Technological advances in the production of high value oxygen-containing chemicals from coal via dimethyl oxalate
-
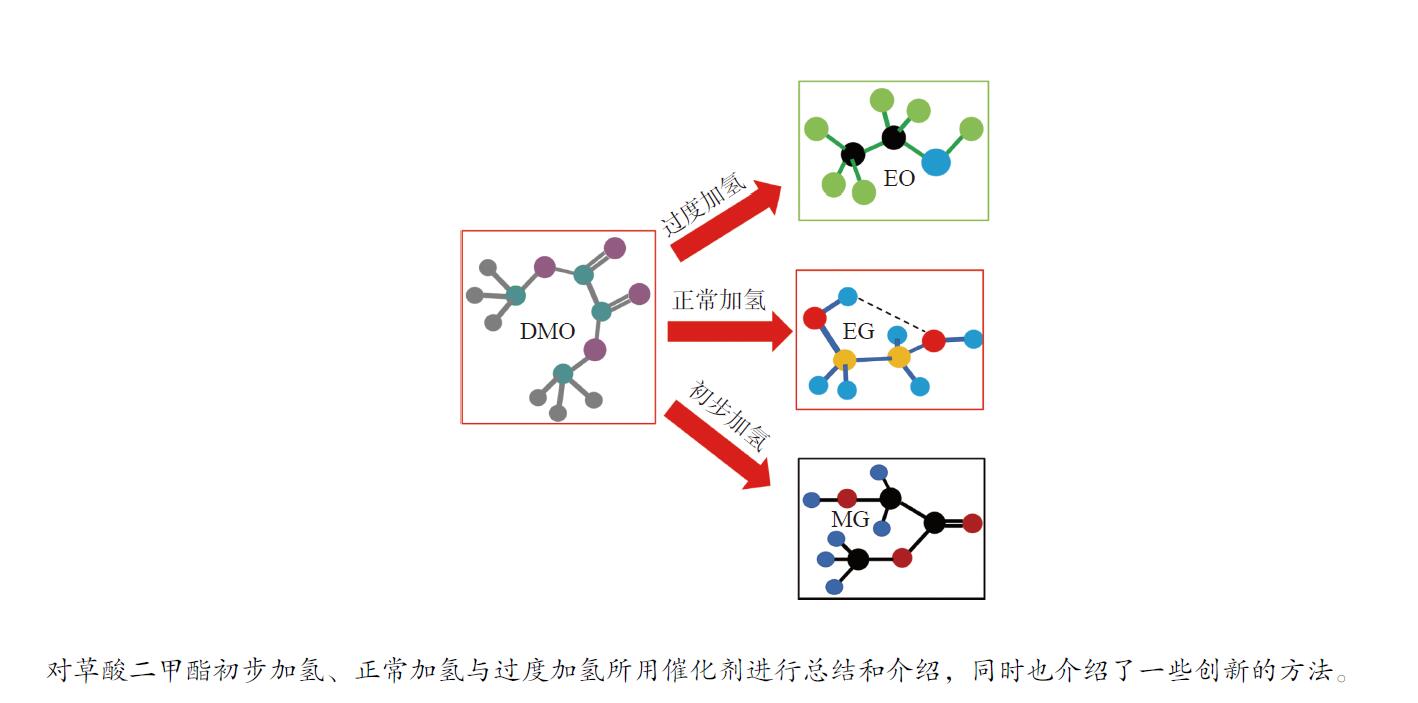
摘要: 中国的能源结构是富煤少油,开发煤炭资源的高效清洁利用是中国重点发展方向。煤经合成气羰基化后可以合成草酸酯(DMO),进一步加氢可获得具有高附加值的含氧化学品:如乙醇酸甲酯(MG)、乙二醇(EG)、乙醇(EO)等。其中,MG可以制备可降解材料聚乙醇酸(PGA),EG可以合成聚乙二醇(PEG),EO可以合成醋酸乙酯(EAC),应用前景十分广泛。本工作围绕DMO加氢反应展开,分析了各个加氢过程中所使用催化剂的研究状况,重点归纳了催化剂的组成调控、催化作用机理以及新催化剂制备技术,分析了DMO加氢催化剂研发过程存在的问题和挑战,指出了加氢产物以及下游产品的应用瓶颈及未来发展趋势。Abstract: Chinese energy structure is rich in coal and less in oil, and the development of efficient and clean utilization of coal resources is a key development direction in China. Coal can be used to synthesize dimethyl oxalate (DMO) after carbonylation by synthesis gas, and DMO can further be hydrogenated to obtain oxygen-containing chemicals with high added value, such as methyl glycolate (MG), ethylene glycol (EG), ethanol (EO), etc. Among them, MG can prepare degradable materials polyglycolic acid (PGA), EG can synthesize polyethylene glycol (PEG), and EO can synthesize ethyl acetate (EAC), which have wide application prospects. This paper focuses on DMO hydrogenation reactions, analyzes the research status of catalysts used in each hydrogenation process, focuses on the regulation of catalyst composition, catalytic mechanism and new catalyst preparation technology, analyzes the problems and challenges in the development of DMO hydrogenation catalysts, and points out the application bottlenecks and future development trends of hydrogenation products and downstream products.
-
Key words:
- coal /
- dimethyl oxalate /
- selective hydrogenation /
- oxygen-containing chemicals /
- copper-based catalysts
-
图 2 冷冻铜催化剂(a)旋转溅射制备方法示意图;(b)Cu原子电子结构重构原理图;(c)冷冻铜的抗氧化性质;(d)冷冻铜催化剂在DMO加氢反应中的产物分布[32]
Figure 2 Catalyst of freezing copper: (a) Schematic diagram of rotation sputtering preparation method; (b) Principle diagram of electronic structure reconstruction of Cu atoms; (c) The resistance properties of freezing copper; (d) Product distrib ution of freezing copper catalyst in DMO hydrogenation reaction[32] (with permission from Science Advances)
表 1 Ag催化剂用于DMO转化为MG的催化
Table 1 Ag catalyst for DMO to MG conversion
Entry Catalyst Conv.DMO /% Selec.MG /% p /MPa t /℃ H2 /DMO LHSV /h−1 Lifetime /h Ref. 1 10Ag/SiO2 54.4 99.8 0.5 180 150 0.5 120 [22] 2 15Ag/SiO2 100.0 99.8 2.5 220 100 0.2 − [23] 3 4.5Ag/SBA-15 99.9 95.6 3.0 235 100 0.6 110 [24] 4 15Ag/KCC-1 97.8 92.2 3.0 200 100 1.75 100 [25] 5 10Ag/0.02Ti-KCC ~ 98.0 ~ 95.0 3.0 200 100 1.75 200 [26] 6 Ag-in/hCNT 100.0 >97.0 3.0 220 80 0.6 150 [27] 7 Ag/AC-N-3 100.0 ~ 95.0 3.0 220 80 0.6 100 [28] 8 5Ag1Ni0.20/SBA-15 97.6 92.8 3.0 200 80 1.0 140 [29] 9 Ag-B2O3/SiO2 98.9 97.2 0.5 180 150 0.5 260 [22] 表 2 Cu用于DMO转化为MG的催化性能
Table 2 Cu catalyst for DMO to MG conversion
Entry Catalyst Conv.DMO /% Selec.MG /% p /MPa t /℃ H2 /DMO LHSV /h−1 Lifetime /h Ref. 1 Cu/SiO2-u 26.5 87 3.0 180 80 4.0 200 [31] 2 SP-Cu-SiO2 20 87.0 3.0 240 150 0.5 ~ 30 [32] 3 6Cu/SBA-15 75.4 60.8 3.0 180 80 0.6 − [33] 4 Cu/AC-673 91.6 88.9 2.5 220 120 0.18 120 [20] 5 Cu-ZrO2-SiO2 90 90 2.0 200 150 0.3 100 [34] 6 Raney Cu-40 80.0 80.0 2.5 210 100 2.0 100 [35] 7 20Cu-HAP 85.0 75.0 2.5 210 150 0.4 120 [36] 8 Cu/MgO ~ 60.0 88.0 2.5 210 200 0.257 300 [37] 9 Cu/RGO 100.0 98.8 2.5 210 200 0.257 264 [38] 10 Cu/SiO2-CeO2 100 95 − 200 80 6 100 [39] -
[1] WANG G, XU Y, REN H. Intelligent and ecological coal mining as well as clean utilization technology in China: Review and prospects[J]. Int J Min Sci Technol,2019,29(2):161−169. doi: 10.1016/j.ijmst.2018.06.005 [2] WAGNER N J, COERTZEN M, MATJIE R H, VAN DYK J C, SUÁREZ-RUIZ I, CRELLING J C. Applied Coal Petrology[M]. America: Academic Press, 2008: 119−144. [3] XU J, YANG Y, LI Y-W. Recent development in converting coal to clean fuels in China[J]. Fuel,2015,152:122−130. doi: 10.1016/j.fuel.2014.11.059 [4] BELL D A, TOWLER B F, FAN M. Coal Gasification And Its Applications[M]. English: William Andrew, 2010: 101–111. [5] TREMEL A, HASELSTEINER T, KUNZE C, SPLIETHOFF H. Experimental investigation of high temperature and high pressure coal gasification[J]. Appl Energy,2012,92:279−285. doi: 10.1016/j.apenergy.2011.11.009 [6] ZHAO Y, ZHANG Y, WANG Y, ZHANG J, XU Y, WANG S, MA X. Structure evolution of mesoporous silica supported copper catalyst for dimethyl oxalate hydrogenation[J]. Appl Catal A: Gen,2017,539:59−69. doi: 10.1016/j.apcata.2017.04.001 [7] CHEN Z, DUN Q, SHI Y, LAI D, ZHOU Y, GAO S, XU G. High quality syngas production from catalytic coal gasification using disposable Ca(OH)2 catalyst[J]. Chem Eng J,2017,316:842−849. doi: 10.1016/j.cej.2017.02.025 [8] LU T, MAO Y, WANG H, LIU L, LI K. Effect of pre-treatment on catalytic coal gasification characteristics of sub-bituminous coal[J]. J Energy Inst,2021,96:173−180. doi: 10.1016/j.joei.2021.03.013 [9] HASANOĞLU A, FAKI E, SEÇER A, TÜRKER ÜZDEN Ş. Co-solvent effects on hydrothermal co-gasification of coal/biomass mixtures for hydrogen production[J]. Fuel, 2023, 331: part 1. [10] HONG Y C, LEE S J, SHIN D H, KIM Y J, LEE B J, CHO S Y, CHANG H S. Syngas production from gasification of brown coal in a microwave torch plasma[J]. Energy,2012,47(1):36−40. doi: 10.1016/j.energy.2012.05.008 [11] MIDILLI A, KUCUK H, TOPAL M E, AKBULUT U, DINCER I. A comprehensive review on hydrogen production from coal gasification: Challenges and opportunities[J]. Int J Hydrogen Energy,2021,46(50):25385−25412. doi: 10.1016/j.ijhydene.2021.05.088 [12] JIANG X Z, SU Y H, LEE B J, CHIEN S H. A study on the synthesis of diethyl oxalate over Pd/α-Al2O3 catalysts[J]. Appl Catal A: Gen,2001,211(1):47−51. doi: 10.1016/S0926-860X(00)00837-1 [13] YANG L, PAN Z, WANG D, WANG S, WANG X, MA H, LIU H, WANG C, QU W, TIAN Z. Highly effective Pd/MgO/γ-Al2O3 catalysts for CO oxidative coupling to dimethyl oxalate: The effect of MgO coating on γ-Al2O3[J]. ACS Appl Mater Interfaces,2021,13(24):28064−28071. doi: 10.1021/acsami.1c04051 [14] WANG Z Q, SUN J, XU Z N, GUO G C. CO direct esterification to dimethyl oxalate and dimethyl carbonate: the key functional motifs for catalytic selectivity[J]. Nanoscale,2020,12(39):20131−20140. doi: 10.1039/D0NR03008B [15] WANG C, HAN L, CHEN P, ZHAO G, LIU Y, LU Y. High-performance, low Pd-loading microfibrous-structured Al-fiber@ns-AlOOH@Pd catalyst for CO coupling to dimethyl oxalate[J]. J Catal,2016,337:145−156. doi: 10.1016/j.jcat.2016.02.008 [16] MA X, CHI H, YUE H, ZHAO Y, XU Y, LV J, WANG S, GONG J. Hydrogenation of dimethyl oxalate to ethylene glycol over mesoporous Cu-MCM-41 catalysts[J]. AlChE J,2013,59(7):2530−2539. doi: 10.1002/aic.13998 [17] YIN A, GUO X, DAI W-L, LI H, FAN K. Highly active and selective copper-containing HMS catalyst in the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Appl Catal A: Gen,2008,349(1):91−99. [18] KONG X, WU Y, DING L, WANG R, CHEN J. Effect of Cu loading on the structural evolution and catalytic activity of Cu-Mg/ZnO catalysts for dimethyl oxalate hydrogenation[J]. New J Chem,2020,44(11):4486−4493. doi: 10.1039/C9NJ06085E [19] ROHMAN F S, SULAIMAN M S, MURAT M N, AZIZ N. Performance evaluation of adaptive based model predictive control for ethylene glycol production from dimethyl oxide hydrogenation [J]. Int J Chem React Eng, 2022, 21: 859–878. [20] CUI Y, WANG B, WEN C, CHEN X, DAI W-L. Investigation of activated-carbon-supported copper catalysts with unique catalytic performance in the hydrogenation of dimethyl oxalate to methyl glycolate[J]. ChemCatChem,2016,8(3):527−531. doi: 10.1002/cctc.201501055 [21] FAN H, TAN J, ZHU Y, ZHENG H, LI Y. Efficient hydrogenation of dimethyl oxalate to methyl glycolate over highly active immobilized-ruthenium catalyst[J]. J Mol Catal A: Chem,2016,425:68−75. doi: 10.1016/j.molcata.2016.09.033 [22] CHEN H, TAN J, CUI J, YANG X, ZHENG H, ZHU Y, LI Y. Promoting effect of boron oxide on Ag/SiO2 catalyst for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. Mol Catal,2017,433:346−353. doi: 10.1016/j.mcat.2017.02.039 [23] YIN A, GUO X, DAI W, FAN K. High activity and selectivity of Ag/SiO2 catalyst for hydrogenation of dimethyl oxalate[J]. Chem Commun,2010,46(24):4348−4350. doi: 10.1039/c0cc00581a [24] ZHENG J, LIN H, WANG Y-N, ZHENG X, DUAN X, YUAN Y. Efficient low-temperature selective hydrogenation of esters on bimetallic Au-Ag/SBA-15 catalyst[J]. J Catal,2013,297:110−118. doi: 10.1016/j.jcat.2012.09.023 [25] OUYANG M, WANG Y, ZHANG J, ZHAO Y, WANG S, MA X. Three dimensional Ag/KCC-1 catalyst with a hierarchical fibrous framework for the hydrogenation of dimethyl oxalate[J]. RSC Adv,2016,6(16):12788−12791. doi: 10.1039/C5RA26602E [26] OUYANG M, WANG J, PENG B, ZHAO Y, WANG S, MA X. Effect of Ti on Ag catalyst supported on spherical fibrous silica for partial hydrogenation of dimethyl oxalate[J]. Appl Surf Sci,2019,466:592−600. doi: 10.1016/j.apsusc.2018.10.065 [27] ZHENG J, DUAN X, LIN H, GU Z, FANG H, LI J, YUAN Y. Silver nanoparticles confined in carbon nanotubes: on the understanding of the confinement effect and promotional catalysis for the selective hydrogenation of dimethyl oxalate[J]. Nanoscale,2016,8(11):5959−5967. doi: 10.1039/C5NR08651E [28] HU M, YAN Y, DUAN X, YE L, ZHOU J, LIN H, YUAN Y. Effective anchoring of silver nanoparticles onto N-doped carbon with enhanced catalytic performance for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. Catal Commun,2017,100:148−152. doi: 10.1016/j.catcom.2017.06.025 [29] ZHOU J, DUAN X, YE L, ZHENG J, LI M M-J, TSANG S C E, YUAN Y. Enhanced chemoselective hydrogenation of dimethyl oxalate to methyl glycolate over bimetallic Ag-Ni/SBA-15 catalysts[J]. Appl Catal A: Gen,2015,505:344−353. doi: 10.1016/j.apcata.2015.08.022 [30] ZHENG J, LIN H, ZHENG X, DUAN X, YUAN Y. Highly efficient mesostructured Ag/SBA-15 catalysts for the chemoselective synthesis of methyl glycolate by dimethyl oxalate hydrogenation[J]. Catal Commun,2013,40:129−133. doi: 10.1016/j.catcom.2013.06.022 [31] ZHENG X, LIN H, ZHENG J, DUAN X, YUAN Y. Lanthanum oxide-modified Cu/SiO2 as a high-performance catalyst for chemoselective hydrogenation of dimethyl oxalate to ethylene glycol[J]. ACS Catal,2013,3(12):2738−2749. doi: 10.1021/cs400574v [32] SUN J, YU J, MA Q, MENG F, WEI X, SUN Y, TSUBAKI N. Freezing copper as a noble metal-like catalyst for preliminary hydrogenation[J]. Sci Adv,2018,4(12):eaau3275. doi: 10.1126/sciadv.aau3275 [33] WANG Y-N, DUAN X, ZHENG J, LIN H, YUAN Y, ARIGA H, TAKAKUSAGI S, ASAKURA K. Remarkable enhancement of Cu catalyst activity in hydrogenation of dimethyl oxalate to ethylene glycol using gold[J]. Catal Sci Technol,2012,2(8):1637−1639. doi: 10.1039/c2cy20154b [34] WANG D, ZHANG C, ZHU M, YU F, DAI B. Highly active and stable ZrO2-SiO2-supported Cu-catalysts for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. ChemistrySelect,2017,2(17):4823−4829. doi: 10.1002/slct.201700895 [35] KONG X, MA C, ZHANG J, SUN J, CHEN J, LIU K. Effect of leaching temperature on structure and performance of Raney Cu catalysts for hydrogenation of dimethyl oxalate[J]. Appl Catal A: Gen,2016,509:153−160. doi: 10.1016/j.apcata.2015.10.029 [36] WEN C, CUI Y, CHEN X, ZONG B, DAI W-L. Reaction temperature controlled selective hydrogenation of dimethyl oxalate to methyl glycolate and ethylene glycol over copper-hydroxyapatite catalysts[J]. Appl Catal B: Environ,2015,162:483−493. doi: 10.1016/j.apcatb.2014.07.023 [37] ABBAS M, ZHANG J, CHEN Z, CHEN J. Sonochemical synthesis of Zn-promoted porous MgO-supported lamellar Cu catalysts for selective hydrogenation of dimethyl oxalate to ethanol and their long-term stability[J]. New J Chem,2018,42(21):17553−17562. doi: 10.1039/C8NJ03766C [38] ABBAS M, CHEN Z, CHEN J. Shape- and size-controlled synthesis of Cu nanoparticles wrapped on RGO nanosheet catalyst and their outstanding stability and catalytic performance in the hydrogenation reaction of dimethyl oxalate[J]. J Mater Chem A,2018,6(39):19133−19142. doi: 10.1039/C8TA07371F [39] YAO D, WANG Y, LI Y, LI A, ZHEN Z, LV J, SUN F, YANG R, LUO J, JIANG Z, WANG Y, MA X. Scalable synthesis of Cu clusters for remarkable selectivity control of intermediates in consecutive hydrogenation[J]. Nat Commun,2023,14(1):1123. doi: 10.1038/s41467-023-36640-8 [40] NORSKOV J K, BLIGAARD T, ROSSMEISL J, CHRISTENSEN C H. Towards the computational design of solid catalysts[J]. Nat Chem,2009,1(1):37−46. doi: 10.1038/nchem.121 [41] YIN A, WEN C, GUO X, DAI W-L, FAN K. Influence of Ni species on the structural evolution of Cu/SiO2 catalyst for the chemoselective hydrogenation of dimethyl oxalate[J]. J Catal,2011,280(1):77−88. doi: 10.1016/j.jcat.2011.03.006 [42] HUANG H, WANG B, WANG Y, ZHAO Y, WANG S, MA X. Partial hydrogenation of dimethyl oxalate on Cu/SiO2 catalyst modified by sodium silicate[J]. Catal Today,2020,358:68−73. doi: 10.1016/j.cattod.2019.08.048 [43] CHEN H, TAN J, ZHU Y, LI Y. An effective and stable Ni2P/TiO2 catalyst for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. Catal Commun,2016,73:46−49. doi: 10.1016/j.catcom.2015.10.010 [44] ZHUANG Z, LI Y, CHEN F, CHEN X, LI Z, WANG S, WANG X, ZHU H, TAN Y, DING Y. Synthesis of methyl glycolate by hydrogenation of dimethyl oxalate with a P modified Co/SiO2 catalyst[J]. Chem Commun,2022,58(12):1958−1961. doi: 10.1039/D1CC07003G [45] YAN W-Q, ZHANG J-B, XIAO L, ZHU Y-A, CAO Y-Q, ZHOU J-H, SUI Z-J, LI W, ZHOU X-G. Toward rational catalyst design for partial hydrogenation of dimethyl oxalate to methyl glycolate: A descriptor-based microkinetic analysis[J]. Catal Sci Technol,2019,9(20):5763−5773. doi: 10.1039/C9CY01198F [46] HAYDEN B E. Particle size and support effects in electrocatalysis[J]. Acc Chem Res,2013,46(8):1858−1866. doi: 10.1021/ar400001n [47] ZHOU M, BAO S, BARD A J. Probing size and substrate effects on the hydrogen evolution reaction by single isolated Pt atoms, atomic clusters, and nanoparticles[J]. J Am Chem Soc,2019,141(18):7327−7332. doi: 10.1021/jacs.8b13366 [48] DONG G, LUO Z, CAO Y, ZHENG S, ZHOU J, LI W, ZHOU X. Understanding size-dependent hydrogenation of dimethyl oxalate to methyl glycolate over Ag catalysts[J]. J Catal,2021,401:252−261. doi: 10.1016/j.jcat.2021.07.028 [49] WANG X, CHEN M, CHEN X, LIN R, ZHU H, HUANG C, YANG W, TAN Y, WANG S, DU Z, DING Y. Constructing copper-zinc interface for selective hydrogenation of dimethyl oxalate[J]. J Catal,2020,383:254−263. doi: 10.1016/j.jcat.2020.01.018 [50] GIORGIANNI G, MEBRAHTU C, PERATHONER S, CENTI G, ABATE S. Hydrogenation of dimethyl oxalate to ethylene glycol on Cu/SiO2 catalysts prepared by a deposition-decomposition method: Optimization of the operating conditions and pre-reduction procedure[J]. Catal Today,2022,390-391:343−353. doi: 10.1016/j.cattod.2021.08.032 [51] SHI J, HE Y, MA K, TANG S, LIU C, YUE H, LIANG B. Cu active sites confined in MgAl layered double hydroxide for hydrogenation of dimethyl oxalate to ethanol[J]. Catal Today,2021,365:318−326. doi: 10.1016/j.cattod.2020.04.042 [52] YIN S, ZHU L, WANG X, LIU Y, WANG S. The influence mechanism of solvent on the hydrogenation of dimethyl oxalate[J]. Chin J Chem Eng,2019,27(2):386−390. doi: 10.1016/j.cjche.2018.04.029 [53] YIN A, WEN C, DAI W-L, FAN K. Ag/MCM-41 as a highly efficient mesostructured catalyst for the chemoselective synthesis of methyl glycolate and ethylene glycol[J]. Appl Catal B: Environ,2011,108-109:90−99. doi: 10.1016/j.apcatb.2011.08.013 [54] DONG G, CAO Y, ZHENG S, ZHOU J, LI W, ZAERA F, ZHOU X. Catalyst consisting of Ag nanoparticles anchored on amine-derivatized mesoporous silica nanospheres for the selective hydrogenation of dimethyl oxalate to methyl glycolate[J]. J Catal,2020,391:155−162. doi: 10.1016/j.jcat.2020.08.018 [55] GÖKTÜRK E, ERDAL H. Poliglikolik Asit’ in (PGA) Biyomedikal Uygulamaları[J]. SAÜ Fen Bilimleri Enstitüsü Dergisi, 2017, 1: 1 [56] SINGH V, TIWARI M. Structure-processing-property relationship of poly(glycolic acid) for drug delivery systems 1: Synthesis and catalysis[J]. Int J Polym Sci,2010,2010:652719. [57] WEI L, MA S, HAO M, MA L, LIN X. Modifying anti-compression property and water-soluble ability of polyglycolic acid via melt blending with polyvinyl alcohol[J]. Polymers (Basel), 2022, 14: 16. [58] GAUTIER E, FUERTES P, CASSAGNAU P, PASCAULT J-P, FLEURY E. Synthesis and rheology of biodegradable poly(glycolic acid) prepared by melt ring-opening polymerization of glycolide[J]. J Polym Sci, Part A: Polym Chem,2009,47(5):1440−1449. doi: 10.1002/pola.23253 [59] BUDAK K, SOGUT O, AYDEMIR SEZER U. A review on synthesis and biomedical applications of polyglycolic acid[J]. J Polym Res,2020,27(8):208. doi: 10.1007/s10965-020-02187-1 [60] JEM K J, TAN B. The development and challenges of poly (lactic acid) and poly (glycolic acid)[J]. Adv Ind Eng Polym Res,2020,3(2):60−70. [61] CELIK F E, LAWRENCE H, BELL A T. Synthesis of precursors to ethylene glycol from formaldehyde and methyl formate catalyzed by heteropoly acids[J]. J Mol Catal A: Chem,2008,288(1):87−96. [62] YU X, VEST T A, GLEASON-BOURE N, KARAKALOS S G, TATE G L, BURKHOLDER M, MONNIER J R, WILLIAMS C T. Enhanced hydrogenation of dimethyl oxalate to ethylene glycol over indium promoted Cu/SiO2[J]. J Catal,2019,380:289−296. doi: 10.1016/j.jcat.2019.10.001 [63] LI Y, YAN S, QIAN L, YANG W, XIE Z, CHEN Q, YUE B, HE H. Effect of tin on Nb2O5/α-Al2O3 catalyst for ethylene oxide hydration[J]. J Catal,2006,241(1):173−179. doi: 10.1016/j.jcat.2006.04.030 [64] KONG X, CHEN Z, WU Y, WANG R, CHEN J, DING L. Synthesis of Cu-Mg/ZnO catalysts and catalysis in dimethyl oxalate hydrogenation to ethylene glycol: enhanced catalytic behavior in the presence of a Mg2 + dopant[J]. RSC Adv,2017,7(78):49548−49561. doi: 10.1039/C7RA09435C [65] ZHAO Y, ZHANG H, XU Y, WANG S, XU Y, WANG S, MA X. Interface tuning of Cu + /Cu0 by zirconia for dimethyl oxalate hydrogenation to ethylene glycol over Cu/SiO2 catalyst[J]. J Energy Chem,2020,49:248−256. doi: 10.1016/j.jechem.2020.02.038 [66] HE Z, LIN H, HE P, YUAN Y. Effect of boric oxide doping on the stability and activity of a Cu-SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol[J]. J Catal,2011,277(1):54−63. doi: 10.1016/j.jcat.2010.10.010 [67] ZHENG J, HUANG L, CUI C-H, CHEN Z-C, LIU X-F, DUAN X, CAO X-Y, YANG T-Z, ZHU H, SHI K, DU P, YING S-W, ZHU C-F, YAO Y-G, GUO G-C, YUAN Y, XIE S-Y, ZHENG L-S. Ambient-pressure synthesis of ethylene glycol catalyzed by C60-buffered Cu/SiO2[J]. Science,2022,376(6590):288−292. doi: 10.1126/science.abm9257 [68] DING J, LIU H, WANG M, TIAN H, WU J, YU G, WANG Y. Enhanced ethylene glycol selectivity of CuO-La2O3/ZrO2 catalyst: The role of calcination temperatures[J]. ACS Omega,2020,5(43):28212−28223. doi: 10.1021/acsomega.0c03982 [69] ZHU J, ZHAO G, MENG C, CHEN P, SHI X-R, LU Y. Superb Ni-foam-structured nano-intermetallic InNi3C0.5 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol[J]. Chem Eng J,2021,426:130857. doi: 10.1016/j.cej.2021.130857 [70] CUI G, MENG X, ZHANG X, WANG W, XU S, YE Y, TANG K, WANG W, ZHU J, WEI M, EVANS D G, DUAN X. Low-temperature hydrogenation of dimethyl oxalate to ethylene glycol via ternary synergistic catalysis of Cu and acid-base sites[J]. Appl Catal B: Environ,2019,248:394−404. doi: 10.1016/j.apcatb.2019.02.042 [71] WANG M, YAO D, LI A, YANG Y, LV J, HUANG S, WANG Y, MA X. Enhanced selectivity and stability of Cu/SiO2 catalysts for dimethyl oxalate hydrogenation to ethylene glycol by using silane coupling agents for surface modification[J]. Ind Eng Chem Res,2020,59(20):9414−9422. doi: 10.1021/acs.iecr.0c00789 [72] YUE H, ZHAO Y, MA X, GONG J. Ethylene glycol: Properties, synthesis, and applications[J]. Chem Soc Rev,2012,41(11):4218−4244. doi: 10.1039/c2cs15359a [73] YU B-Y, CHIEN I L. Design and optimization of dimethyl oxalate (DMO) hydrogenation process to produce ethylene glycol (EG)[J]. Chem Eng Res Des,2017,121:173−190. doi: 10.1016/j.cherd.2017.03.012 [74] ZALIPSKY S, HARRIS J M. Introduction to Chemistry and Biological Applications of Poly(ethylene glycol)[M]. Poly(ethylene glycol), 1997, 680: 1–13. [75] ZHANG B, ZHANG Y, ZHANG N, LIU J, CONG L, LIU J, SUN L, MAUGER A, JULIEN C M, XIE H, PAN X. Synthesis and interface stability of polystyrene-poly(ethylene glycol)-polystyrene triblock copolymer as solid-state electrolyte for lithium-metal batteries[J]. J Power Sources,2019,428:93−104. doi: 10.1016/j.jpowsour.2019.04.033 [76] GEDDES C C, NIEVES I U, INGRAM L O. Advances in ethanol production[J]. Curr Opin Biotechnol,2011,22(3):312−319. doi: 10.1016/j.copbio.2011.04.012 [77] CHEN C-C, LIN L, YE R-P, SUN M-L, YANG J-X, LI F, YAO Y-G. Mannitol as a novel dopant for Cu/SiO2: A low-cost, environmental and highly stable catalyst for dimethyl oxalate hydrogenation without hydrogen prereduction[J]. J Catal,2020,389:421−431. doi: 10.1016/j.jcat.2020.06.008 [78] SUN Y, MA Q, GE Q, SUN J. Tunable synthesis of ethanol or methyl acetate via dimethyl oxalate hydrogenation on confined iron catalysts[J]. ACS Catal,2021,11(8):4908−4919. doi: 10.1021/acscatal.1c00339 [79] AI P, TAN M, ISHIKURO Y, HOSOI Y, YANG G, YONEYAMA Y, TSUBAKI N. Design of an autoreduced copper in carbon nanotube catalyst to realize the precisely selective hydrogenation of dimethyl oxalate[J]. ChemCatChem,2017,9(6):1067−1075. doi: 10.1002/cctc.201601503 [80] DING J, WANG M, LIU H, GUO X, YU G, WANG Y. Effect of Cu content on Ce-doping CuO/ZrO2 catalysts for low-temperature hydrogenation of dimethyl oxalate to ethanol[J]. Asia-Pac J Chem Eng, 2021, 16: 5. [81] ZHU Y, KONG X, LI X, DING G, ZHU Y, LI Y-W. Cu nanoparticles inlaid mesoporous Al2O3 As a high-performance bifunctional catalyst for ethanol synthesis via dimethyl oxalate hydrogenation[J]. ACS Catal,2014,4(10):3612−3620. doi: 10.1021/cs5009283 [82] ZHAO S, YUE H, ZHAO Y, WANG B, GENG Y, LV J, WANG S, GONG J, MA X. Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2: Enhanced stability with boron dopant[J]. J Catal,2013,297:142−150. doi: 10.1016/j.jcat.2012.10.004 [83] ROOM R, BABOR T, REHM J. Alcohol and public health[J]. Lancet,2005,365(9458):519−530. doi: 10.1016/S0140-6736(05)17870-2 [84] YUE H, MA X, GONG J. An alternative synthetic approach for efficient catalytic conversion of syngas to ethanol[J]. Acc Chem Res,2014,47(5):1483−1492. doi: 10.1021/ar4002697 [85] INUI K, KURABAYASHI T, SATO S. Direct synthesis of ethyl acetate from ethanol carried out under pressure[J]. J Catal,2002,212(2):207−215. doi: 10.1006/jcat.2002.3769 [86] GASPAR A B, ESTEVES A M L, MENDES F M T, BARBOSA F G, APPEL L G. Chemicals from ethanol—The ethyl acetate one-pot synthesis[J]. Appl Catal A: Gen,2009,363(1):109−114. [87] LIU W-B, ZHANG X, DAI L-X, YOU S-L. Asymmetric N-allylation of indoles through the iridium-catalyzed allylic alkylation/oxidation of indolines[J]. Angew,2012,51(21):5183−5187. doi: 10.1002/anie.201200649 [88] WANG A, HE P, YUNG M, ZENG H, QIAN H, SONG H. Catalytic co-aromatization of ethanol and methane[J]. Appl Catal B: Environ,2016,198:480−492. doi: 10.1016/j.apcatb.2016.06.013 [89] LI S, WANG Y, ZHANG J, WANG S, XU Y, ZHAO Y, MA X J I, RESEARCH E C. Kinetics study of hydrogenation of dimethyl oxalate over Cu/SiO2 catalyst[J]. Ind Eng Chem,2015,54(4):1243−1250. [90] ZHANG L, HAN L, ZHAO G, CHAI R, ZHANG Q, LIU Y, LU Y. Structured Pd-Au/Cu-fiber catalyst for gas-phase hydrogenolysis of dimethyl oxalate to ethylene glycol[J]. Chem Commun (Camb),2015,51(52):10547−10550. doi: 10.1039/C5CC03009A [91] MARTíN A J, MITCHELL S, MONDELLI C, JAYDEV S, PÉREZ-RAMÍREZ J. Unifying views on catalyst deactivation[J]. Nat Catal,2022,5(10):854−866. doi: 10.1038/s41929-022-00842-y [92] ZHAO Y, KONG L, XU Y, HUANG H, YAO Y, ZHANG J, WANG S, MA X. Deactivation mechanism of Cu/SiO2 catalysts in the synthesis of ethylene glycol via methyl glycolate hydrogenation[J]. Ind Eng Chem Res,2020,59(27):12381−12388. doi: 10.1021/acs.iecr.0c01619 -





 下载:
下载: