Catalytic soot combustion performance of core-shell Co3O4@MnOx monolithic catalyst
-
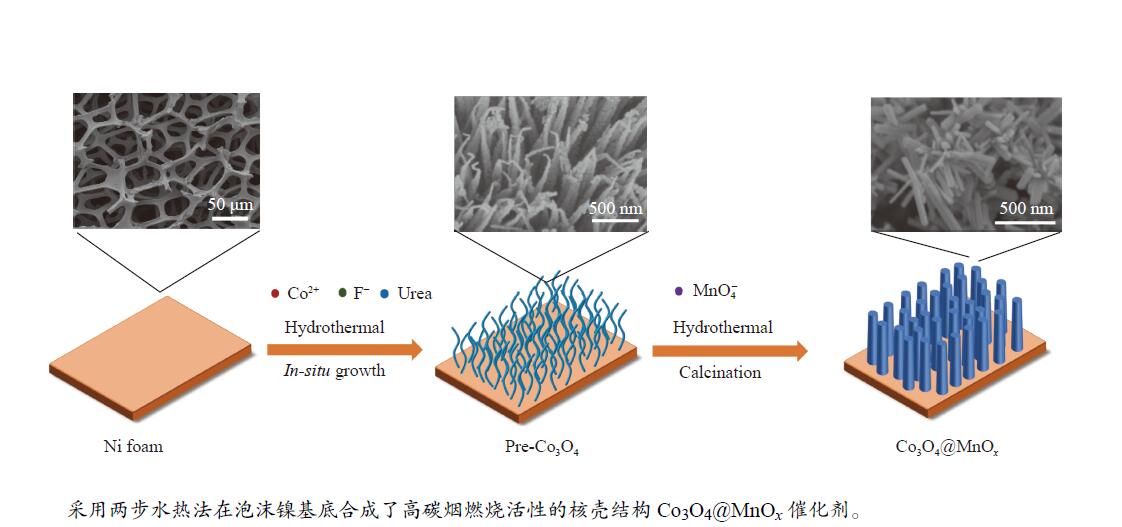
摘要: 采用两步水热法在泡沫镍基底上合成了具有纳米棒形貌的 Co3O4@MnOx 整体式催化剂,通过X射线衍射、X射线能谱分析、氢气-程序升温还原、X射线光电子能谱、拉曼光谱和碳烟-程序升温还原等手段对催化剂进行表征,在微型固定床反应器上评价了其催化碳烟燃烧性能,通过等温动力学实验探究了催化剂的本征活性。结果表明,Co3O4@MnOx 催化剂呈现了以 Co3O4 为核、以 MnOx 为壳的核壳结构。与催化剂 Co-NW 相比,Co3O4@MnOx 催化剂中 Co3O4 与 MnOx 之间的相互作用使其表面产生了更多高价物种 Mn4+ 和 Mn3+ 以及更多的表面氧空位,其氧化还原性能提高,催化剂的活性氧物种数量增加了两倍,催化性能得到改善,在 NO 存在的反应气氛中使碳烟起燃温度降低148 ℃。此外,相比催化剂 Co-NW,Co3O4@MnOx 催化剂使碳烟燃烧反应的活化能从113.6 kJ/mol降低至102.2 kJ/mol,催化剂的本征活性提高了两倍。Abstract: The Co3O4@MnOx monolithic catalyst with nanorod morphology was synthesized on nickel foam by a two-step hydrothermal process and characterized by XRD, EDS-mapping, H2-TPR, XPS, Raman and Soot-TPR. The catalytic soot combustion activities of the catalysts were investigated in a fixed-bed micro-reactor and the intrinsic activities of the catalysts were investigated by the isothermal kinetic experiments. The results reveal that the Co3O4@MnOx catalyst shows a core-shell structure with Co3O4 as the core and MnOx as the shell. Compared with Co-NW, there exist more high-valent state species of Mn4+ and Mn3+ and oxygen vacancies on the Co3O4@MnOx catalyst surface due to the interaction between Co3O4 and MnOx, which improve the redox performance of Co3O4@MnOx. Compared with Co-NW, the active oxygen species amount of Co3O4@MnOx is increased by two times, and Co3O4@MnOx shows enhanced activity, which lowers the ignition temperature by 148 ℃ in the presence of NO. Also, compared with Co-NW, Co3O4@MnOx decreases the activation energy of soot combustion reaction from 113.6 to 102.2 kJ/mol, and its intrinsic activity is increased by two times.
-
图 5 催化剂((a1)–(a3))Co-NW和((b1)–(b3))Co3O4@MnOx的扫描电镜照片((a1)和(b1))、透射电镜图((a2)和(b2))和高分辨透射电镜照片((a3)和(b3));(c)催化剂Co3O4@MnOx的TEM-EDS能谱图
Figure 5 SEM (a1 and b1), TEM (a2 and b2) and HRTEM ((a3) and (b3)) images of ((a1)–(a3)) Co-NW and ((b1)–(b3)) Co3O4@MnOx, and (c) SEM-EDS elemental mapping images of the Co3O4@MnOx
表 1 在有/无6.0×10−4 NO存在时催化剂的碳烟燃烧活性
Table 1 Activities of the catalysts in the presence or absence of 6.0×10−4 NO for soot combustion
Catalyst t10/℃ t50/℃ sCO2 0 6.0×10−4 0 6.0×10−4 0 6.0×10−4 Blank 475 447 562 542 55 60 Ni-foam 428 396 517 475 100 100 Co-NW 400 337 461 387 100 100 Co3O4@MnOx 321 299 371 334 100 100 表 2 松接触模式下,催化剂的碳烟燃烧活性
Table 2 Catalytic activities of catalysts for soot combustion in loose contact
Catalyst Soot/catalyst weight ratio Reaction gas Characteristic temperature (t50 or tm) Co3O4@MnOx (this work) 1/10 10% O2+6.0×10−4 NO 334 Co3O4-C[10] 1/10 5% O2+2.5×10−3 NO 421 K0.1Co[21] 1/9 8% O2+5.0×10−4 NO 365 5KCo-NW* [16] 1/10 5% O2+6.0×10−4 NO 324 Mn2O3-C[11] 1/10 5% O2+2.5×10−3 NO 435 3DOM Mn0.3Ce0.7Oδ[22] 1/10 5% O2+2.0×10−3 NO 356 Mn3O4-HNS[23] 1/10 5% O2+2.5×10−3 NO 408 K-OMS-2/SiO2-50[24] 1/10 10% O2+2.0×10−3 NO 328 K-Mn/3DOM La0.8Ce0.2FeO3[25] 1/9 5% O2+5.0×10−4 NO 377 3DOMM PdCo2O4/CZO[26] 1/9 5% O2+2.0×10−3 NO 367 0.6Co/Fe-NF* [27] 1/10 5% O2+3.0×10−4 NO 382 *: These targets are monolithic catalysts. 表 3 催化剂的元素含量和表面组成
Table 3 Elemental content and surface composition of the catalysts
Catalyst Total Co/Mn ratioa Surface Co/Mn
ratiobCo2+/
Co3+Oads/Olatt Co3O4-NW − − 0.5 0.66 Co3O4@MnOx 0.91 0.037 − 0.81 a: Obtained by ICP;b: Obtained by XPS. 表 4 催化剂的反应速率、活性氧数量(O*)和 TOF 值
Table 4 The reaction rate, active oxygen (O*) amounts, and TOF of the catalysts
Catalyst v/(mol·g−1·s−1) O* amount/(mol·g−1) TOF/s−1 Co-NW 4.7×10−7 5.6×10−5 8×10−4 Co3O4@MnOx 1.95×10−6 1.15×10−4 1.7×10−3 -
[1] 王磊, 曹春梅, 邢令利, 等. 锰铈相互作用对催化碳烟燃烧性能的影响[J]. 化学工业与工程,2018,35(4):32−37. doi: 10.13353/j.issn.1004.9533.20161063WANG Lei, CAO Chunmei, XING Lingli, et al. The effect of manganese/cerium interactions in catalytic soot combustion[J]. Chem Ind Eng,2018,35(4):32−37. doi: 10.13353/j.issn.1004.9533.20161063 [2] PENG C, YU D, ZHANG C, et al. Alkali/alkaline-earth metal-modified MnOx supported on three-dimensionally ordered macroporous-mesoporous TixSi1-xO2 catalysts: Preparation and catalytic performance for soot combustion[J]. J Environ Sci,2023,125:82−94. doi: 10.1016/j.jes.2021.10.029 [3] DHAL G C, MOHAN D, PRASAD R. Preparation and application of effective different catalysts for simultaneous control of diesel soot and NOx emissions: An overview[J]. Catal Sci Technol,2017,7(9):1803−1825. doi: 10.1039/C6CY02612E [4] REN W, DING T, YANG Y X, et al. Identifying oxygen activation/oxidation sites for efficient soot combustion over silver catalysts interacted with nanoflower-like hydrotalcite-derived CoAlO metal oxides[J]. ACS Catal,2019,9(9):8772−8784. doi: 10.1021/acscatal.9b01897 [5] YU Q, XIONG J, LI Z, et al. Optimal exposed crystal facets of α-Mn2O3 catalysts with enhancing catalytic performance for soot combustion[J]. Catal Today,2021,376:229−238. doi: 10.1016/j.cattod.2020.05.039 [6] PENG R, LI S, SUN X, et al. Size effect of Pt nanoparticles on the catalytic oxidation of toluene over Pt/CeO2 catalysts[J]. Appl Catal B: Environ,2018,220:462−470. doi: 10.1016/j.apcatb.2017.07.048 [7] LEE J H, JO D Y, CHOUNG J W, et al. Roles of noble metals (M=Ag, Au, Pd, Pt and Rh) on CeO2 in enhancing activity toward soot oxidation: Active oxygen species and DFT calculations[J]. J Haz Mater,2021,403:124085. doi: 10.1016/j.jhazmat.2020.124085 [8] RONG S P, ZHANG P Y, LIU F, et al. Engineering crystal facet of α-MnO2 nanowire for highly efficient catalytic oxidation of carcinogenic airborne formaldehyde[J]. ACS Catal,2018,8:3435−3446. doi: 10.1021/acscatal.8b00456 [9] LEGUTKO P, STELMACHOWSKI P, YU X H, et al. Catalytic soot combustion-general concepts and alkali promotion[J]. ACS Catal,2023,13:3395−3418. doi: 10.1021/acscatal.2c05994 [10] ZHAI G J, WANG J G, CHEN Z M, et al. Boosting soot combustion efficiency of Co3O4 nanocrystals via tailoring crystal facets[J]. Chem Eng J,2018,337:488−498. doi: 10.1016/j.cej.2017.12.141 [11] CHENG L, MEN Y, WANG J G, et al. Crystal facet-dependent reactivity of α-Mn2O3 microcrystalline catalyst for soot combustion[J]. Appl Catal B: Environ,2017,204:374−384. doi: 10.1016/j.apcatb.2016.11.041 [12] CAO C M, YANG H, XIAO J Y, et al. Catalytic diesel soot elimination over potassium promoted transition metal oxide (Co/Mn/Fe) nanosheets monolithic catalysts[J]. Fuel,2021,305(9):121446. [13] YU Y F, MENG M, DAI F F. The monolithic lawn-like CuO-based nanorods array used for diesel soot combustion under gravitational contact mode[J]. Nanoscale,2013,5(3):904−909. doi: 10.1039/C2NR33269H [14] ZHAO P, FENG N J, FANG F, et al. Surface acid etching for efficient anchoring of potassium on 3DOM La0.8Sr0.2MnO3 catalyst: An integration strategy for boosting soot and NOx simultaneous elimination[J]. J Haz Mater,2021,409:124916. [15] XING L L, YANG Y X, CAO C M, et al. Decorating CeO2 nanoparticles on Mn2O3 nanosheets to improve catalytic soot combustion[J]. ACS Sustainable Chem Eng,2018,6(12):16544−16554. doi: 10.1021/acssuschemeng.8b03645 [16] CAO C M, XING L L, YANG Y X, et al. Diesel soot elimination over potassium-promoted Co3O4 nanowires monolithic catalysts under gravitation contact mode[J]. Appl Catal B: Environ,2017,218:32−45. doi: 10.1016/j.apcatb.2017.06.035 [17] ZHAO Y, HU L F, ZHAO S Y, et al. Preparation of MnCo2O4@Ni(OH)2 core-shell flowers for asymmetric supercapacitor materials with ultrahigh specific capacitance[J]. Adv Funct Mater,2016,26(23):4085−4093. doi: 10.1002/adfm.201600494 [18] XU K B, ZOU R J, LI W Y, et al. Design and synthesis of 3D interconnected mesoporous NiCo2O4@CoxNi1-x(OH)2 core-shell nanosheet arrays with large areal capacitance and high rate performance for supercapacitors[J]. J Mater Chem A,2014,2(26):10090−10097. doi: 10.1039/c4ta01489h [19] KONG D Z, LUO J S, WANG Y L, et al. Three-dimensional Co3O4@MnO2 hierarchical nanoneedle arrays: Morphology control and electrochemical energy storage[J]. Adv Funct Mater,2014,24(24):3815−3826. doi: 10.1002/adfm.201304206 [20] ZHANG Z L, ZHANG Y X, WANG Z P, et al. Catalytic performance and mechanism of potassium-promoted Mg-Al hydrotalcite mixed oxides for soot combustion with O2[J]. J Catal,2010,271(1):12−21. doi: 10.1016/j.jcat.2010.01.022 [21] SHANG Z, SUN M, CHE X, et al. The existing states of potassium species in K-doped Co3O4 catalysts and their influence on the activities for NO and soot oxidation[J]. Catal Sci Technol,2017,7(20):4710−4719. doi: 10.1039/C7CY01444A [22] YU X H, LI J M, WEI Y C, et al. Three dimensionally ordered macroporous MnxCe1–xOδ and Pt/Mn0.5Ce0.5Oδ catalysts: Synthesis and catalytic performance for soot oxidation[J]. Ind Eng Chem Res,2014,53(23):9653−9664. doi: 10.1021/ie500666m [23] JI F, MEN Y, WANG J G, et al. Promoting diesel soot combustion efficiency by tailoring the shapes and crystal facets of nanoscale Mn3O4[J]. Appl Catal B: Environ,2018,242:227−237. [24] YU X H, ZHAO Z, WEI Y C, et al. Ordered micro/macro porous K-OMS-2/SiO2 nanocatalysts: Facile synthesis, low cost and high catalytic activity for diesel soot combustion[J]. Sci Rep,2017,7:43894. doi: 10.1038/srep43894 [25] FENG N J, CHEN C, MENG J, et al. K-Mn supported on three-dimensionally ordered macroporous La0.8Ce0.2FeO3 catalysts for the catalytic combustion of soot[J]. Appl Surf Sci,2017,399:114−122. doi: 10.1016/j.apsusc.2016.12.066 [26] XIONG J, WU Q Q, MEI X L, et al. Fabrication of spinel-type PdxCo3-xO4 binary active sites on 3D ordered meso-macroporous Ce-ZrO2 with enhanced activity for catalytic soot oxidation[J]. ACS Catal,2018,8(9):7915−7930. doi: 10.1021/acscatal.8b01924 [27] CAO C M, LI X G, ZHA Y Q, et al. Crossed ferric oxide nanosheets supported cobalt oxide on 3-dimensional macroporous Ni foam substrate used for diesel soot elimination under self-capture contact mode[J]. Nanoscale,2016,8(11):5857−5864. doi: 10.1039/C5NR05310B [28] ZHAO S, HU F Y, LI J H. Hierarchical core-shell Al2O3@Pd-CoAlO microspheres for low-temperature toluene combustion[J]. ACS Catal,2016,6:3433−3441. doi: 10.1021/acscatal.6b00144 [29] LIU Y, XU J, LI H R, et al. Rational design and in situ fabrication of MnO2@NiCo2O4 nanowire arrays on Ni foam as high-performance monolith de-NOx catalysts[J]. J Mater Chem A,2015,3:11543−11553. doi: 10.1039/C5TA01212K [30] WANG C L, WANG D D, YANG Y, et al. Enhanced CO oxidation on CeO2/Co3O4 nanojunctions derived from annealing of metal organic frameworks[J]. Nanoscale,2016,8(47):19761−19768. doi: 10.1039/C6NR07725K [31] JIANG M, ABUSHRENTA N, Wu X C, et al. Investigation for the synthesis of hierarchical Co3O4@MnO2 nanoarrays materials and their application for super-capacitor[J]. J Mater Sci: Mater Electron,2017,28:1281−1287. doi: 10.1007/s10854-016-5656-1 [32] FEDYNA M, LEGUTKO P, GRYBOS J, et al. Multiple doping of cryptomelane catalysts by Co, Cu, Ag and Ca for efficient soot oxidation and its effect on NO2 formation and SO2 resistance[J]. Fuel,2023,348:128553. doi: 10.1016/j.fuel.2023.128553 [33] DAI W Y, LI Z H, LI C C, et al. Revealing the effects of preparation methods over Ce-MnOx catalysts for soot combustion: Physicochemical properties and catalytic performance[J]. J Ind Eng Chem,2023,121:15−26. doi: 10.1016/j.jiec.2023.01.013 [34] MANE R, KIM H, HAN K, et al. Pivotal role of MnOx physicochemical structure in soot oxidation activity[J]. Fuel,2023,346:128287. doi: 10.1016/j.fuel.2023.128287 [35] TANG W X, XIAO W, Wang S B, et al. Boosting catalytic propane oxidation over PGM-free Co3O4 nanocrystal aggregates through chemical leaching: A comparative study with Pt and Pd based catalysts[J]. Appl Catal B: Environ,2018,226:585−595. doi: 10.1016/j.apcatb.2017.12.075 [36] ZHU Z Z, LU G Z, ZHANG Z G, et al. Highly active and stable Co3O4/ZSM-5 catalyst for propane oxidation: Effect of the preparation method[J]. ACS Catal,2013,3(6):1154−1164. doi: 10.1021/cs400068v [37] TANG W X, Wu X F, Li S D, et al. Porous Mn-Co mixed oxide nanorod as a novel catalyst with enhanced catalytic activity for removal of VOCs[J]. Catal Commun,2014,56:134−138. doi: 10.1016/j.catcom.2014.07.023 [38] ZHAO S, LI K Z, JIANG S, et al. Pd-Co based spinel oxides derived from Pd nanoparticles immobilized on layered double hydroxides for toluene combustion[J]. Appl Catal B: Environ,2016,181:236−248. doi: 10.1016/j.apcatb.2015.08.001 [39] ZHAO K, LI J M, WANG L Y, et al. Preparation of cordierite monolith catalysts with the coating of K-modified spinel MnCo2O4 oxide and their catalytic performances for soot combustion[J]. Catalysts,2022,12(3):295. doi: 10.3390/catal12030295 [40] GAO Q, RANJAN C, PAVLOVIC Z, et al. Enhancement of stability and activity of MnOx/Au electrocatalysts for oxygen evolution through adequate electrolyte composition[J]. ACS Catal,2015,5:7265−7275. doi: 10.1021/acscatal.5b01632 [41] HONG F, YUE B B, HIRAO N, et al. Significant improvement in Mn2O3 transition metal oxide electrical conductivity via high pressure[J]. Nature,2017,7:1−7. -





 下载:
下载: