Experimental study of hydrogen production via the steam reforming of hydrogen-rich biomass pyrolysis gas under the catalysis of Ni/γ-Al2O3
-
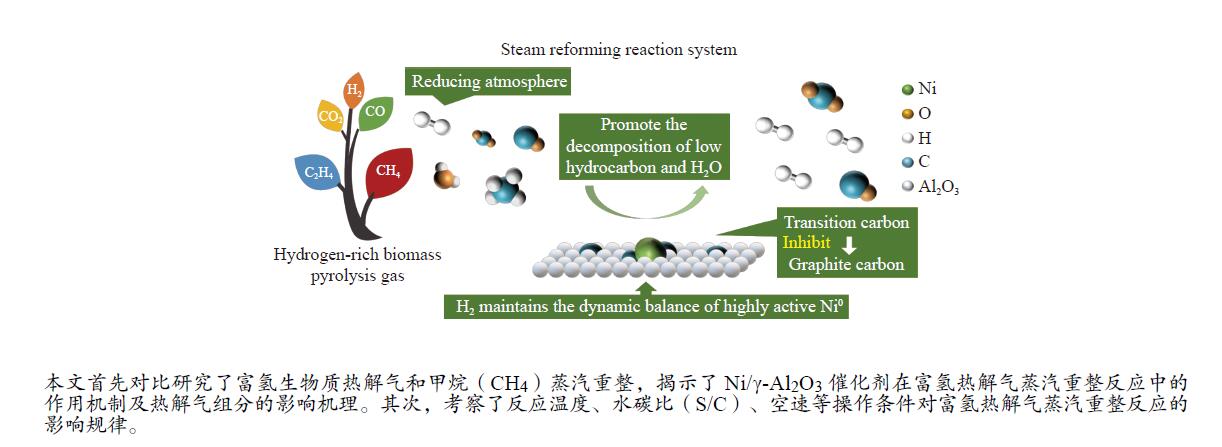
摘要: 本实验对比研究了富氢生物质热解气和甲烷(CH4)蒸汽重整,探讨了富氢生物质热解气组分对CH4等低烃重整反应的影响机理,并揭示了Ni/γ-Al2O3催化剂在富氢热解气蒸汽重整反应中的作用机制。H2通过提供还原气氛使得催化剂表面高活性Ni0维持动态平衡,从而提高其催化活性;同时,生物质热解气对过渡碳向石墨碳的转化产生抑制作用,降低了积炭对Ni/γ-Al2O3催化活性的影响。其次,考察了反应温度、水碳比(S/C)、空速等操作条件对富氢热解气蒸汽重整反应的影响规律。反应温度和S/C的提高有效促进了CH4蒸汽重整反应,同时抑制了积炭的产生;随着反应空速的提高,CH4蒸汽重整反应的竞争性减弱,水煤气变换反应、CH4干重整反应的竞争性逐渐增加,使得CH4转化受到抑制。本实验为生物质热解气蒸汽重整反应机理研究及高效催化剂开发奠定了基础。
-
关键词:
- 生物质热解气 /
- 蒸汽重整 /
- Ni/γ-Al2O3 /
- 氢气
Abstract: In this paper, the steam reforming reactions of the hydrogen-rich biomass pyrolysis gas and methane (CH4) were compared. The influence mechanism of hydrogen-rich biomass pyrolysis gas components on the reforming reaction of CH4 and other low hydrocarbons was discussed, and the catalytic effect of Ni/γ-Al2O3 catalyst was revealed. H2 could provide a reductive atmosphere to maintain the dynamic balance of the highly active Ni0 on the catalyst surface, so as to improve its catalytic activity. At the same time, biomass pyrolysis gas could inhibit the conversion of transition carbon to graphitic carbon, reducing the influence of carbon deposition on the catalytic activity of Ni/γ-Al2O3. In addition, the influence of operating conditions such as reaction temperature, the ratio of steam and carbon (S/C), as well as space velocity on the steam reforming reaction of hydrogen-rich pyrolysis gas was investigated. The increase of reaction temperature and S/C ratio effectively promoted the steam reforming of CH4 and inhibited the production of carbon deposition. With the increase of space velocity, the competitiveness of CH4 steam reforming reaction was weakened, whereas that of water gas shift reaction and CH4 dry reforming was increased. Hence, the transformation of CH4 was inhibited. This paper cound lay a foundation for the research on the mechanism of biomass pyrolysis gas steam reforming reaction and the development of high-efficiency catalysts.-
Key words:
- biomass pyrolysis gas /
- steam reforming /
- Ni/γ-Al2O3 /
- hydrogen
-
图 6 不同Ni负载量Ni/γ-Al2O3催化剂催化生物质热解气重整对比
Figure 6 Comparative analysis of biomass pyrolysis gas reforming catalyzed by Ni/γ-Al2O3 catalyst with different Ni loading
(a): Variation of $ {x}_{\text{CH}_{4}} $ and $ {{n}}_{\text{H}_{2}}/{{n}}_{\text{CO}} $ with reaction time; (b): Components of gas product and $ {{I}}_{\text{H}_{2}} $
图 7 不同反应温度下14Ni/γ-Al2O3催化剂催化生物质热解气重整对比
Figure 7 Comparative analysis of biomass pyrolysis gas reforming catalyzed by 14Ni/γ-Al2O3 catalyst at different reaction temperature
(a): Variation of $ {x}_{\text{CH}_{4}} $ and $ {{n}}_{\text{H}_{2}}/{{n}}_{\text{CO}} $ with reaction time; (b): Components of gas product and $ {{I}}_{\text{H}_{2}} $
图 8 不同S/C比下14Ni/γ-Al2O3催化生物质热解气重整对比
Figure 8 Comparative analysis of biomass pyrolysis gas reforming catalyzed by 14Ni/γ-Al2O3 catalyst at different S/C ratios
(a): Variation of $ {x}_{\text{CH}_{4}} $ and $ {{n}}_{\text{H}_{2}}/{{n}}_{\text{CO}} $ with reaction time; (b): Components of gas product and $ {{I}}_{\text{H}_{2}} $
图 9 不同反应空速下14Ni/γ-Al2O3催化生物质热解气重整对比
Figure 9 Comparative analysis of biomass pyrolysis gas reforming catalyzed by 14Ni/γ-Al2O3 catalyst at different Reaction space velocity
(a): Variation of $ {x}_{\text{CH}_{4}} $ and $ {{n}}_{\text{H}_{2}}/{\text{n}}_{\text{CO}} $ with reaction time; (b): Components of gas product and $ {{I}}_{\text{H}_{2}} $
表 1 生物质热解气和CH4蒸汽催化重整进出口流量和积炭量对比
Table 1 Comparison of inlet and outlet flow and carbon deposition for the steam catalytic reforming of biomass pyrolysis gas and CH4
Type of gas Carbon deposition /(g·gcatal−1·h−1) CH4 H2 CO CO2 C2H4 Biomass pyrolysis gas inlet flow /(mol·min−1) 0.38 0.57 0.34 0.23 0.07 0.147 outlet flow /(mol·min−1) 0.03 2.34 0.59 0.34 − CH4 inlet flow /(mol·min−1) 1.57 − − − − 0.037 outlet flow /(mol·min−1) 0.38 2.97 0.98 0.11 − 表 2 催化剂的物理吸附
Table 2 Physical adsorption test results of catalysts
Sample BET surface area /
(m2·g−1)Pore volume /
(cm3·g−1)Average pore diameter /nm 14Ni/Al2O3
before reaction127.7 0.49 15.2 14Ni/Al2O3
after CH4 reforming103.5 0.47 17.7 14Ni/Al2O3
after biomass pyrolysis gas reforming103.1 0.49 18.5 表 3 不同Ni负载量下的积炭量
Table 3 Catalyst deposition under different Ni loading
Nickel loading w/% 10 14 18 Catalyst deposition /(g·gcata−1·h−1) 0.100 0.147 0.107 表 4 不同反应条件下的积炭量
Table 4 Deposition under different reaction conditions
Reaction conditions Deposition /(g·gcata−1·h−1) Temperature /℃ 600 700 800 900 0.147 0.080 0.060 0.040 S/C ratio 1 3 5 10 0.147 0.092 0.064 0.036 Space velocity /h−1 1200 4800 9600 12800 0.147 0.120 0.162 0.190 -
[1] TAIBI E M R, VANHOUDT W. Hydrogen from renewable power: Technology outlook for the energy transition[R]. IRENA, 2018. [2] PIVOVAR B R N, SATYAPAL S. Hydrogen at scale (H2@scale): Key to a clean, economic, and sustainable energy system[J]. Electrochem Soc Inte,2018,27(1):47−52. doi: 10.1149/2.F04181if [3] CHAUBEY R, SAHU S, JAMES O O, MAITY J. A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources[J]. Renewable Sustainable Energy Rev,2013,23:443−462. doi: 10.1016/j.rser.2013.02.019 [4] 刘尚泽, 于青, 管健. 氢能利用与产业发展现状及展望[J]. 能源与节能,2022,(11):18−21. doi: 10.3969/j.issn.2095-0802.2022.11.004LIU Shang-ze, YU Qing, GUAN Jian. Current situation and prospects of hydrogen energy utilization and industrial development[J]. Energy Energy Cons,2022,(11):18−21. doi: 10.3969/j.issn.2095-0802.2022.11.004 [5] 田江南, 蒋晶, 罗扬, 马雄. 绿色氢能技术发展现状与趋势[J]. 分布式能源,2021,6(2):8−13.TIAN Jiang-nan, JIANG Jin, LUO Yang, MA Xiong. Development status and trend of green hydrogen energy technology[J]. Distr Energy,2021,6(2):8−13. [6] 李果, 张安东, 万震, 李志合, 王绍庆, 李宁, 张鹏. 生物油及其衍生物催化重整制氢研究进展[J]. 燃料化学学报,2023,51(4):444−457.LI Guo, ZHANG An-dong, WANG Zhen, LI Zhi-he, WANG Shao-qin, LI Ning, ZHANG Peng. Research progress on catalytic reforming of bio-oil and its derivatives for hydrogen production[J]. J Fuel Chem Technol,2023,51(4):444−457. [7] 宋春山. 面向氢能源、燃料电池和二氧化碳减排的制氢途径的选择(英文)[J]. 燃料化学学报,2005,33(6):641−649.SONG Chun-shan. Overview of hydrogen production options for developing hydrogen energy, fuel processing for fuel cells and mitigation of CO2 emissions[J]. J Fuel Chem Technol,2005,33(6):641−649. [8] QUAN C, GAO N, WU C. Utilization of NiO/porous ceramic monolithic catalyst for upgrading biomass fuel gas[J]. J Energy Inst,2018,91(3):331−338. doi: 10.1016/j.joei.2017.02.008 [9] ZENG W, LI L, SONG M, WU X, LI G, HU C. The effect of different atmosphere treatments on the performance of Ni/Nb-Al2O3 catalysts for methane steam reforming[J]. Int J Hydrogen Energy,2022,48(16):6358−6369. [10] HIBLOT H, ZIEGLER-DEVIN I, FOURNET R, GLUADE P. A. Steam reforming of methane in a synthesis gas from biomass gasification[J]. Int J Hydrogen Energy,2016,41(41):18329−18338. doi: 10.1016/j.ijhydene.2016.07.226 [11] HAO Q, WANG C, LU D, WANG Y, LI D, LI G. Production of hydrogen-rich gas from plant biomass by catalytic pyrolysis at low temperature[J]. Int J Hydrogen Energy,2010,35(17):8884−8890. doi: 10.1016/j.ijhydene.2010.06.039 [12] LUO S, FU J, ZHOU Y, YI C. The production of hydrogen-rich gas by catalytic pyrolysis of biomass using waste heat from blast-furnace slag[J]. Renewable Energy,2017,101:1030−1036. doi: 10.1016/j.renene.2016.09.072 [13] WANG Y, HUANG L, ZHANG T, WANG Q. Hydrogen-rich syngas production from biomass pyrolysis and catalytic reforming using biochar-based catalysts[J]. Fuel,2022,313:123006. doi: 10.1016/j.fuel.2021.123006 [14] 尚双, 郭朝强, 兰奎, 李泽善, 秦振华, 贺维韬, 李建芬. Ni/Zr-MOF催化剂的制备及其在生物质热解中的应用[J]. 燃料化学学报,2019,47(9):1067−1074.SHANG Shuang, GUO Chao-qiang, LAN Kui, LI Ze-shan, QIN Zhen-hua, HE Wei-tao, LI Jian-fen. Preparation of Ni/Zr-MOF catalyst and its application in pyrolysis of biomass[J]. J Fuel Chem Technol,2019,47(9):1067−1074. [15] 陈冠益, 李强, SPLIETHOFF H, 王福全. 生物质热解气化制取氢气[J]. 太阳能学报,2004,(6):776−781.CHEN Guan-yi, LI Qiang, SPLIETHOFF H, WANG Fu-quan. Hydrogen production from biomass pyrolysis and gasification[J]. Acta Energ Sol Sin,2004,(6):776−781. [16] XU X, JIANG E, WANG M, XU Y. Dry and steam reforming of biomass pyrolysis gas for rich hydrogen gas[J]. Biomass Bioenergy,2015,78:6−16. doi: 10.1016/j.biombioe.2015.03.015 [17] ARKATOVA L A. The deposition of coke during carbon dioxide reforming of methane over intermetallides[J]. Catal Today,2010,157(1/4):170−176. doi: 10.1016/j.cattod.2010.03.003 [18] 赵云莉. 甲烷重整制氢镍基催化剂制备及活性评价研究[D]. 太原: 太原理工大学, 2009.ZHAO Yun-li. Study of methane catalytic reforming to hydrogen on nickel-based catalysts[D]. Taiyuan: Taiyuan University of Technology, 2009 [19] XU J, CHEN L, TAN K, BORGNA A, SAEYS M. Effect of boron on the stability of Ni catalysts during steam methane reforming[J]. J Catal,2009,261(2):158−165. doi: 10.1016/j.jcat.2008.11.007 [20] KOO K Y, LEE S-H, JUNG U H, ROH H-S. Syngas production via combined steam and carbon dioxide reforming of methane over Ni–Ce/MgAl2O4 catalysts with enhanced coke resistance[J]. Fuel Process Technol,2014,119:151−157. doi: 10.1016/j.fuproc.2013.11.005 [21] SON I H, LEE S J, SOON A, ROH H, LEE H. Steam treatment on Ni/γ-Al2O3 for enhanced carbon resistance in combined steam and carbon dioxide reforming of methane[J]. Appl Catal B: Environ,2013,134-135:103−109. doi: 10.1016/j.apcatb.2013.01.001 [22] 侯悦, 张荣俊, 陆强, 杨少霞, 李明丰. 基于改性Ni/γ-Al2O3催化剂的电催化甲烷水蒸气重整的研究[J]. 燃料化学学报,2018,46(4):489−499.HOU Yue, ZHANG Rong-jun, LU Qiang, YANG Shao-xia, LI Ming-feng. Research on electro-catalytic steam reforming of methane with modified Ni/γ-Al2O3 catalysts[J]. J Fuel Chem Technol,2018,46(4):489−499. [23] ZHAO Q, WANG Y, WANG Y, LI L, ZENG W, LI G, HU C. Steam reforming of CH4 at low temperature on Ni/ZrO2 catalyst: Effect of H2O/CH4 ratio on carbon deposition[J]. Int J Hydrogen Energy,2020,45(28):14281−14292. doi: 10.1016/j.ijhydene.2020.03.112 [24] HE L, HU S, YIN X, XU J, HAN H, LI H, REN Q. SU S, WANG Y, XIANG J. Promoting effects of Fe-Ni alloy on co-production of H2 and carbon nanotubes during steam reforming of biomass tar over Ni-Fe/α-Al2O3[J]. Fuel,2020,276:118116. doi: 10.1016/j.fuel.2020.118116 [25] RAN M, SUN W, LIU Y, CHU W, JIANG C. Functionalization of multi-walled carbon nanotubes using water-assisted chemical vapor deposition[J]. J Solid State Chem,2013,197:517−522. doi: 10.1016/j.jssc.2012.08.014 [26] 李春义, 余长春, 沈师孔. Ni/Al2O3催化剂上CH4部分氧化制合成气反应积碳的原因[J]. 催化学报,2001,(4):377−382.LI Chun-yi, YU Chang-chun, SHEN Shi-kong. Causes of carbon deposition in partial oxidation of CH4 to syngas over Ni/Al2O3 catalyst[J]. Chin J Catal,2001,(4):377−382. [27] BEJ B, PRADHAN N C, NEOGI S. Production of hydrogen by steam reforming of methane over alumina supported nano-NiO/SiO2 catalyst[J]. Catal Today,2013,207:28−35. doi: 10.1016/j.cattod.2012.04.011 [28] 胡捷, 贺德华, 李映伟, 张昕, 王晖. Ni/ZrO2催化剂上甲烷水蒸气重整反应的研究[J]. 燃料化学学报,2004,32(1):98−103.HU Jie, HE De-hua, LI Ying-wei, ZHANG xin, WANG Hui. Study on steam reforming of methane over Ni/ZrO2 catalyst[J]. J Fuel Chem Technol,2004,32(1):98−103. -





 下载:
下载: