Research on mechanism and catalysts of CO2 electroreduction to formate
-
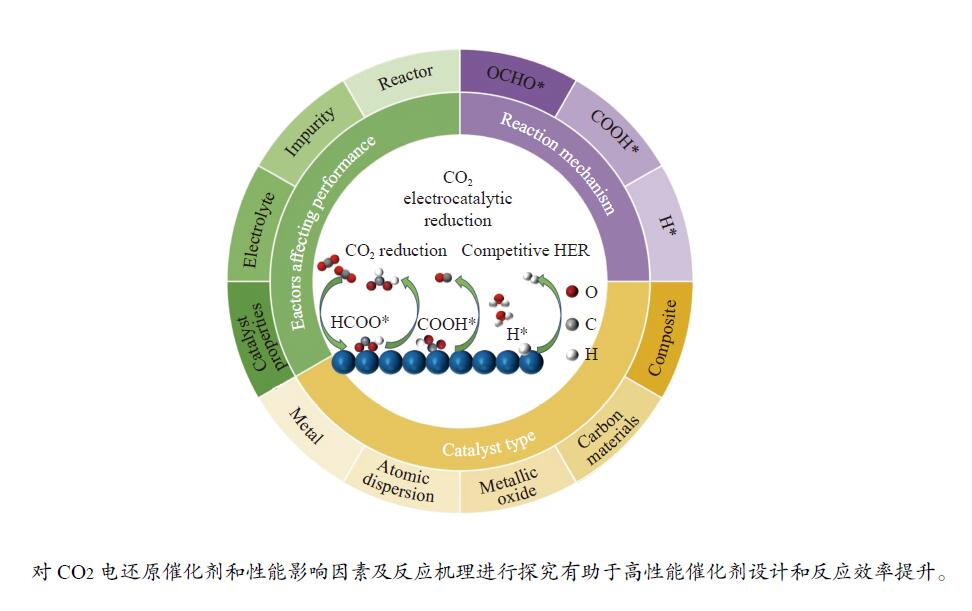
摘要: 本工作综述了近五年来CO2电催化还原生成甲酸盐领域取得的最新进展。介绍了CO2电还原生成甲酸盐的反应机理,以及该过程所使用的催化剂的类型,包括金属催化剂、原子分散催化剂、金属氧化物催化剂、炭材料及复合材料催化剂。从催化剂、电解液、反应气氛和电解池等多角度详细分析了影响产物选择性、催化活性和稳定性的主要因素。针对目前CO2电还原生成甲酸盐研究现状,提出对纳米材料和复合材料进行创新,借助原位表征技术探究活性位点及反应路径,并用于指导高效催化剂的设计与合成,改进电化学反应器组件以提高催化效率等问题可以作为未来的研究重点和发展方向。Abstract: This paper reviews the latest progress in the field of CO2 electrocatalytic reduction to formate in the past five years. The reaction mechanism of CO2 electroreduction to formate and the types of catalysts used in this process are introduced, including metal catalysts, atomic dispersion catalysts, metal oxide catalysts, carbon materials, and composite material catalysts. The main factors affecting product selectivity, catalytic activity and stability are analyzed in detail from the perspectives of catalyst, electrolyte, reaction atmosphere and electrolytic cell. In view of the current research status of carbon dioxide electroreduction to formate, it is proposed to innovate on nanomaterials and composites, explore the active site and reaction path with the help of in-situ characterization technology, and guide the design and synthesis of efficient catalysts, improve the electrochemical reactor module to improve the catalytic efficiency and other issues can be regarded as the future research focus and development direction.
-
Key words:
- CO2 electroreduction /
- reaction mechanism /
- catalyst /
- formate
-
图 4 (a)原位拉曼测试装置示意图;在0.5 mol/L KHCO3电解液中,CO2鼓泡条件下,BiCuSeO催化剂的原位拉曼光谱谱图:(b)不同还原电位,((c)−(d))不同反应时间[28]
Figure 4 (a) Schematic illustration of the in situ Raman measurement device during the CO2-ECR; (b) potential- dependent and ((c)−(d)) time-dependent in situ Raman spectra of BiCuSeO catalysts in 0.5 mol/L KHCO3 solution under CO2 bubbling[28] (with permission from Nature publication)
图 5 (a)基于Poisson-Nernst-Planck模拟得到的H + 离子浓度CH + 与外亥姆霍兹面之间距离的关系;(b)不同K + 浓度下的产物选择性;(c)在没有或存在K + 的情况下,CO2-ECR生成HCOOH的吉布斯自由能[37]
Figure 5 (a) The ions concentration CH + as function of distance from the outer Helmholtz plane (OHP) based Poisson-Nernst-Planck simulations; (b) product selectivity at different K + concentrations; (c) Gibbs free energy diagram of CO2-ECR to HCOOH in the absence or presence of K + cations[37] (with permission from ACS publication)
图 6 Sn-Bi界面结构催化剂的(a)扫描电镜照片;(b)透射电镜照片;不同样品(Sn-Bi界面,Sn-Bi合金,电沉积ED-Bi,水热法SnOx,电沉积ED-Sn)的(c)FEHCOOH和(d)PCDHCOOH[41]
Figure 6 (a) SEM image and (b) TEM image of Sn-Bi interfacial catalyst; (c) FEHCOOH and (d) PCDHCOOH over various samples (Sn-Bi interface, Sn-Bi alloy, electrodeposition ED-Bi, hydrothermal SnOx and electrodeposition ED-Sn)[41] (with permission from Nature publication)
图 7 (a)Pd 3d精细XPS光谱谱图;(b)PdCuAuAgBiIn HEAAs的伏安曲线;(c)PdCuAuAgBiIn HEAAs和(d)Pd金属气凝胶(Pd MAs)在不同电位下各种产物的法拉第效率[27]
Figure 7 (a) Pd 3d fine XPS spectra; (b) CO stripping curve of PdCuAuAgBiIn HEAAs; Reduction potential dependent FEs measured on (c) PdCuAuAgBiIn HEAAs and (d) Pd MAs[27] (with permission from Wiley publication)
图 8 (a)*OCHO和*COOH在In-N-C表面的稳定吸附的几何结构;(b)在In-N-C、In(101)、In(110)和In(112)表面CO2-ECR自由能[51]
Figure 8 (a) Stable adsorption geometry of *OCHO and *COOH on the surface of In-N-C; (b) free energy diagram on the surface of In-N-C, In(101), In(110) and In(112) under CO2-ECR[51] (with permission from ACS publication)
图 10 在含0.5 mol/L KHCO3电解液的H型电解池中,Bi2O3@C/HB、Bi2O3@C/HL、C/HB、C/HL的(a)线性扫描伏安曲线,(b)甲酸盐法拉第效率,(c)甲酸盐部分电流密度;在含1 mol/L KOH电解液的流动池中,Bi2O3@C/HB和Bi2O3@C/HB的(d)线性扫描伏安曲线,(e)甲酸盐法拉第效率,(f)甲酸盐部分电流密度[74]
Figure 10 (a) Linear sweep voltammetry curve, (b) FEformate, (c) jformate of Bi2O3@C/HB, Bi2O3@C/HL, C/HB, C/HL in a H-cell containing 0.5 mol/L KHCO3 electrolyte, (d) Linear sweep voltammetry curve, (e) FEformate, (f) jformate of Bi2O3@C/HB, Bi2O3@C/HL in a flow cell containing 1 mol/L KOH electrolyte[74] (with permission from ACS publication)
图 14 (a)电解质类型和阳离子尺寸对电流密度和FEHCOOH的影响 0.1 mol/L的MHCO3(M= Li + 、Na + 、K + 、Rb + 、Cs + )电解液,−1.4 V vs. RHE电位,SnO2/C电极;(b)0.1 mol/L的NaX和KX(X =
${\rm{HCO}}_3^- $ 、Cl−、Br− and I−)电解液中,−1.4 V vs. RHE电位下,SnO2/C电极上得到的电流密度和FEHCOOH[93]Figure 14 (a) Current density and FEHCOOH as functions of electrolyte type and the cation size, measured using an SnO2/C electrode at −1.4 V in 0.1 mol/L MHCO3 (M = Li + , Na + , K + , Rb + , Cs + ) electrolytes; (b) current density and FEHCOOH at the SnO2/C electrode at −1.4 V in 0.1 mol/L NaX and KX (X =
${\rm{HCO}}_3^- $ , Cl−, Br− and I−), respectively[93] (with permission form Elsevier publication)表 1 不同催化剂电还原CO2生成甲酸盐的性能
Table 1 Performance of electroreduction CO2 towards formate over various catalysts
Catalyst Potential
(V vs. RHE)FEHCOO−/% JHCOO−/(mA·cm−2) Cell type Electrolyte Reference PdCuAuAgBiIn HEAAs −1.1 98.1 − H-cell 0.1 mol/L KHCO3 [27] hp-In −1.2 90 67.5 H-cell 0.1 mol/L KHCO3 [33] Bi NS −1.23 92.2 237.1 flow cell 0.5 mol/L H2SO4(K + ) [37] CuSn −1.4 87.3 140.6 H-cell 0.5 mol/L KHCO3 [40] Sn-Bi −0.84 96.4 − H-cell 0.5 mol/L KHCO3 [41] Sn SA/ZnO −1.2 80 − flow cell 0.5 mol/L KHCO3 [50] In-N-C −0.79 80 6.8 H-cell 0.5 mol/L KHCO3 [51] BiOn − 90 500 MEA 0.1 mol/L KOH [58] Bi2O3-A −1.2 91 22 H-cell 0.5 mol/L KHCO3 [59] NW-SnO2 −1.0 87.4 22 H-cell 0.5 mol/L KHCO3 [60] BDD − 90 2 flow cell 0.5 mol/L KCl [67] Bi-HTC/OCNTs −1.01 94 17.8 H-cell 0.5 mol/L KHCO3 [72] In2S3-rGO −1.2 91 − H-cell 0.1 mol/L KHCO3 [73] Bi2O3@C/HB −0.7 93.8 − H-cell 0.5 mol/L KHCO3 [74] −1.5 − 285 flow cell 1 mol/L KOH Bi2O3@C −1.1 93 208 flow cell 1 mol/L KOH [76] In2O3@C −1.1 94 − flow cell 1 mol/L KOH [77] Bi2S3 −0.95 93 1900 flow cell 1 mol/L KOH [84] CuS/SnO2-S −0.8 84.9 18.8 H-cell 0.5 mol/L KHCO3 [85] SnCu-CNS −0.9 95.1 − H-cell 0.1 mol/L KHCO3 [86] -
[1] LIU Z, DENG Z, DAVIS S J, GIRON C, CIAIS P. Monitoring global carbon emissions in 2021[J]. Nat Rev Earth Environ,2022,3:217−219. doi: 10.1038/s43017-022-00285-w [2] SHEN H, WANG Y Z, CHAKRABORTY T, ZHOU G Y, WANG C H, FU X B, WANG Y X, ZHANG J Y, LI C Y, XU F, CAO L, MUELLER T, WANG C. Asymmetrical C-C coupling for electroreduction of CO on bimetallic Cu-Pd catalysts[J]. ACS Catal,2022,12(9):5275−5283. doi: 10.1021/acscatal.2c00646 [3] GUO C Y, GUO Y H, SHI Y M, LAN X E, WANG Y T, YU Y F, ZHANG B. Electrocatalytic reduction of CO2 to ethanol at close to theoretical potential via engineering abundant electron-donating Cuδ + species[J]. Angew Chem Int Ed,2022,61(32):202205909. [4] ZHAO Z, PENG X Y, LIU X J, SUN X M, SHI J, HAN L L, LI G L, LUO J. Efficient and stable electroreduction of CO2 to CH4 on CuS nanosheet arrays[J]. J Mater Chem A,2017,5:20239−20243. doi: 10.1039/C7TA05507B [5] HAN L L, SONG S J, LIU M J, YAO S Y, LIANG Z X, CHENG H, REN Z H, LIU W, LIN R Q, QI G C, LIU X J, WU Q, LUO J, XIN H L L. Stable and efficient single-atom Zn catalyst for CO2 reduction to CH4[J]. JACS,2020,142(29):12563−12567. doi: 10.1021/jacs.9b12111 [6] CHEN C, KOTYK J F K, SHEEHAN S W. Progress toward commercial application of electrochemical carbon dioxide reduction[J]. Chem,2018,4(11):2571−2586. doi: 10.1016/j.chempr.2018.08.019 [7] SONG C F, LIU Q L, QI Y, CHEN G Y, SONG Y J, KANSHA Y, KITAMURA Y. Absorption-microalgae hybrid CO2 capture and biotransformation strategy-a review[J]. Int J Greenh Gas Con,2019,88:109−117. doi: 10.1016/j.ijggc.2019.06.002 [8] LI C F, GUO R T, ZHANG Z R, WU T, PAN W G. Converting CO2 into value-added products by Cu2O-based catalysts: from photocatalysis, electrocatalysis to photoelectrocatalysis[J]. Small,2023,19(19):2207875. doi: 10.1002/smll.202207875 [9] 付豪, 廉红蕾. CO2甲烷化反应路径的研究进展[J]. 燃料化学学报,2023,51(4):428−443.FU Hao, LIAN Hong-lei. Research progress on the reaction pathway of CO2 methanation[J]. J Fuel Chem Technol,2023,51(4):428−443. [10] ZHOU Z Y, SUN S G. A breakthrough in electrocatalysis of CO2 conversion[J]. NSR,2017,4(2):155−156. [11] DU C, WANG X, CHEN W, FENG S, WEN J, WU Y A. CO2 transformation to multicarbon products by photocatalysis and electrocatalysis[J]. Mater Today Adv,2020,6:100071. doi: 10.1016/j.mtadv.2020.100071 [12] GODIN J, LIU W Z, REN S, XU C C. Advances in recovery and utilization of carbon dioxide: A brief review[J]. J Environ Chem Eng,2021,9(4):105644. doi: 10.1016/j.jece.2021.105644 [13] CHEN S Y, LI X Q, LI H M, CHEN K J, LUO T, FU J W, LIU K, WANG Q Y, ZHU M S, LIU M. Proton transfer dynamics mediated CO2 electroreduction reaction[J]. ChemSusChem, 2023, 16(12): e202202251. [14] HAN N, DING P, HE L, LI Y Y, LI Y G. Promises of main group metal-based nanostructured materials for electrochemical CO2 reduction to formate[J]. Adv Energy Mater,2020,10(11):1902338. doi: 10.1002/aenm.201902338 [15] HSIEH Y C, SENANAYAKE S D, ZHANG Y, XU W Q, POLYANSKY D. Effect of chloride anions on the synthesis and enhanced catalytic activity of silver nanocoral electrodes for CO2 electroreduction[J]. ACS Catal,2015,5(9):5349−5356. doi: 10.1021/acscatal.5b01235 [16] LIU G B, LI Z H, SHI J J, SUN K, JI Y J, WANG Z G, QIU Y F, LIU Y Y, WANG Z J, HU P A. Black reduced porous SnO2 nanosheets for CO2 electroreduction with high formate selectivity and low overpotential[J]. Appl Catal B: Environ,2020,260:118134. doi: 10.1016/j.apcatb.2019.118134 [17] KLINKOVA A, LUNA P D, DINH C T, VOZNYY O, LARIN E M, KUMACHEVA E, SARGENT E H. Rational design of efficient palladium catalysts for electroreduction of carbon dioxide to formate[J]. ACS Catal,2016,6(12):8115−8120. doi: 10.1021/acscatal.6b01719 [18] WANG J G, NING S L, LUO M, XIANG D, CHEN W, KANG X W, JIANG Z, CHEN S W. In-Sn alloy core-shell nanoparticles In-doped SnOx shell enables highstability and activity towards selective formate production from electrochemical reduction of CO2[J]. Appl Catal B: Environ,2021,288:119979. doi: 10.1016/j.apcatb.2021.119979 [19] CHEN S Y, LI X Q, LI H M, CHEN K J, LUO T, FU J W, LIU K, WANG Q Y, ZHU M S, LIU M. Proton transfer dynamics mediated CO2 electroreduction reaction[J]. ChemSusChem,2023,16(12):e202202251. [20] LUO W, XIE W, MUTSCHLER R, OVEISI E, GREGORIO G L D, BUONSANTI R, ZÜTTEL A. Selective and stable electroreduction of CO2 to CO at the copper/indium interface[J]. ACS Catal,2018,8:6571−6581. doi: 10.1021/acscatal.7b04457 [21] YANG H, HU Y W, CHEN J J, BALOGUN M S, FANG P P, ZHANG S Q, CHEN J T, Y X. CO2 electroreduction: intermediates adsorption engineering of CO2 electroreduction reaction in highly selective heterostructure Cu-based electrocatalysts for CO production[J]. Adv Energy Mater,2019,9(27):1970107. [22] LIU T Y, OHASHI K, NAGITA K, HARADA T, NAKANISHI S, KAMIYA K. A tin oxide-coated copper foam hybridized with a gas diffusion electrode for efficient CO2 reduction to formate with a current density exceeding 1 A cm−2[J]. Small,2022,18(50):2205323. doi: 10.1002/smll.202205323 [23] DENG P L, WANG H M, QI R J, ZHU J X, CHEN S H, YANG F, ZHOU L, QI K, LIU H F, XIA B Y. Bismuth oxides with enhanced bismuth-oxygen structure for efficient electrochemical reduction of carbon dioxide to formate[J]. ACS Catal,2020,10(1):743−750. doi: 10.1021/acscatal.9b04043 [24] LIU S, FAN Y P, WANG Y, JIN S, HOU M C, ZENG W J, LI K, JIANG T L, QIN L, YAN Z H, TAO Z L, ZHENG X H, SHEN C Y, LIU Z C, AHMAD T, ZHANG K, CHEN W. Surface-oxygen-rich Bi@C nanoparticles for high-efficiency electroreduction of CO2 to formate[J]. Nano Lett,2022,22(22):9107−9114. doi: 10.1021/acs.nanolett.2c03573 [25] FIGUEIREDO M C, LEDEZMA-YANEZ I, KOPER M T M. In situ spectroscopic study of CO2 electroreduction at copper electrodes in acetonitrile[J]. ACS Catal,2016,6(4):2382−2392. doi: 10.1021/acscatal.5b02543 [26] MOTOBAYASHI K, MAENO Y, IKEDA K. In situ spectroscopic characterization of an intermediate of CO2 electroreduction on a Au electrode in room-temperature ionic liquids[J]. J Phys Chem C,2022,126(29):11981−11986. doi: 10.1021/acs.jpcc.2c03012 [27] LI H J, HUANG H G, CHEN Y, LAI F L, FU H, ZHANG L S, ZHANG N, BAI S X, LIU T X. High-entropy alloy aerogels: a new platform for carbon dioxide reduction[J]. Adv Mater,2023,35(2):2209242. doi: 10.1002/adma.202209242 [28] DUAN J Y, LIU T Y, ZHAO Y H, YANG R O, ZHAO Y, WANG W B, LIU Y W, LI H Q, LI Y F, ZHAI T Y. Active and conductive layer stacked superlattices for highly selective CO2 electroreduction[J]. Nat Commun,2022,13:2039. doi: 10.1038/s41467-022-29699-2 [29] YOO J S, CHRISTENSEN, R, VEGGE T, NØRSKOV J K, STUDT F. Theoretical insight into the trends that guide the electrochemical reduction of carbon dioxide to formic acid[J]. ChemSusChem,2016,9(4):358−363. doi: 10.1002/cssc.201501197 [30] CHEN Y H, KANAN M W. Tin oxide dependence of the CO2 reduction efficiency on tin electrodes and enhanced activity for tin/tin oxide thin-film catalysts[J]. JACS,2012,134(4):1986−1989. doi: 10.1021/ja2108799 [31] HAN N, WANG Y, YANG H, DENG J, WU J H, LI Y F, LI Y G. Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate[J]. Nat Commun,2018,9(1):1320−1328. doi: 10.1038/s41467-018-03712-z [32] VASILEFF A, XU C C, JIAO Y, ZHENG Y, QIAO S Z. Surface and interface engineering in copper-based bimetallic materials for selective CO2 electroreduction[J]. Chem,2018,4(8):1809−1831. doi: 10.1016/j.chempr.2018.05.001 [33] LUO W, XIE W, LI M, ZHANG J, ZÜTTEL A. 3D hierarchical porous indium catalyst for highly efficient electroreduction of CO2[J]. J Mater Chem A,2019,7:4505−4515. doi: 10.1039/C8TA11645H [34] GREELEY J, JARAMILLO T F, BONDE J, CHORKENDORFF I, NØRSKOV J K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution[J]. Nat Mater,2006,5:909−913. doi: 10.1038/nmat1752 [35] YANG J, WANG X L, QU Y T, WANG X, HUO H, FAN Q K, WANG J, YANG L M, WU Y E. Bi-based metal-organic framework derived leafy bismuth nanosheets for carbon dioxide electroreduction[J]. Adv Energy Mater,2019,45(9):11861−11867. [36] YI L C, CHEN J X, SHAO P, HUANG J H, PENG X X, LI J W, WANG G X, ZHANG C, WEN Z H. Molten-salt-assisted synthesis of bismuth nanosheets for long-term continuous electrocatalytic conversion of CO2 to formate[J]. Angew Chem Int Ed,2020,59(45):20112−20119. doi: 10.1002/anie.202008316 [37] QIAO Y, LAI W C, HUANG K, YU T T, WANG Q Y, GAO L, YANG Z L, MA Z S, SUN T L, LIU M, LIAN C, HUANG H W. Engineering the local microenvironment over Bi nanosheets for highly selective electrocatalytic conversion of CO2 to HCOOH in strong acid[J]. ACS Catal,2022,12(4):2357−2364. doi: 10.1021/acscatal.1c05135 [38] JOUNY M, LUC W, JIAO F. General techno-economic analysis of CO2 electrolysis systems[J]. Ind Eng Chem Res,2018,57(6):2165−2177. doi: 10.1021/acs.iecr.7b03514 [39] FENG G H, CHEN W, WANG B Y, SONG Y F, LI G H, FANG J H, WEI W, SUN Y H. Oxygenates from the electrochemical reduction of carbon dioxide[J]. Chem-Asian J,2018,13(16):1992−2008. doi: 10.1002/asia.201800637 [40] LI Y, HUO C Z, WANG H J, YE Z X, LUO P P, CAO X X, LU T B. Coupling CO2 reduction with CH3OH oxidation for efficient electrosynthesis of formate on hierarchical bifunctional CuSn alloy[J]. Nano Energy,2022,98:107277. doi: 10.1016/j.nanoen.2022.107277 [41] REN B H, WEN G B, GAO R, LUO D, ZHANG Z, QIU W B, MA Q Y, WANG X, CUI Y, RICARDEZ-SANDOVAL L, YU A P, CHEN Z W. Nano-crumples induced Sn-Bi bimetallic interface pattern with moderate electron bank for highly efficient CO2 electroreduction[J]. Nat Commun,2022,13:2486. doi: 10.1038/s41467-022-29861-w [42] GEORGE E P, RAABE D, RITCHIE R O. High-entropy alloys[J]. Nat Rev Mater,2019,4:515−534. doi: 10.1038/s41578-019-0121-4 [43] WANG X, GUO W, FU Y Z. High-entropy alloys: Emerging materials for advanced functional applications[J]. J Mater Chem A,2021,9:663−701. doi: 10.1039/D0TA09601F [44] LU X L, RONG X, ZHANG C, LU T B. Carbon-based single-atom catalysts for CO2 electroreduction progress and optimization strategies[J]. J Mater Chem A,2020,8:10695−10708. doi: 10.1039/D0TA01955K [45] LI X, PEREIRA-HERNÁNDEZ X I, CHEN Y Z, XU J, ZHAO J K, PAO C W, FANG C Y, ZENG J, WANG Y, GATES B C, LIU J Y. Functional CeOx nanoglues for robust atomically dispersed catalysts[J]. Nature,2022,611:284−288. doi: 10.1038/s41586-022-05251-6 [46] HERZING A A, KIELY C J, CARLEY A F, LANDON P, HUTCHINGS G J. Identification of active gold nanoclusters on Iron oxide supports for CO oxidation[J]. Science,2008,321(5894):1331−1335. doi: 10.1126/science.1159639 [47] CREISSEN C E, FONTECAVE M. Keeping sight of copper in single-atom catalysts for electrochemical carbon dioxide reduction[J]. Nat Commun,2022,13:2280. doi: 10.1038/s41467-022-30027-x [48] CHEN Y, LIN J, JIA B H, WANG X D, JIANG S Y, MA T Y. Isolating single and few atoms for enhanced catalysis[J]. Adv Mater,2022,34(39):2201796. doi: 10.1002/adma.202201796 [49] YANG X F, WANG A Q, QIAO B T, LI J, LIU J Y, ZHANG T. Single-atom catalysts: A new frontier in heterogeneous catalysis[J]. Accounts Chem Res,2013,46(8):1740−1748. doi: 10.1021/ar300361m [50] ZHANG Y Z, JANG H, GE X, ZHANG W, LI Z J, HOU L Q, ZHAI L, WEI X Q, WANG Z, KIM M G, LIU S G, QIN Q, LIU X E, CHO J. Single-atom Sn on tensile-strained ZnO nanosheets for highly efficient conversion of CO2 into formate[J]. Adv Energy Mater,2022,12(45):2202695. doi: 10.1002/aenm.202202695 [51] LU P L, TAN X, ZHAO H T, XIANG Q, LIU K L, ZHAO X X, YIN X M, LI X Z, HAI X, XI S B, WEE A T S, PENNYCOOK S J, YU X F, YUAN M L, WU J B, ZHANG G J, SMITH S C, YIN Z Y. Atomically dispersed indium sites for selective CO2 electroreduction to formic acid[J]. ACS Nano,2021,15(3):5671−5678. doi: 10.1021/acsnano.1c00858 [52] LIANG X M, WANG H J, ZHANG C, ZHONG D C, LU T B. Controlled synthesis of a Ni2 dual-atom catalyst for synergistic CO2 electroreduction[J]. Appl Catal B: Environ,2023,322:122073. doi: 10.1016/j.apcatb.2022.122073 [53] LI Y F, CHEN C, CAO R, PAN Z W, HE H, ZHOU K B. Dual-stom Ag2/graphene catalyst for efficient electroreduction of CO2 to CO[J]. Appl Catal B: Environ,2020,268:118747. doi: 10.1016/j.apcatb.2020.118747 [54] YAO D Z, TANG C, ZHI X, JOHANNESSEN B, SLATTERY A, CHERN S, QIAO S Z. Inter-metal interaction with a threshold effect in NiCu dual-atom catalysts for CO2 electroreduction[J]. Adv Mater,2022,35(11):2209386. [55] GONG Y N, CAO C Y, SHI W J, ZHANG J H, DENG J H, LU T B, ZHONG D C. Modulating the electronic structures of dual-atom catalysts via coordination environment engineering for boosting CO2 electroreduction[J]. Angew Chem Int Ed,2022,61(51):202215187. [56] LIU L J, WANG Z Y, WANG Z Y, WANG R, ZANG S Q, MAK T C W. Mediating CO2 electroreduction activity and selectivity over atomically precise copper clusters[J]. Angew Chem Int Ed,2022,61(35):202205626. [57] WANG Y X, CUI X Z, ZHANG J Q, QIAO J L, HUANG H T, SHI J L, WANG G X. Advances of atomically dispersed catalysts from single-atom to clusters in energy storage and conversion applications[J]. Prog Mater,2022,128:100964. doi: 10.1016/j.pmatsci.2022.100964 [58] JIANG X L, LIN L, RONG Y W, LI R T, JIANG Q K, YANG Y Y, GAO D F. Boosting CO2 electroreduction to formate via bismuth oxide clusters[J]. Nano Res, 2023, 16: 12050–12057. [59] MIAO C C, YUAN G Q. Morphology-controlled Bi2O3 nanoparticles as catalysts for selective electrochemical reduction of CO2 to formate[J]. ChemElectroChem,2018,5(23):3741−3747. doi: 10.1002/celc.201801036 [60] CHEN Z, FAN T T, ZHANG Y Q, XIAO J, GAO M R, DUAN N Q, ZHANG J W, LI J H, LIU Q X, YI X D, LUO J L. Wavy SnO2 catalyzed simultaneous reinforcement of carbon dioxide adsorption and activation towards electrochemical conversion of CO2 to HCOOH[J]. Appl Catal B: Environ,2020,261:118243. doi: 10.1016/j.apcatb.2019.118243 [61] SHU Z Y, YE G Y, WANG J, LIU S Q, HE Z, ZHU W W, LIU B, LIU M. Nitrogen-doped carbon with high graphitic-N exposure for electroreduction of CO2 to CO[J]. Ionics,2021,27:3089−3098. doi: 10.1007/s11581-021-04077-y [62] SONG Y F, CHEN W, ZHAO C C, LI S G, WEI W, SUN Y H. Metal-free nitrogen-doped Mesoporous carbon for electroreduction of CO2 to ethanol[J]. Angew Chem Int Ed,2017,56(36):10840−10844. doi: 10.1002/anie.201706777 [63] JIA C, DASTAFKAN K, REN W H, YANG W F, ZHAO C. Carbon-based catalysts for electrochemical CO2 reduction[J]. Sustainable Energy Fuels,2019,3(11):2890−2906. doi: 10.1039/C9SE00527G [64] DUAN X C, XU J T, WEI Z X, MA J M, GUO S J, WANG S Y, LIU H K, DOU S X. Metal-free carbon materials for CO2 electrochemical reduction[J]. Adv Mater,2017,29(41):1701784. doi: 10.1002/adma.201701784 [65] LIN J, YAN S L, ZHANG C X, HU Q, CHENG Z M. Electroreduction of CO2 toward high current density[J]. Processes,2022,10(5):826. doi: 10.3390/pr10050826 [66] ZANG D J, GAO X J J, LI L Y, WEI Y G, WANG H Q. Confined interface engineering of self-supported Cu@N-doped graphene for electrocatalytic CO2 reduction with enhanced selectivity towards ethanol[J]. Nano Res,2022,15:8872−8879. doi: 10.1007/s12274-022-4698-3 [67] NATSUI K, IWAKAWA H, IKEMIYA N, NAKATA K, EINAGA Y. Stable and highly efficient electrochemical production of formic acid from carbon dioxide using diamond electrodes[J]. Angew Chem Int Ed,2018,57(10):2693−2643. [68] CAO W T, HUANG Y F, LI D, CHEN W H, QIE Z P, PI X X, DU Q J, LAI X Y, LI Y H. N/S co-doped microporous zeolite-templated carbon for efficient CO2 adsorption and separation[J]. J Energy Inst,2023,106:101159. doi: 10.1016/j.joei.2022.101159 [69] LIANG X D, TIAN N, ZHOU Z Y, SUN S G. N, P dual-doped porous carbon nanosheets for high-efficiency CO2 electroreduction[J]. ACS Sustainable Chem Eng,2022,10(5):1880−1887. doi: 10.1021/acssuschemeng.1c07601 [70] MA X Y, DU J J, SUN H, YE F H, WANG X, XU P F, HU C G, ZHANG L P, LIU D. Boron, nitrogen co-doped carbon with abundant mesopores for efficient CO2 electroreduction[J]. Appl Catal B: Environ,2021,298:120543. doi: 10.1016/j.apcatb.2021.120543 [71] LI W B, YU C, TAN X Y, CUI S, ZHANG Y F, QIU J S. Recent advances in the electroreduction of carbon dioxide to formic acid over carbon-based materials[J]. New Carbon Mater,2022,37(2):277−287. doi: 10.1016/S1872-5805(22)60592-4 [72] YANG F F, ZHANG W F, MA X Y, ZHANG D J. Ultrafine Bi nanoparticles confined in hydrothermal carbon-modified carbon nanotubes for highly efficient CO2 electroreduction to formate[J]. J Electrochem Soc,2022,169:44524. doi: 10.1149/1945-7111/ac6703 [73] NING H, FEI X, TAN Z H, WANG W H, YANG Z X, WU M B. In situ-fabricated In2S3-reduced graphene oxide nanosheet composites for enhanced CO2 electroreduction to formate[J]. ACS Appl Nano Mater,2022,5(2):2335−2342. doi: 10.1021/acsanm.1c04016 [74] LIU S Q, SHAHINI E, GAO M R, GONG L, SUI P F, TANG T, ZENG H B, LUO J L. Bi2O3 nanosheets grown on carbon nanofiber with inherent hydrophobicity for high-performance CO2 electroreduction in a wide potential window[J]. ACS Nano,2021,15(11):17757−17768. doi: 10.1021/acsnano.1c05737 [75] ABDELKADER-FERNÁNDEZ V K, FERNANDES D M, FREIRE C. Carbon-based electrocatalysts for CO2 electroreduction produced via MOF, biomass, and other precursors carbonization: A review[J]. J CO2 Util,2020,42:101350. doi: 10.1016/j.jcou.2020.101350 [76] DENG P L, YANG F, WANG Z T, CHEN S H, ZHOU Y Z, ZAMAN S, XIA B Y. Metal-organic frameworks-derived carbon nanorods encapsulated bismuth oxides for rapid and selective CO2 electroreduction to formate[J]. Angew Chem Int Ed,2020,59(27):10807−10813. doi: 10.1002/anie.202000657 [77] WANG Z T, ZHOU Y S, LIU D Y, QI R J, XIA C F, LI M T, YOU B, XIA B Y. Carbon-confined indium oxides for efficient carbon dioxide reduction in a solid-state electrolyte flow cell[J]. Angew Chem Int Ed,2022,61(21):202200552. [78] OHYA S, KANECO S, KATSUMATA H, SUZUKI T, OHTA K. Electrochemical reduction of CO2 in methanol with aid of CuO and Cu2O[J]. Catal Today,2009,148(3):329−334. [79] LIU S Y, PANG F J, ZHANG Q W, GUO R J, WANG Z F, WANG Y C, ZHANG W Q, OU J Z. Stable nanoporous Sn/SnO2 composites for efficient electroreduction of CO2 to formate over wide potential range[J]. Appl Mater Today,2018,13:135−143. doi: 10.1016/j.apmt.2018.08.014 [80] DAIYAN R, CHEN R, KUMAR P, BEDFORD N M, QU J T, CAIRNEY J M, LU X Y, AMAL R. Tunable syngas production through CO2 electroreduction on cobalt-carbon composite electrocatalyst[J]. ACS Appl Mater Inter,2020,12(8):9370−9315. [81] ZHANG Y Y, LI K, CHEN M M, WANG J, LIU J D, ZHANG Y T. Cu/Cu2O nanoparticles supported on vertically ZIF-L-coated nitrogen-doped graphene nanosheets for electroreduction of CO2 to ethanol[J]. ACS Appl Nano Mater,2019,3(1):257−263. [82] JIANG X X, LI X, KONG Y, DENG C, LI X J, HU Q, YANG H P, HE C X. A hierarchically structured tin-cobalt composite with an enhanced electronic effect for high-performance CO2 electroreduction in a wide potential range[J]. J Energy Chem,2023,76:462−469. doi: 10.1016/j.jechem.2022.10.008 [83] CHAIKA M Y, VOLKOV V V, KRAVCHENKO T A, KONEV D V, GORSHKOV V S, KRYSANOV V A, BOSYACHENKO A A. Oxygen electroreduction on the anthraquinone-modified thin-film carbon-polymer composite in alkaline solution[J]. Russ J Electrochem,2019,55:1284−1291. doi: 10.1134/S102319351911003X [84] LIN L, HE X Y, ZHANG X G, MA W C, ZHANG B, WEI D Y, XIE S J, ZHANG Q H, YI X D, WANG Y. A nanocomposite of Bi clusters and Bi2O2CO3 sheets for highly efficient electrocatalytic reduction of CO2 to formate[J]. Angew Chem Int Ed,2023,62(3):202214959. [85] DOU T, HE J Q, DIAO S T, WANG Y P, ZHAO X H, ZHANG F Z, LEI X D. Dynamic reconstructuring of CuS/SnO2-S for promoting CO2 electroreduction to formate[J]. J Energy Chem,2023,82:497−506. doi: 10.1016/j.jechem.2023.03.016 [86] JIANG X X, LI X, KONG Y, DENG C, LI X J, HU Q, YANG H P, HE C X. Oxidation state modulation of bimetallic tin-copper oxide nanotubes for selective CO2 electroreduction to formate[J]. Small,2022,18(47):2204148. doi: 10.1002/smll.202204148 [87] MENG F L, ZHANG Q, LIU K H, ZHANG X B. Integrated bismuth oxide ultrathin nanosheets/carbon foam electrode for highly selective and energy-efficient electrocatalytic conversion of CO2 to HCOOH[J]. Chem Eur J,2020,26(18):4013−4018. doi: 10.1002/chem.201903158 [88] WANG M, LIU S, CHEN B, HUANG M J, PENG C. Co-regulation of intermediate binding and water activation in sulfur-doped bismuth nanosheets for electrocatalytic CO2 reduction to formate[J]. Chem Eng J,2023,451:139056. doi: 10.1016/j.cej.2022.139056 [89] QIU Y, DU J, DONG W, DAI C N, TAO C Y. Selective conversion of CO2 to formate on a size tunable nano-Bi electrocatalyst[J]. J CO2 Util,2017,20:328−335. doi: 10.1016/j.jcou.2017.05.024 [90] YANG H, HAN N, DENG J, WU J H, WANG Y, HU Y P, DING P, LI Y F, LI Y G, LU J. Selective CO2 reduction on 2D mesoporous Bi nanosheets[J]. Adv Energy Mater,2018,8(35):1801536. doi: 10.1002/aenm.201801536 [91] WEI X Y, ZHANG W N, LIU D P, LIU D D, YAN Y D, ZHANG J, YANG Y D, YAN S C, ZOU Z G. Bi particles with exposed (012) facet on 3D substrate as highly active and durable electrode for CO2 reduction to formate[J]. J CO2 Util,2022,55:101797. doi: 10.1016/j.jcou.2021.101797 [92] ZHANG B, CHANG Y, WU Y Z, FAN Z Z, ZHAI P L, WANG C, GAO J F, SUN L C, HOU J G. Regulating OCHO Intermediate as rate-determining step of defective oxynitride nanosheets enabling robust CO2 electroreduction[J]. Adv Energy Mater,2022,12(27):2200321. doi: 10.1002/aenm.202200321 [93] ZHANG Q, SHAO X L, YI J, LIU Y Y, ZHANG J J. An experimental study of electroreduction of CO2 to HCOOH on SnO2/C in presence of alkali metal cations (Li + , Na + , K + , Rb + and Cs + ) and anions (HCO3−, Cl−, Br− and I−)[J]. Chin J Chem Eng,2020,28(10):2549−2554. doi: 10.1016/j.cjche.2020.04.015 [94] CHEN S Y, LI X Q, KAO C W, LUO T, CHEN K J, FU J W, MA C, LI H M, LI M, CHAN T S, LIU M. Unveiling the proton-feeding effect in sulfur-doped Fe−N−C single-atom catalyst for enhanced CO2 electroreduction[J]. Angew Chem Int Ed,2022,61(32):202206233. [95] CHEN X, CHEN J X, CHEN H Y, ZHANG Q Q, LI J X, CUI J W, SUN Y H, WANG D F, YE J H, LIU L Q. Promoting water dissociation for efficient solar driven CO2 electroreduction via improving hydroxyl adsorption[J]. Nat Commun,2023,14:751. doi: 10.1038/s41467-023-36263-z [96] HORI Y, KONISHI H, FUTAMURA T, MURATA A, KOGA O, SAKURAI H, OGUMA K. “Deactivation of copper electrode” in electrochemical reduction of CO2[J]. Electrochimica Acta,2005,50(27):5354−5369. doi: 10.1016/j.electacta.2005.03.015 [97] LUC W, KO B H, KATTEL S, LI S, SU D, CHEN J G, JIAO F. SO2-induced selectivity change in CO2 electroreduction[J]. JACS,2019,141(25):9902−9909. doi: 10.1021/jacs.9b03215 [98] QIAN Y, LIU Y F, TANG H H, LIN B L. Highly efficient electroreduction of CO2 to formate by nanorod@2D nanosheets SnO[J]. J CO2 Util,2020,42:101287. doi: 10.1016/j.jcou.2020.101287 [99] XIA C, ZHU P, JIANG Q, PAN Y, LIANG W T, STAVITSKI E, ALSHAREEF H N, WANG H T. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices[J]. Nat Energy,2019,4:776−785. doi: 10.1038/s41560-019-0451-x -





 下载:
下载: