem.2020.581512 [8] SUN D, BI Q Y, DENG M X, JIA B Q, HUANG F Q. Atomically dispersed Pd-Ru dual sites in an amorphous matrix towards efficient phenylacetylene semi-hydrogenation[J]. Chem Commun,2021,57(46):5670−5673. doi: 10.1039/D1CC00923K [9] LEITMANNOVA E, SVOBODA J, SEDLACEK J, VOHLIDAL J, KACER P, CERVENY L. Hydrogenation of phenylacetylene and 3-phenylpropyne using Rh(diene) complexes under homogeneous and heterogeneous conditions[J]. Appl Catal A: Gen,2010,372:34−39. [10] EROKHIN A V, LOKTEVA E S, YERMAKOV A Y, BOUKHVALOV D W, MASLAKOV K I, GOOLUBINA E V, UIMIN M A. Phenylacetylene hydrogenation on Fe@C and Ni@C core-shell nanoparticles: About intrinsic activity of graphene-like carbon layer in H2 activation[J]. Carbon,2014,74:291−301. doi: 10.1016/j.carbon.2014.03.034 [11] HUANG Y, YAN H T, ZHANG C Y, WANG Y, WEI Q H, ZHANG R K. Interfacial electronic effects in Co@N-doped carbon shells heterojunction catalyst for semihydrogenation of phenylacetylene[J]. Nanomaterials,2021,11(11):2776. doi: 10.3390/nano11112776 [12] SUN Y, LUO B, XU S, GUO W, HUANG X, SHAO L. Atomic Cu on nanodiamond-based sp2/sp3 hybrid nanostructures for selective hydrogenation of phenylacetylene[J]. Chem Phys Lett,2019,723:39−43. doi: 10.1016/j.cplett.2019.03.015 [13] PANG M, SHAO Z, WANG X, LIANG C, XIA W. Toward economical purification of styrene monomers: Eggshell Mo2C for front-end hydrogenation of phenylacetylene[J]. AIChE J,2015,61(8):2522−2531. doi: 10.1002/aic.14822 [14] LV S, DU Y, WU F, CAI Y, ZHOU T. Review on LSPR assisted photocatalysis: Effects of physical fields and opportunities in multifield decoupling[J]. Nanoscale Adv,2022,4(12):2608−2631. doi: 10.1039/D2NA00140C [15] BUENO-ALEJO C J, ARCA-RAMOS A, HUESO J L, SANTAMARIA J. LED-driven continuous flow carbon dioxide hydrogenation on a nickel-based catalyst[J]. Catal Today,2020,355:678−684. [16] WANG J, WANG M, LI X, GU X, KONG X, WANG R, KE X, YU G, ZHENG Z. Bidentate ligand modification strategy on supported Ni nanoparticles for photocatalytic selective hydrogenation of alkynes[J]. Appl Catal B: Environ,2022,313:121449. doi: 10.1016/j.apcatb.2022.121449 [17] JIA T, MENG D, JI H, SHENG X, CHEN C, SONG W, ZHAO J. Visible-light-driven semihydrogenation of alkynes via proton reduction over carbon nitride supported nickel[J]. Appl Catal B: Environ,2022,304:121004. doi: 10.1016/j.apcatb.2021.121004 [18] GONG J, LIU J, CHEN X, JIANG Z, WEN X, MIJOWSKA E, TANG T. One-pot synthesis of core/shell Co@C spheres by catalytic carbonization of mixed plastics and their application in the photo-degradation of Congo red[J]. J Mater Chem A,2014,2(20):7461−7470. doi: 10.1039/C4TA00173G [19] XU S, TANG J, ZHOU Q, DU J, LI H. Interfacing anatase with carbon layers for photocatalytic nitroarene hydrogenation[J]. ACS Sustainable Chem Eng,2019,7(19):16190−16199. doi: 10.1021/acssuschemeng.9b03149 [20] XIN J, CUI H, CHENG Z, ZHOU Z. Bimetallic Ni-Co/SBA-15 catalysts prepared by urea co-precipitation for dry reforming of methane[J]. Appl Catal A: Gen,2018,554:95−104. doi: 10.1016/j.apcata.2018.01.033 [21] LUCCHINI M A, TESTINO A, LUDWIG C, KAMBOLIS A, EI-KAZZI M, CERVELLINO A, RIANI P, CANEPA F. Continuous synthesis of nickel nanopowders: Characterization, process optimization, and catalytic properties[J]. Appl Catal B: Environ,2014,156−157:404−415. doi: 10.1016/j.apcatb.2014.03.045 [22] LIU S, KIM K H, YUN J M, KUNDU A, SANKAR K V, PATIL U M, RAY C, JUN S C. 3D yolk-shell NiGa2S4 microspheres confined with nanosheets for high performance supercapacitors[J]. J Mater Chem A,2017,5(13):6292−6298. [23] LIU Y, ZHAO J, FENG J, HE Y, DU Y, LI D. Layered double hydroxide-derived Ni-Cu nanoalloy catalysts for semihydrogenation of alkynes: Improvement of selectivity and anti-coking ability via alloying of Ni and Cu[J]. J Catal,2018,359:251−260. doi: 10.1016/j.jcat.2018.01.009 [24] 宋娟娟, 朱宝林, 胡晓静, 石玉坤, 黄唯平. 碳修饰二氧化钛纳米管的光催化性能研究[C]//国家自然科学基金委员会, 中国化学会. 第六届全国物理无机化学会议论文摘要集, 2012: 1.SONG Juan-juan, ZHU Bao-lin, HU Xiao-jing, SHI Yu-kun, HUANG Wei-ping. Photocatalytic properties of carbon-modified titanium dioxide nanotubes[C]//National Natural Science Foundation of China, Chinese Chemical Society. Abstracts of the 6th National Conference on Physical Inorganic Chemistry, 2012: 1. [25] TORRES C C, ALDERETE J B, MELLA C, PAWELEC B. Maleic anhydride hydrogenation to succinic anhydride over mesoporous Ni/TiO2 catalysts: Effects of Ni loading and temperature[J]. J Mol Catal A: Chem,2016,423:441−448. doi: 10.1016/j.molcata.2016.07.037 [26] CAPORALI M, SERRANO-RUIZ M, TELESIO F, HEUN S, NICOTRA G, SPINELLA C, PERUZZINI M. Decoration of exfoliated black phosphorus with nickel nanoparticles and its application in catalysis[J]. Chem Commun,2017,53(79):10946−10949. doi: 10.1039/C7CC05906J [27] MURUGESAN K, ALSHAMMARI A S, SOHAIL M, BELLER M, JAGADEESH R V. Monodisperse nickel-nanoparticles for stereo- and chemoselective hydrogenation of alkynes to alkenes[J]. J Catal,2019,370:372−377. doi: 10.1016/j.jcat.2018.12.018 [28] GUO X, CHEN X, SU D, LIANG C. Preparation of Ni/C core-shell nanoparticles through MOF pyrolysis for phenylacetylene hydrogenation reaction[J]. Acta Chim Sin,2018,76(1):22−29. doi: 10.6023/A17070339 [29] DONPHAI W, KAMEGAWA T, CHAREONPANICH M, YAMASHITA H. Reactivity of Ni-carbon nanofibers/mesocellular silica composite catalyst for phenylacetylene hydrogenation[J]. Ind Eng Chem Res,2014,53(24):10105−10111. [30] WANG N, LIU J, ZHANG M, WANG C, LI X, MA L. Non-noble nickel-modified covalent organic framework for partial hydrogenation of aromatic terminal alkynes[J]. ACS Appl Mater Interfaces,2021,13(50):60135−60143. doi: 10.1021/acsami.1c22069 [31] FU W, ZHANG L, TAO T, TANG T. Highly dispersed Ni2P clusters inlaid in micropore openings on mesoporous ZSM-5 zeolite and its catalytic performance in the phenylacetylene semi-hydrogenation[J]. J Ind Eng Chem,2021,95:376−387. [32] CHEN Y, LI C, ZHOU J, ZHANG S, RAO D, HE S, WEI M, EVANS D G, DUAN X. Metal phosphides derived from hydrotalcite precursors toward the selective hydrogenation of phenylacetylene[J]. ACS Catal,2015,5(10):5756−5765. doi: 10.1021/acscatal.5b01429 [33] YANG K, CHEN X, GUAN J, LIANG C. Nickel silicides prepared from organometallic polymer as efficient catalyst towards hydrogenation of phenylacetylene[J]. Catal Today,2015,246:176−183. doi: 10.1016/j.cattod.2014.09.027 [34] CHEN X, ZHAO A, SHAO Z, MA Z, LIANG C. A novel approach to synthesize highly selective nickel silicide catalysts for phenylacetylene semihydrogenation[C]// Scientific Bases for the Preparation of Heterogeneous Catalysts-Proceedings of the 10th International Symposium. Louvain-la-Neuve, Belgium, 2010: 77−84. [35] BAO Z, YANG L, CHENG Z, ZHOU Z. Selective hydrogenation of the C8 aromatic fraction of pyrolysis gasoline over NiZn3/α-Al2O3: Experimental and modeling studies[J]. Ind Eng Chem Res,2020,59(10):4322−4332. doi: 10.1021/acs.iecr.9b06476 [36] LIU Y, LIU X, FENG Q, HE D, ZHANG L, LIAN C, SHEN R, ZHAO G, JI Y, WANG D, ZHOU G, LI Y. Intermetallic Nix My (M = Ga and Sn) nanocrystals: A non-precious metal catalyst for semi-hydrogenation of alkynes[J]. Adv Mater,2016,28(23):4747−4754. doi: 10.1002/adma.201600603 [37] LI C, CHEN Y, ZHANG S, ZHOU J, WANG F, HE S, WEI M, EVANS D G, DUAN S. Nickel-gallium intermetallic nanocrystal catalysts in the semihydrogenation of phenylacetylene[J]. ChemCatChem,2014,6(3):824−831. doi: 10.1002/cctc.201300813 [38] ZHU P, GAO M, ZHANG J, WU Z, WANG R, WANG Y, WACLAWIK E R, ZHENG Z. Synergistic interaction between Ru and MgAl-LDH support for efficient hydrogen transfer reduction of carbonyl compounds under visible light[J]. Appl Catal B: Environ,2021,283:119640. doi: 10.1016/j.apcatb.2020.119640 [39] WANG R, LIU H, WANG X, LI X, GU X, ZHENG Z. Plasmon-enhanced furfural hydrogenation catalyzed by stable carbon-coated copper nanoparticles driven from metal–organic frameworks[J]. Catal Sci Technol,2020,10(19):6483−6494. [40] WANG J, GU X, PEI L, KONG P, ZHANG J, WANG X, WANG R, WACLAWIK E R, ZHENG Z. Strong metal-support interaction induced O2 activation over Au/MNb2O6 (M = Zn2 + , Ni2 + and Co2 + ) for efficient photocatalytic benzyl alcohol oxidative esterification[J]. Appl Catal B: Environ,2021,283:119618. doi: 10.1016/j.apcatb.2020.119618 [41] LIU W, OTERO AREAN C, BORDIGA S, GROPPO E, ZECCHINA A. Selective phenylacetylene hydrogenation on a polymer-supported palladium catalyst monitored by FTIR spectroscopy[J]. ChemCatChem,2011,3(1):222−226. doi: 10.1002/cctc.201000244 [42] HE P, CHEN B, HUANG L, LIU X, QIN J, ZHANG Z, DAI W. Heterogeneous manganese-oxide-catalyzed successive cleavage and functionalization of alcohols to access amides and nitriles[J]. Chem,2022,8(7):1906−1927. doi: 10.1016/j.chempr.2022.02.021 [43] SOLIS-GARCIA A, LOUVIER-HERNANDEZ J F, ALMENDAREZ-CAMARILLO A, FIERRO-GONZALEZ J C. Participation of surface bicarbonate, formate and methoxy species in the carbon dioxide methanation catalyzed by ZrO2-supported Ni[J]. Appl Catal B: Environ,2017,218:611−620. doi: 10.1016/j.apcatb.2017.06.063 -




 下载:
下载: