Research progress of homogeneous and heterogeneous catalysts in CO2 cycloaddition reactions
-
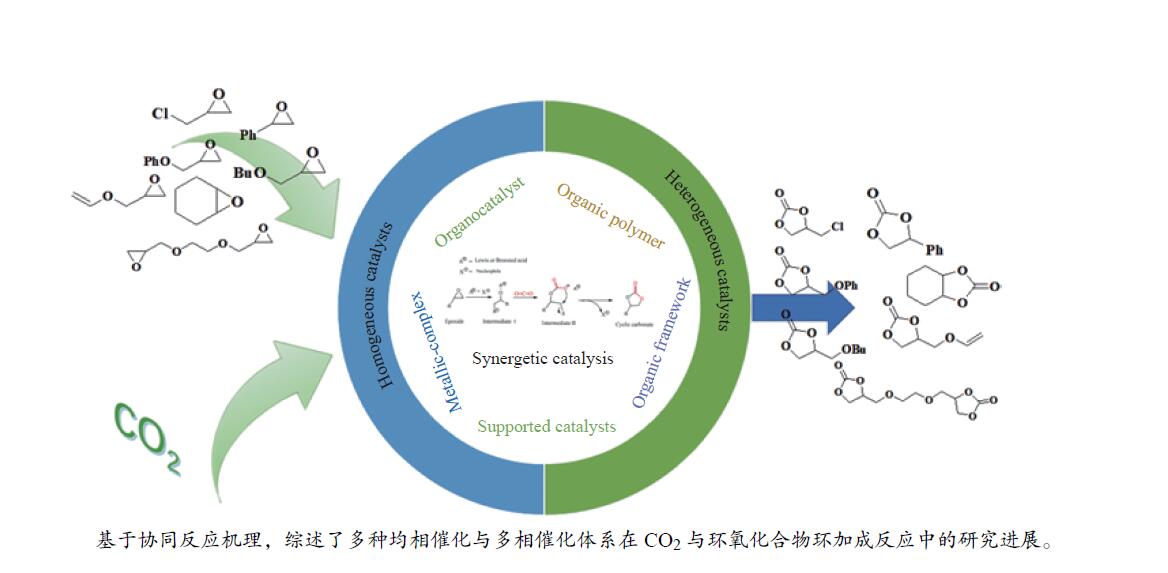
摘要: 二氧化碳(CO2)是一种主要的人为温室气体,主要由化学、热电和钢铁工业以及运输等部门产生。大气层中CO2浓度的增加是导致诸多环境问题的主要原因,如全球变暖、海平面上升和全球气温升高。然而,CO2作为一种可再生、廉价和无毒的化学原料,可用来生产具有高附加值的化学品,进而降低碳浓度。五元环碳酸酯由于其优越的物理化学特性,如高沸点、高偶极矩和生物降解等性能而被广泛应用。由环氧化合物和CO2合成环碳酸酯是迄今为止研究较多的方法。然而,由于CO2的高热稳定性和动力学惰性,使其作为反应原料需要大量的能量投入,可能导致的结果是CO2浓度是一个净增长过程。因此,利用CO2作为C1构筑单元是一个长期的挑战。本工作基于CO2固定反应机制,概述了各种类型的均相和多相催化剂在CO2固定反应合成精细化学品环状碳酸酯中的研究进展,包括有机催化剂、离子液体、金属有机框架化合物、多孔有机聚合物等。目前,几乎所有类别催化剂均可以在室温和低压力下,以实验室规模成功地使用纯CO2将其固定到末端环氧化合物上,对于非末端环氧化合物通过更高的温度和压力以实现相应转化。同时,分析了催化剂在多取代环氧化合物或生物衍生环氧化合物转化、低浓度CO2转化和实现工业化三个方面所面临的挑战,并提出了未来相关研究努力的方向。Abstract: Carbon dioxide (CO2) is a major anthropogenic greenhouse gas produced by chemical, thermoelectric and steel industries as well as transport sector. The increasing concentration of CO2 in atmosphere is responsible for plenty of environmental problems such as global warming, rising sea levels and increasing global temperatures. However, CO2 could consider as renewable, cheap and non-toxic chemical raw material, using CO2 to produce high value-added chemicals to reduce carbon concentrations is a highly desirable strategy. Five-membered cyclic carbonates have a wide range of applications due to their superior physicochemical properties such as high boiling point, high dipole moment and biodegradability. The synthesis of cyclic carbonates from epoxides and CO2 is by far the most approved method. Nevertheless, due to high thermal stability and kinetic inertness, it is necessary to activate CO2 as feedstock for organic synthesis with large energy, which may result in the release of more CO2 than is actually. Therefore, the use of CO2 as C1 building block is long-term challenged. This paper outlines the progress of research on various types of homogeneous and heterogeneous catalysts for CO2 fixation to generate cyclic carbonates, including organocatalysts, ionic liquids, metal-organic frameworks, and porous organic polymers. Almost all of these catalysts are currently available for the successful fixation of CO2 to terminal epoxides on laboratory scale using pure CO2 at ambient temperatures. For internal epoxides higher reaction conditions are usually required to achieve the desired conversion. It was analyzed three areas of present major challenges in catalyzing multi-substituted epoxides or bio-derived epoxides, diluted CO2 conversion and industrialization, and the directions for future research efforts on the subject were suggested.
-
Key words:
- carbon dioxide /
- epoxide /
- catalyst /
- cycloaddition reaction /
- cyclic carbonates
-
图 20 化合物Cu MOF单晶结构:(a) 两种Cu配位模式和CPTPTA5−配体的多面体简化;(b) 球棒视图和(c) 沿[001]框架的多面体视图[85]
Figure 20 Single-crystal structure of compound Cu MOF: (a) polyhedral simplifications of two kinds of Cu coordination modes and CPTPTA5-ligand; (b) ball-and-stick view and (c) polyhedral view of the framework along [001][85] (with permission from RSC Publications)
表 1 不同多相催化剂催化活性的比较
Table 1 Comparison of catalytic activity of different heterogeneous catalysts
Catalyst Co-catalyst Reaction conditions
catalyst amount/epoxide amount
(mmol)/temperature(℃)/CO2 pressure(bar)/time(h)Yield
/%References Support Salophen-MnCl TBAB 0.05 mmol/6.1 mmol/120/15/4 100 [77] KIT-6@ILCH3CH(OH)COO(0.6) − 0.15 g/0.01/90/7/2 99 [78] ZrO2/g-C3N4−400 − 200 mg/16.6/140/20/6 69 [79] Cu MOF TBAB 0.5%/20/60/20/6 97 [85] PMo12@Zr-FcMOF TBAB 10.26%/12.5/80/1/8 86.77 [86] Co(II)MOF TBAB 15 mg/31/80/10/3 97 [87] COF-SO3H TBAB 0.01 mmol/20/80/1/24 99 [89] Zn@TpTta TBAB 10 mmol/10/60/1/4 100 [90] PIL-HPCOF − 15 mg/5/90/10/24 99 [91] 2,5-DCP-CTF − 100 mg/18/130/6.9/4 99.1 [93] CTF-TPM-400 − 3%/10/100/7/24 99 [94] CTF-IM − 100 mg/35.7/120/20/2.5 94.6 [95] IL-ZIF-8(0.3) − 30 mg/25/110/10/4 97 [99] ZIF-67@CeO2 − 4.6%/-/120/7.5/8 100 [100] ML-ZIF 5Co − 0.2%/-/120/14/0.5 94 [101] Co/Zn-ZIF-A-24 h − 50 mg/25/80/1/24 99 [102] DHI-CSU-3-Br TBAB 30 mg/3/70/1/4 99 [110] Py-HCP-Br − 0.4 g/34.5/120/20/4 97 [111] IHCP-1 − 100 mg/10/140/10/24 99 [112] DTBBQ-CMP TBAB 5 mg/2/25/1/48 99 [115] Zn-Salen-CMP TBAB 0.1 mmol/50/120/30/1 90 [116] CMP-Salen-Zn TBAB 0.025 mmol/5/120/1/12 99 [117] Co-PPOPs − 20 mg/25/25/1/48 98 [122] PPh2PStR-PMtVPP − 0.0424 mmol/20/80/48/1 98.9 [123] COCP-OH KI 71 mg/10/70/1/24 94.8 [124] 1D-UCP KI 0.1 mmol/10/80/1/24 90.1 [125] PMP-TDNs − 61.3 mg/50/110/10/8 98.6 [126] PIMBr-COOH − 1%/43/100/1/6 94 [128] PIP-urea 0.3%/10/100/10/5 93 [129] H-MOP-BA TBAI 1.25 mmol/4/50/10/24 96 [130] PIM2 − 0.2%/10/130/10/4 92 [133] PIL-DVB-IV − 0.5 g/34.5/110/20/6 93 [134] PILMs − 0.319 mmol/28.6/110/25/3 87.3 [135] [G3−PAMAM-HADMAB-C18]Br − 0.3125%/50/110/10/3 94.9 [136] -
[1] YANG Z Q, HE C Q, SUI H, et al. Recent advances of CO2-responsive materials in separations[J]. J CO2 Util,2019,30:79−99. doi: 10.1016/j.jcou.2019.01.004 [2] PARKINSON B, BALCOMBE P, SPEIRS J F, et al. Levelized cost of CO2 mitigation from hydrogen production routes[J]. Energy Environ Sci,2019,12(1):19−40. doi: 10.1039/C8EE02079E [3] FAJARDY M, MAC DOWELL N. The energy return on investment of BECCS: Is BECCS a threat to energy security?[J]. Energy Environ Sci,2018,11(6):1581−1594. doi: 10.1039/C7EE03610H [4] SZIMA S, NAZIR S M, CLOETE S, et al. Gas switching reforming for flexible power and hydrogen production to balance variable renewables[J]. Renewable Sustainable Energy Rev,2019,110:207−219. doi: 10.1016/j.rser.2019.03.061 [5] CHATTERJEE R, BHATTACHARJEE S, BHAUMIK A. Bifunctional metal-free heterogeneous catalyst for the synthesis of methanol and diols from CO2 and Epoxide[J]. Asian J Org Chem,2022,11(9):e202200317. [6] LIU R, XU S, HAO G P, et al. Recent advances of porous solids for ultradilute CO2 capture[J]. Chem Res Chin Univ,2022,38(1):18−30. doi: 10.1007/s40242-021-1394-x [7] KHOO H H, BU J, WONG R L, et al. Carbon capture and utilization: Preliminary life cycle CO2, energy, and cost results of potential mineral carbonation[J]. Energy Procedia,2011,4:2494−2501. doi: 10.1016/j.egypro.2011.02.145 [8] ALLURI S, CLAREMBOUX, V, KAWATRA S. Opportunities and challenges in CO2 utilization[J]. J Environ Sci,2022,113:322−344. doi: 10.1016/j.jes.2021.05.043 [9] CUÉLLAR-FRANCA R M, AZAPAGIC A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts[J]. J CO2 Util,2015,9:82−102. doi: 10.1016/j.jcou.2014.12.001 [10] 姜秀云, 杨文兵, 宋昊, 等. 甲酸辅助Cu-ZnO-Al2O3催化剂制备及其CO2加氢制甲醇性能研究[J]. 燃料化学学报(中英文),2023,51(1):120−128. doi: 10.1016/S1872-5813(22)60041-0JIANG Xiuyun, YANG Wenbing, SONG Hao, et al. Formic acid assisted synthesis of Cu-ZnO-Al2O3 catalyst and its performance in CO2 hydrogenation to methanol[J]. J Fuel Chem Technol,2023,51(1):120−128. doi: 10.1016/S1872-5813(22)60041-0 [11] 周程, 南永永, 查飞, 等. 金属有机骨架材料在二氧化碳加氢中的应用[J]. 燃料化学学报,2021,49(10):1444−1457. doi: 10.1016/S1872-5813(21)60097-XZHOU Cheng, NAN Yongyong, ZHA Fei, et al. Application of metal-organic frameworks in CO2 hydrogenation[J]. J Fuel Chem Technol,2021,49(10):1444−1457. doi: 10.1016/S1872-5813(21)60097-X [12] 毛瑀中, 查飞, 田海锋, 等. 热催化CO2加氢制乙醇的研究进展[J]. 燃料化学学报(中英文),2023,51(5):1−15.MAO Yuzhong, ZHA Fei, TIAN Haifeng, et al. Progress in thermal catalysis hydrogenation of CO2 to ethanol[J]. J Fuel Chem Technol,2023,51(5):1−15. [13] LI G Q, DONG S, FU P, et al. Synthesis of porous poly(ionic liquid)s for chemical CO2 fixation with epoxides[J]. Green Chem,2022,24(9):3433−3460. doi: 10.1039/D2GC00324D [14] LI N N, QIN S J, HAO Y J, et al. Nanoarchitectonics of polymeric crown-ether analog of Tröger base combined with potassium iodide and acids synergy in fixation of CO2 and epoxides[J]. Mol Catal,2023,545:113241. doi: 10.1016/j.mcat.2023.113241 [15] KULAL N, VASISTA V, SHANBHAG G V. Identification and tuning of active sites in selected mixed metal oxide catalysts for cyclic carbonate synthesis from epoxides and CO2[J]. J CO2 Util,2019,33:434−444. doi: 10.1016/j.jcou.2019.07.018 [16] GAO L Y, ZHOU Y, LI Z J, et al. Nicotinamide onium halide bidentate hybrid H-bond donor organocatalyst for CO2 fixation[J]. J CO2 Util,2022,65:102196. doi: 10.1016/j.jcou.2022.102196 [17] SONG X H, WU Y F, PAN D H, et al. Melem based multifunctional catalyst for chemical fixation of carbon dioxide into cyclic carbonate[J]. J CO2 Util,2018,24:287−297. doi: 10.1016/j.jcou.2018.01.017 [18] KAMPHUIS A J, PICCHIONI F, PESCARMONA P P. CO2-fixation into cyclic and polymeric carbonates: principles and applications[J]. Green Chem,2019,21(3):406−448. doi: 10.1039/C8GC03086C [19] LEU M K, VICENTE I, FERNANDES J A, et al. On the real catalytically active species for CO2 fixation into cyclic carbonates under near ambient conditions: Dissociation equilibrium of [BMIm][Fe(NO)2Cl2] dependant on reaction temperature[J]. Appl Catal B: Environ,2019,245:240−250. doi: 10.1016/j.apcatb.2018.12.062 [20] LIU J, YANG G Q, LIU Y, et al. Efficient conversion of CO2 into cyclic carbonates at room temperature catalyzed by Al-salen and imidazolium hydrogen carbonate ionic liquids[J]. Green Chem,2020,22(14):4509−4515. doi: 10.1039/D0GC00458H [21] REHMAN A, SALEEM F, JAVED F, et al. Recent advances in the synthesis of cyclic carbonates via CO2 cycloaddition to epoxides[J]. J Environ Chem Eng,2021,9(2):105113. doi: 10.1016/j.jece.2021.105113 [22] LU Z, HE J, GUO B G, et al. Efficient homogenous catalysis of CO2 to generate cyclic carbonates by heterogeneous and recyclable polypyrazoles[J]. Chin J Chem Eng,2022,43:110−115. doi: 10.1016/j.cjche.2022.01.009 [23] 李东娜, 邱明月, 张奇日, 等. 一步法构筑离子液体功能化MOF材料用于常压催化CO2环加成反应[J]. 燃料化学学报,2022,50(7):824−831. doi: 10.1016/S1872-5813(21)60195-0LI Dongna, QIU Mingyue, ZHANG Qiri, et al. One-pot construction of ionic liquid-functionalized MOF material as the catalyst for CO2 cycloaddition under atmospheric pressure[J]. J Fuel Chem Technol,2022,50(7):824−831. doi: 10.1016/S1872-5813(21)60195-0 [24] 胡凤群, 邱明月, 易群, 等. 核壳型MOFs@离子液体复合材料的制备及其常压下催化CO2环加成反应性能探究[J]. 燃料化学学报(中英文),2023,51(11):1673−1682.HU Fengqun, QIU Mingyue, YI Qun, et al. Construction of core-shell MOFs@ionic liquid materials and their performance for CO2 cycloaddition reaction under atmospheric pressure[J]. J Fuel Chem Technol,2023,51(11):1673−1682. [25] KEMBER M R, BUCHARD A, WILLIAMS C K. Catalysts for CO2/epoxide copolymerisation[J]. Chem Commun,2011,47(1):141−163. doi: 10.1039/C0CC02207A [26] 王献红, 王佛松. CO2的固定和利用[M]. 北京: 化学工业出版社, 2011: 64.WANG Xianhong, WANG Fosong. CO2 Fixation and Utilization[M]. Beijing: Chemical Industry Press, 2011: 64. [27] GUO L P, LAMB K J, NORTH M. Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates[J]. Green Chem,2021,23(1):77−118. doi: 10.1039/D0GC03465G [28] YUE S, WANG P, HAO X. Synthesis of cyclic carbonate from CO2 and epoxide using bifunctional imidazolium ionic liquid under mild conditions[J]. Fuel,2019,251:233−241. doi: 10.1016/j.fuel.2019.04.039 [29] MUJMULE R B, KIM H. Efficient imidazolium ionic liquid as a tri-functional robust catalyst for chemical fixation of CO2 into cyclic carbonates[J]. J Environ Manage,2022,314:115045. doi: 10.1016/j.jenvman.2022.115045 [30] ZHANG A, CHEN C J, ZUO C S, et al. Imidazolium-based ionic liquids containing multipoint hydrogen bond donors as bifunctional organocatalysts for efficient cooperative conversion of CO2 to cyclic carbonates[J]. Green Chem,2022,24(18):7194−7207. doi: 10.1039/D2GC02517E [31] de ALMEIDA BEZERRA W, MILANI J L S, de JESUS FRANCO C H, et al. Bis-benzimidazolium salts as bifunctional organocatalysts for the cycloaddition of CO2 with epoxides[J]. Mol Catal,2022,530:112632. doi: 10.1016/j.mcat.2022.112632 [32] CHENG W G, XIAO B N, SUN J, et al. Effect of hydrogen bond of hydroxyl-functionalized ammonium ionic liquids on cycloaddition of CO2[J]. Tetrahedron Lett,2015,56(11):1416−1419. doi: 10.1016/j.tetlet.2015.01.174 [33] PENG J, WANG S, YANG H J, et al. Highly efficient fixation of carbon dioxide to cyclic carbonates with new multi-hydroxyl bis-(quaternary ammonium) ionic liquids as metal-free catalysts under mild conditions[J]. Fuel,2018,224:481−488. doi: 10.1016/j.fuel.2018.03.119 [34] BÜTTNER H, STEINBAUER J, WULF C, et al. Organocatalyzed synthesis of oleochemical carbonates from CO2 and renewables[J]. ChemSusChem,2017,10(6):1076−1079. doi: 10.1002/cssc.201601163 [35] LIU Y, CHENG W G, ZHANG Y Q, et al. Controllable preparation of phosphonium-based polymeric ionic liquids as highly selective nanocatalysts for the chemical conversion of CO2 with epoxides[J]. Green Chem,2017,19(9):2184−2193. doi: 10.1039/C7GC00444C [36] SHAIKH A C, VELETA J M, BLOCH J, et al. Syntheses of phosphonium salts from phosphines and carbenium: efficient CO2 fixation and phase-transfer catalysts[J]. Eur J Org Chem,2020,2020(17):2553−2559. doi: 10.1002/ejoc.202000221 [37] ZHANG Z G, FAN F J, XING H B, et al. Efficient synthesis of cyclic carbonates from atmospheric CO2 using a positive charge delocalized ionic liquid catalyst[J]. ACS Sustainable Chem Eng,2017,5(4):2841−2846. doi: 10.1021/acssuschemeng.7b00513 [38] ROSTAMI A, MAHMOODABADI M, HOSSEIN E A, et al. An electrostatically enhanced phenol as a simple and efficient bifunctional organocatalyst for carbon dioxide fixation[J]. ChemSusChem,2018,11(24):4262−4268. doi: 10.1002/cssc.201802028 [39] NOROUZI F, ABDOLMALEKI A. CO2 conversion into carbonate using pyridinium-based ionic liquids under mild conditions[J]. Fuel,2023,334:126641. doi: 10.1016/j.fuel.2022.126641 [40] LI W, CHENG W G, YANG X, et al. Synthesis of cyclic carbonate catalyzed by DBU derived basic ionic liquids[J]. Chin J Chem,2018,36(4):293−298. doi: 10.1002/cjoc.201700747 [41] FANJUL-MOSTEIRÍN N, JEHANNO C, RUIPÉREZ F, et al. Rational study of DBU salts for the CO2 insertion into epoxides for the synthesis of cyclic carbonates[J]. ACS Sustainable Chem Eng,2019,7(12):10633−10640. doi: 10.1021/acssuschemeng.9b01300 [42] CHOWDHURY B, ZVINCHUK A A, AYSIN R R, et al. Amine-iodine adducts as simple but effective catalysts for the synthesis of organic carbonates from epoxides and CO2[J]. Catal Surv Asia,2021,25(4):419−423. doi: 10.1007/s10563-021-09341-9 [43] HE L, ZHANG W W, YANG Y F, et al. Novel biomass-derived deep eutectic solvents promoted cycloaddition of CO2 with epoxides under mild and additive-free conditions[J]. J CO2 Util,2021,54:101750. doi: 10.1016/j.jcou.2021.101750 [44] YANG X Q, ZOU Q Z, ZHAO T X, et al. Deep eutectic solvents as efficient catalysts for fixation of CO2 to cyclic carbonates at ambient temperature and pressure through synergetic catalysis[J]. ACS Sustainable Chem Eng,2021,9(31):10437−10443. doi: 10.1021/acssuschemeng.1c03187 [45] SHENG T, OU J L, ZHAO T X, et al. Efficient fixation of CO2 into cyclic carbonate catalyzed by choline bromide/imidazole derivatives-based deep eutectic solvents[J]. Mol Catal,2023,536:112907. doi: 10.1016/j.mcat.2022.112907 [46] NG C K, TOH R W, LIN T T, et al. Metal-salen molecular cages as efficient and recyclable heterogeneous catalysts for cycloaddition of CO2 with epoxides under ambient conditions[J]. Chem Sci,2019,10(5):1549−1554. doi: 10.1039/C8SC05019H [47] CASTRO-OSMA J A, LAMB K J, NORTH M. Cr (salophen) complex catalyzed cyclic carbonate synthesis at ambient temperature and pressure[J]. ACS Catal,2016,6(8):5012−5025. doi: 10.1021/acscatal.6b01386 [48] DELLA MONICA F, CAPACCHIONE C. Recent advancements in metal-catalysts design for CO2/epoxide reactions[J]. Asian J Org Chem,2022,11(8):e202200300. [49] AOMCHAD V, Del GOBBO S, YINGCHAROEN P, et al. Exploring the potential of group III salen complexes for the conversion of CO2 under ambient conditions[J]. Catal Today,2021,375:324−334. doi: 10.1016/j.cattod.2020.01.021 [50] KUZNETSOVA S A, GORODISHCH I V, GAK A S. Chiral titanium(IV) and vanadium(V) salen complexes as catalysts for carbon dioxide and epoxide coupling reactions[J]. Tetrahedron,2021,82:131929. doi: 10.1016/j.tet.2021.131929 [51] KIRIRATNIKOM J, LAIWATTANAPAISARN N, VONGNAM K, et al. Highly active chromium complexes supported by constrained schiff-base ligands for cycloaddition of carbon dioxide to epoxides[J]. Inorg Chem,2021,60(9):6147−6151. doi: 10.1021/acs.inorgchem.0c03732 [52] KANG Y R, WANG B N, NAN R X, et al. Cyclic carbonate synthesis from epoxides and CO2 catalyzed by aluminum-salen complexes bearing a nido-C2B9 carborane ligand[J]. Inorg Chem,2022,61(23):8806−8814. doi: 10.1021/acs.inorgchem.2c00797 [53] CONTENTO I, LAMPARELLI D H, BUONERBA A, et al. New dinuclear chromium complexes supported by thioether-triphenolate ligands as active catalysts for the cycloaddition of CO2 to epoxides[J]. J CO2 Util,2022,66:102276. doi: 10.1016/j.jcou.2022.102276 [54] NORTH M, QUEK S C Z, PRIDMORE N E, et al. Aluminum (salen) complexes as catalysts for the kinetic resolution of terminal epoxides via CO2 coupling[J]. ACS Catal,2015,5(6):3398−3402. doi: 10.1021/acscatal.5b00235 [55] SU Y C, TSUI C H, TSAI C Y, et al. Highly active bimetallic nickel catalysts for alternating copolymerization of carbon dioxide with epoxides[J]. Polym Chem,2020,11(18):3225−3236. doi: 10.1039/D0PY00174K [56] DEACY A C, MOREBY E, PHANOPOULOS A, et al. Co(III)/alkali-metal(I) heterodinuclear catalysts for the ring-opening copolymerization of CO2 and propylene oxide[J]. J Am Chem Soc,2020,142(45):19150−19160. doi: 10.1021/jacs.0c07980 [57] CHEN J, WU X M, DING H N, et al. Tolerant bimetallic macrocyclic -type zinc complexes for efficient CO2 fixation into cyclic carbonates[J]. ACS Sustainable Chem Eng,2021,9(48):16210−16219. doi: 10.1021/acssuschemeng.1c05469 [58] MAEDA C, SHIMONISHI J, MIYAZAKI R, et al. Highly active and robust metalloporphyrin catalysts for the synthesis of cyclic carbonates from a broad range of epoxides and carbon dioxide[J]. Chem-Eur J,2016,22(19):6556−6563. doi: 10.1002/chem.201600164 [59] MILANI J L S, MEIRELES A M, CABRAL B N, et al. Highly active Mn(III) meso-tetrakis(2, 3-dichlorophenyl) porphyrin catalysts for the cycloaddition of CO2 with epoxides[J]. J CO2 Util,2019,30:100−106. doi: 10.1016/j.jcou.2018.12.017 [60] SCHOEPFF L, MONNEREAU L, DUROT S, et al. A flexible bis-Co(III) porphyrin cage as a bimetallic catalyst for the conversion of CO2 and epoxides into cyclic carbonates[J]. ChemCatChem,2020,12(22):101635. [61] LEAL J P S C, BEZERRA W A, CHAGAS R P, et al. Metal–cocatalyst interaction governs the catalytic activity of MII-Porphyrazines for chemical fixation of CO2[J]. Inorg Chem,2021,60(16):12263−12273. doi: 10.1021/acs.inorgchem.1c01462 [62] MARTÍNEZ J, DE LA CRUZ-MARTÍNEZ F, GAONA M A, et al. Influence of the counterion on the synthesis of cyclic carbonates catalyzed by bifunctional aluminum complexes[J]. Inorg Chem,2019,58(5):3396−3408. doi: 10.1021/acs.inorgchem.8b03475 [63] PANZA N, BIASE A, GALLO E, et al. Unexpected “ferrate” species as single-component catalyst for the cycloaddition of CO2 to epoxides[J]. J CO2 Util,2021,51:101635. doi: 10.1016/j.jcou.2021.101635 [64] KIM J H, LEE S H, KIM N H, et al. Sustainable synthesis of five-membered heterocycles using carbon dioxide and Fe-iminopyridine catalysts[J]. J CO2 Util,2021,50:101595. doi: 10.1016/j.jcou.2021.101595 [65] MILOCCO F, CHIARIONI G, PESCARMONA P P. Heterogeneous catalysts for the conversion of CO2 into cyclic and polymeric carbonates[J]. Adv Catal,2022,70:151−187. [66] DU Y, CAI F, KONG D L, et al. Organic solvent-free process for the synthesis of propylene carbonate from supercritical carbon dioxide and propylene oxide catalyzed by insoluble ion exchange resins[J]. Green Chem,2005,7(7):518−523. doi: 10.1039/b500074b [67] ZHANG Q, ZHANG S B, LI S H. Novel functional organic network containing quaternary phosphonium and tertiary phosphorus[J]. Macromol,2012,45(7):2981−2988. doi: 10.1021/ma300278d [68] GHAZALI-ESFAHANI S, SONG H B, PĂUNESCU E, et al. Cycloaddition of CO2 to epoxides catalyzed by imidazolium-based polymeric ionic liquids[J]. Green Chem,2013,15(6):1584−1589. doi: 10.1039/c3gc37085b [69] HAN L, PARK S W, PARK D W. Silica grafted imidazolium-based ionic liquids: Efficient heterogeneous catalysts for chemical fixation of CO2 to a cyclic carbonate[J]. Energy Environ Sci,2009,2(12):1286−1292. doi: 10.1039/b910763k [70] XIE Y, ZHANG Z F, JIANG T, et al. CO2 cycloaddition reactions catalyzed by an ionic liquid grafted onto a highly crosslinked polymer matrix[J]. Angew Chem Int Ed,2007,46(38):7255−7258. doi: 10.1002/anie.200701467 [71] APRILE C, GIACALONE F, AGRIGENTO P, et al. Multilayered supported ionic liquids as catalysts for chemical fixation of carbon dioxide: A high-throughput study in supercritical conditions[J]. ChemSusChem,2011,4(12):1830−1837. doi: 10.1002/cssc.201100446 [72] CHANG T, LI X P, HAO Y J, et al. Pyrene-based ammonium bromides combined with g-C3N4 for the synergistically enhanced fixation reaction of CO2 and epoxides[J]. RSC Adv,2021,11(48):30222−30228. doi: 10.1039/D1RA05328K [73] ZANDA N, SOBOLEWSKA A, ALZA E, et al. Organocatalytic and halide-free synthesis of glycerol carbonate under continuous flow[J]. ACS Sustainable Chem Eng,2021,9(12):4391−4397. doi: 10.1021/acssuschemeng.1c01060 [74] BARBARINI A, MAGGI R, MAZZACANI A, et al. Cycloaddition of CO2 to epoxides over both homogeneous and silica-supported guanidine catalysts[J]. Tetrahedron Lett,2003,44(14):2931−2934. doi: 10.1016/S0040-4039(03)00424-6 [75] UDAYAKUMAR S, LEE M K, SHIM H L, et al. Imidazolium derivatives functionalized MCM-41 for catalytic conversion of carbon dioxide to cyclic carbonate[J]. Catal Commun,2009,10(5):659−664. doi: 10.1016/j.catcom.2008.11.017 [76] CHENG W G, CHEN X, SUN J, et al. SBA-15 supported triazolium-based ionic liquids as highly efficient and recyclable catalysts for fixation of CO2 with epoxides[J]. Catal Today,2013,200:117−124. doi: 10.1016/j.cattod.2012.10.001 [77] BALAS M, BEAUDOIN S, PROUST A, et al. Advantages of covalent immobilization of metal‐salophen on amino-functionalized mesoporous silica in terms of recycling and catalytic activity for CO2 cycloaddition onto epoxides[J]. Eur J Inorg Chem,2021,2021(16):1581−1591. doi: 10.1002/ejic.202100150 [78] LIU Y, HU Y L. Novel and high efficient cycloaddition of CO2 with epoxides to cyclic carbonates over reusable mesoporous KIT-6 supported imidazolium lactate catalyst[J]. Bull Chem Soc Ethiop,2022,36(2):433−450. doi: 10.4314/bcse.v36i2.16 [79] LIU N, WU F, XU J, et al. ZrO2 supported on graphitic carbon nitride based on metal-nitrogen interaction for enhanced catalytic cycloaddition of CO2 to cyclic carbonates[J]. Catal Lett,2023,153(5):1483−1494. doi: 10.1007/s10562-022-04083-3 [80] HE H M, PERMAN J A, ZHU G S, et al. Metal-organic frameworks for CO2 chemical transformations[J]. Small,2016,12(46):6309−6324. doi: 10.1002/smll.201602711 [81] LIANG J, HUANG Y B, CAO R. Metal-organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates[J]. Coord Chem Rev,2019,378:32−65. doi: 10.1016/j.ccr.2017.11.013 [82] PAL T K, DE D, BHARADWAJ P K C. Metal-organic frameworks for the chemical fixation of CO2 into cyclic carbonates[J]. Coord Chem Rev,2020,408:213173. doi: 10.1016/j.ccr.2019.213173 [83] SONG X J, WANG J, YANG L B, et al. The transformation strategies between homogeneous and heterogeneous catalysts for the coupling reactions of CO2 and epoxides/olefins[J]. Inorg Chem Commun,2020,121:108197. doi: 10.1016/j.inoche.2020.108197 [84] QI X P, CHANG T, ZHU Z, et al. Post-synthetic modification of Isomorphic coordination polymers with metal ion exchange and catalytic cycloaddition of CO2[J]. J Solid State Chem,2021,304:122604. doi: 10.1016/j.jssc.2021.122604 [85] ZHU Y Y, GU J M, YU X Y, et al. The multifunctional design of metal-organic framework by applying linker desymmetrization strategy: synergistic catalysis for high CO2-epoxide conversion[J]. Inorg Chem Front,2021,8(23):4990−4997. doi: 10.1039/D1QI00960E [86] FANG Z, DENG Z, WAN X Y, et al. Keggin-type polyoxometalates molecularly loaded in Zr-ferrocene metal organic framework nanosheets for solar-driven CO2 cycloaddition[J]. Appl Catal B: Environ,2021,296:120329. doi: 10.1016/j.apcatb.2021.120329 [87] ULLAH N, RAMIERE A, RAZA W, et al. Cobalt-based MOF nanoribbons with abundant O/N species for cycloaddition of carbon dioxide to epoxides[J]. J Colloid Interf Sci,2022,623:752−761. doi: 10.1016/j.jcis.2022.05.082 [88] DALIRAN S, OVEISI A R, PENG Y, et al. Metal-organic-framework (MOF), covalent-organic-framework (COF), and porous-organic-polymers (POP) catalyzed selective C–H bond activation and functionalization reactions[J]. Chem Soc Rev,2022,51(18):7810−7882. doi: 10.1039/D1CS00976A [89] SINGH G, NAGARAJA C M. Highly efficient metal/solvent-free chemical fixation of CO2 at atmospheric pressure conditions using functionalized porous covalent organic frameworks[J]. J CO2 Util,2021,53:101716. doi: 10.1016/j.jcou.2021.101716 [90] SARKAR S, GHOSH S, ISLAM S M. A Zn (ii)-functionalized COF as a recyclable catalyst for the sustainable synthesis of cyclic carbonates and cyclic carbamates from atmospheric CO2[J]. Org Biomol Chem,2022,20(8):1707−1722. doi: 10.1039/D1OB01938D [91] DU Y R, YANG X, WANG Y F, et al. Immobilization poly(ionic liquid)s into hierarchical porous covalent organic frameworks as heterogeneous catalyst for cycloaddition of CO2 with epoxides[J]. Mol Catal,2022,520:112164. doi: 10.1016/j.mcat.2022.112164 [92] LUO R C, XU W, CHEN M, et al. Covalent triazine frameworks obtained from nitrile monomers for sustainable CO2 catalysis[J]. ChemSusChem,2020,13(24):6509−6522. doi: 10.1002/cssc.202002422 [93] LI Y M, YANG L, SUN L, et al. Chemical fixation of carbon dioxide catalyzed via covalent triazine frameworks as metal free heterogeneous catalysts without a cocatalyst[J]. J Mater Chem A,2019,7(45):26071−26076. doi: 10.1039/C9TA07266G [94] ZHAO Y L, HUANG H L, ZHU H, et al. Design and synthesis of novel pyridine-rich cationic covalent triazine framework for CO2 capture and conversion[J]. Micropor ous Mesopor ous Mater,2022,329:111526. doi: 10.1016/j.micromeso.2021.111526 [95] DAI W L, LI Q, LONG J F, et al. Hierarchically mesoporous imidazole-functionalized covalent triazine framework: An efficient metal-and halogen-free heterogeneous catalyst towards the cycloaddition of CO2 with epoxides[J]. J CO2 Util,2022,62:102101. doi: 10.1016/j.jcou.2022.102101 [96] HWANG G Y, ROSHAN R, RYU H S, et al. A highly efficient zeolitic imidazolate framework catalyst for the co-catalyst and solvent free synthesis of cyclic carbonates from CO2[J]. J CO2 Util,2016,15:123−130. doi: 10.1016/j.jcou.2016.02.005 [97] KURUPPATHPARAMBIL R R, BABU R, JEONG H M, et al. A solid solution zeolitic imidazolate framework as a room temperature efficient catalyst for the chemical fixation of CO2[J]. Green Chem,2016,18(23):6349−6356. doi: 10.1039/C6GC01614F [98] MARCINIAK A A, LAMB K J, OZORIO L P, et al. Heterogeneous catalysts for cyclic carbonate synthesis from carbon dioxide and epoxides[J]. Curr Opin Green Sustainable Chem,2020,26:100365. doi: 10.1016/j.cogsc.2020.100365 [99] XIANG W L, SHEN C Y, LU Z, et al. CO2 cycloaddition over ionic liquid immobilized hybrid zeolitic imidazolate frameworks: Effect of Lewis acid/base sites[J]. Chem Eng Sci,2021,233:116429. doi: 10.1016/j.ces.2020.116429 [100] HU L H, MO X H, CHEN L, et al. Core-Shell ZIF-67@ CeO2 Nanosphere as efficient acid‐base catalyst for the cycloaddition of CO2 and epoxides[J]. ChemistrySelect,2022,7(20):e202200823. [101] ABRAHA Y W, TSAI C W, LANGNER E H G. De novo syntheses of multi-linker Zn-and Co-based ZIFs with application in CO2 fixation[J]. Microporous Mesoporous Mater,2022,346:112319. doi: 10.1016/j.micromeso.2022.112319 [102] NGUYEN Q T, DO X H, CHO K Y, et al. Amine-functionalized bimetallic Co/Zn-zeolitic imidazolate frameworks as an efficient catalyst for the CO2 cycloaddition to epoxides under mild conditions[J]. J CO2 Util,2022,61:102061. doi: 10.1016/j.jcou.2022.102061 [103] DU J, OUYANG H, TAN B. Porous organic polymers for catalytic conversion of carbon dioxide[J]. Chem Asian J,2021,16(23):3833−3850. doi: 10.1002/asia.202100991 [104] ZHANG J S, QIAO Z A, MAHURIN S M, et al. Hypercrosslinked phenolic polymers with well‐developed mesoporous frameworks[J]. Angew Chem Int Ed,2015,54(15):4582−4586. doi: 10.1002/anie.201500305 [105] XU S J, LUO Y L, TAN B. Recent development of hypercrosslinked microporous organic polymers[J]. Macromol Rap Commun,2013,34(6):471−484. doi: 10.1002/marc.201200788 [106] LIANG J, CHEN R P, WANG X Y, et al. Postsynthetic ionization of an imidazole-containing metal-organic framework for the cycloaddition of carbon dioxide and epoxides[J]. Chem Sci,2017,8(2):1570−1575. doi: 10.1039/C6SC04357G [107] HOU S S, RAZZAQUE S, TAN B. Effects of synthesis methodology on microporous organic hyper-cross-linked polymers with respect to structural porosity, gas uptake performance and fluorescence properties[J]. Poly Chem,2019,10(11):1299−1311. doi: 10.1039/C8PY01730A [108] FU Z Y, JIA J Z, LI J, et al. Transforming waste expanded polystyrene foam into hyper-crosslinked polymers for carbon dioxide capture and separation[J]. Chem Eng J,2017,323:557−564. doi: 10.1016/j.cej.2017.04.090 [109] PENG R X, CHEN G, ZHOU F, et al. Catalyst-free synthesis of triazine-based porous organic polymers for Hg2+ adsorptive removal from aqueous solution[J]. Chem Eng J,2019,371:260−266. doi: 10.1016/j.cej.2019.04.063 [110] SANG Y F, SHU Z, WANG Y, et al. Bifunctional ionic hyper-cross-linked polymers for CO2 capture and catalytic conversion[J]. Appl Surf Sci,2022,585:152663. doi: 10.1016/j.apsusc.2022.152663 [111] LIU C, SHI L, ZHANG J X, et al. One-pot synthesis of pyridine-based ionic hyper-cross-linked polymers with hierarchical pores for efficient CO2 capture and catalytic conversion[J]. Chem Eng J,2022,427:131633. doi: 10.1016/j.cej.2021.131633 [112] GU J R, YUAN Y X, ZHAO T X, et al. Ionic-containing hyper-crosslinked polymer: A promising bifunctional material for CO2 capture and conversion[J]. Sep Purif Technol,2022,301:121971. doi: 10.1016/j.seppur.2022.121971 [113] GAO R D, ZHANG G, LU F L, et al. Pyrrole-based conjugated microporous polymers as efficient heterogeneous catalysts for knoevenagel condensation[J]. Front Chem,2021,9:687183. doi: 10.3389/fchem.2021.687183 [114] DONG X Z, HUI Y H, XIE S L, et al. Schiff base supported MCM-41 catalyzed the Knoevenagel condensation in water[J]. RSC Adv,2013,3(10):3222−3226. doi: 10.1039/c3ra00138e [115] ZHANG X F, LIU H, AN P F, et al. Delocalized electron effect on single metal sites in ultrathin conjugated microporous polymer nanosheets for boosting CO2 cycloaddition[J]. Sci Adv,2020,6(17):eaaz4824. doi: 10.1126/sciadv.aaz4824 [116] BHUNIA S, MOLLA R A, KUMARI V, et al. Zn (ii) assisted synthesis of porous salen as an efficient heterogeneous scaffold for capture and conversion of CO2[J]. Chem Commun,2015,51(86):15732−15735. doi: 10.1039/C5CC06868A [117] ZHOU F Y, DENG Q W, HUANG N Y, et al. CO2 fixation into cyclic carbonates by a Zn-salen based conjugated microporous polymer[J]. ChemistrySelect,2020,5(34):10516−10520. doi: 10.1002/slct.202001538 [118] JU P Y, QI W, GUO B X, et al. Highly stable and versatile conjugated microporous polymer for heterogeneous catalytic applications[J]. Catal Lett,2023,153:2125−2136. [119] LI H, LI C Z, CHEN J, et al. Synthesis of a pyridine-zinc-based porous organic polymer for the Co-catalyst-free cycloaddition of epoxides[J]. Chem Asian J,2017,12(10):1095−1103. doi: 10.1002/asia.201700258 [120] WANG W L, WANG Y Q, LI C Y, et al. State-of-the-art multifunctional heterogeneous POP catalyst for cooperative transformation of CO2 to cyclic carbonates[J]. ACS Sustainable Chem Eng,2017,5(6):4523−4528. doi: 10.1021/acssuschemeng.7b00947 [121] CHEN Y J, LUO R C, XU Q H, et al. Charged metalloporphyrin polymers for cooperative synthesis of cyclic carbonates from CO2 under ambient conditions[J]. ChemSusChem,2017,10(11):2534−2541. doi: 10.1002/cssc.201700536 [122] GUO D, LI C, ZHANG J, et al. Metalloporphyrin-based porous organic polymer as an efficient catalyst for cycloaddition of epoxides and CO2[J]. J Solid State Chem,2021,293:121770. doi: 10.1016/j.jssc.2020.121770 [123] DAI Z F, WANG S T, ZHOU N, et al. Novel porous organic polymers functionalized by metalloporphyrin and phosphonium salt for efficient synergistic catalysis of CO2 conversion under mild conditions[J]. New J Chem,2022,46(46):22151−22161. doi: 10.1039/D2NJ04210J [124] HAO Y J, YAN X L, CHANG T, et al. Hydroxyl-anchored covalent organic crown-based polymers for CO2 fixation into cyclic carbonates under mild conditions[J]. Sustainable Energy Fuels,2022,6(1):121−127. doi: 10.1039/D1SE01120K [125] HAO Y J, YAN X L, LIU X H, et al. Urea-based covalent organic crown polymers and KI electrostatic synergy in CO2 fixation reaction: A combined experimental and theoretical study[J]. J CO2 Util,2022,56:101867. doi: 10.1016/j.jcou.2021.101867 [126] YUE Z X, HU T D, ZHAO W B, et al. Triazinyl-imidazole polyamide network as an efficient multi-hydrogen bond donor catalyst for additive-free CO2 cycloaddition[J]. Appl Catal A: Gen,2022,643:118748. doi: 10.1016/j.apcata.2022.118748 [127] QIU Y J, CHEN Y J, LEI L, et al. Bottom-up oriented synthesis of metalloporphyrin-based porous ionic polymers for the cycloaddition of CO2 to epoxides[J]. Mol Catal,2022,521:112171. doi: 10.1016/j.mcat.2022.112171 [128] CHEN Y, LI Y J, WANG H, et al. Facile construction of carboxyl-functionalized ionic polymer towards synergistic catalytic cycloaddition of carbon dioxide into cyclic carbonates[J]. Int J Mol Sci,2022,23(18):10879. doi: 10.3390/ijms231810879 [129] WANG Y P, DUAN J X. Urea and thiourea-functionalized, pyridinium-based ionic polymers convert CO2 to cyclic carbonate under mild conditions[J]. ACS Appl Polym Mater,2022,4(8):5851−5860. doi: 10.1021/acsapm.2c00734 [130] BANG S, JANG J Y, KO Y J K, et al. Hydroboration of hollow microporous organic polymers: A promising postsynthetic modification method for functional materials[J]. ACS Macro Lett,2022,11(8):1034−1040. doi: 10.1021/acsmacrolett.2c00385 [131] ZHANG J W, LI X P, ZHU Z, et al. Hydroxylamino-anchored poly(ionic liquid)s for CO2 fixation into cyclic ccarbonates at mild conditions[J]. Adv Sustainable Syst,2021,5(1):202000133. [132] WANG P, Lv Q, TAO Y J, et al. One-pot efficient fixation of low-concentration CO2 into cyclic carbonate by mesoporous pyridine-functionalized binuclear poly (ionic liquid)s[J]. Mol Catal,2023,544:113157. doi: 10.1016/j.mcat.2023.113157 [133] WANG Y P, NIE J Q, LU C F, et al. Imidazolium-based polymeric ionic liquids for heterogeneous catalytic conversion of CO2 into cyclic carbonates[J]. Microporous Mesoporous Mater,2020,292:109751. doi: 10.1016/j.micromeso.2019.109751 [134] DU H L, YE Y F, XU P, et al. Experimental and theoretical study on dicationic imidazolium derived poly(ionic liquid)s for catalytic cycloaddition of CO2-epoxide[J]. J CO2 Util,2023,67:102325. doi: 10.1016/j.jcou.2022.102325 [135] LIU Y F, DONG L, WANG Y C, et al. Polymeric ionic liquid membranes for the absorption-conversion of CO2 and epoxides into cyclic carbonates[J]. Mol Catal,2022,530:112597. doi: 10.1016/j.mcat.2022.112597 [136] CHANG T, YAN X L, LI Y, et al. Quaternary ammonium immobilized PAMAM as efficient catalysts for conversion of carbon dioxide[J]. J CO2 Util,2022,58:101913. doi: 10.1016/j.jcou.2022.101913 [137] YAN T, LIU H, ZENG Z X, et al. Recent progress of catalysts for synthesis of cyclic carbonates from CO2 and epoxides[J]. J CO2 Util,2023,68:102355. doi: 10.1016/j.jcou.2022.102355 -





 下载:
下载: