Study on co/competitive adsorption mechanism of CO2/C3H6O on the surface of metal oxide-coupled pyrrole nitrogen biochar
-
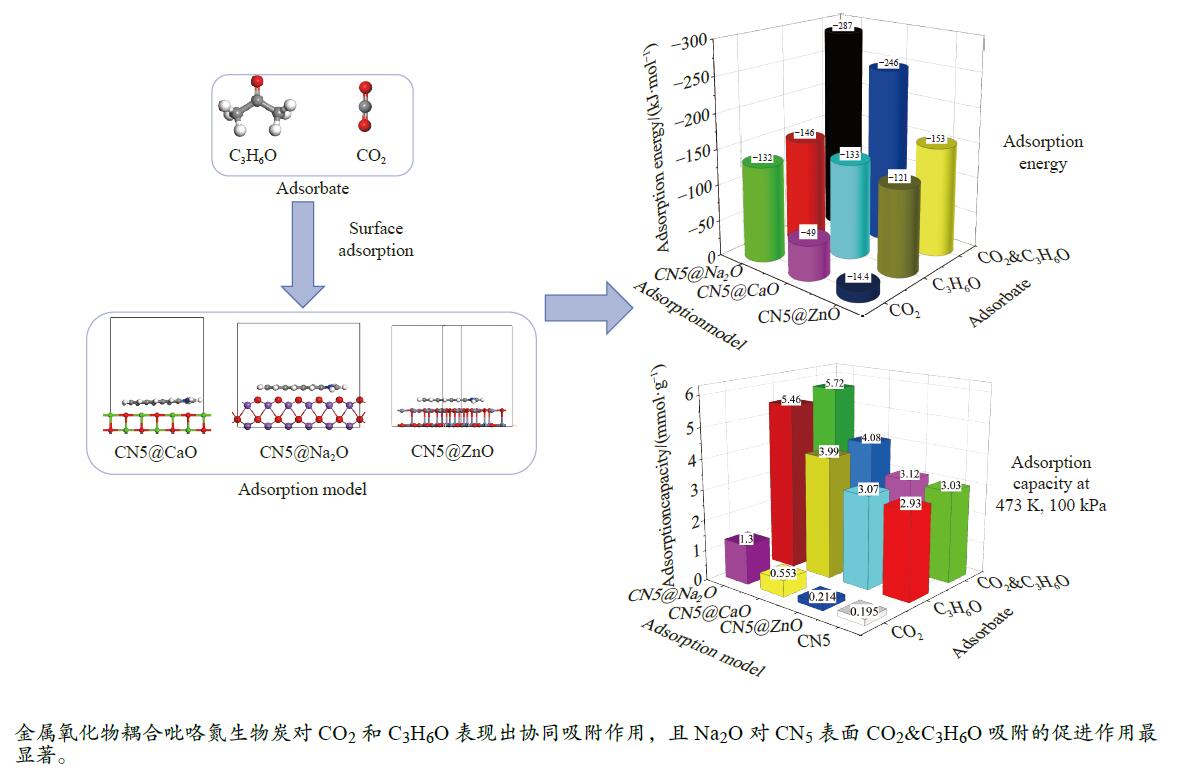
摘要: 本研究采用密度泛函理论,通过比较吸附量、吸附能以及态密度和电荷差分密度的分析,探究了不同金属氧化物耦合吡咯氮生物炭(CN5@MOx,MOx=ZnO、CaO、Na2O)表面CO2与C3H6O(CO2&C3H6O)的吸附机理。首先从CO2/C3H6O单组分方面计算了其在CN5@MOx表面吸附量和吸附能,计算结果表明,在333 K、100 kPa时CN5@Na2O表面对CO2/C3H6O单组分吸附量分别为3.65、15.34 mmol/g,吸附能分别为−145.86、−132.47 kJ/mol,均高于CO2/C3H6O单组分在CN5@CaO及CN5@ZnO表面吸附。得出Na2O掺杂吡咯氮生物炭对CO2/C3H6O单组分吸附效果最优。进一步研究了CO2&C3H6O在CN5@MOx表面共/竞吸附及机理。计算结果表明,CO2&C3H6O在CN5@Na2O、CN5@CaO、CN5@ZnO表面吸附存在临界温度(分别为333、353、393 K),超过临界温度以后CO2&C3H6O共存体系在CN5@MOx表面吸附量较CO2/C3H6O单组分有所提高。CO2&C3H6O在CN5@Na2O、CN5@CaO、CN5@ZnO表面吸附能分别比CO2或C3H6O单组分吸附时至少高141.59、112.77、31.75 kJ/mol,CN5@MOx表面对CO2和C3H6O的吸附表现为协同促进作用,且CN5@Na2O对CO2&C3H6O共同吸附效果最佳。采用电荷差分密度和态密度分析CO2&C3H6O在CN5@MOx表面协同吸附作用机理,得出CO2的吸附作用力是通过C3H6O与CO2的间接相互作用产生的,Na2O中Na与C3H6O电子云重叠,发生电荷转移,增强了两者间相互作用力,CN5@Na2O表面C3H6O与CN5在p轨道主要共振峰结合能较CN5@ZnO低了3.43 eV,使得C3H6O在CN5@Na2O表面吸附最稳定。Abstract: Biomass has a wide range of sources and is porous, and it is a raw material for the preparation of adsorbents with high application value. The adsorption effect of metal oxide-modified biochar on CO2 and acetone can be significantly improved, but the together/competitive relationship and adsorption mechanism of metal oxide-modified biomass-based adsorbent for simultaneous adsorption of multiple components are not clear. Based on this, the co-adsorption relationship between CO2 and C3H6O on the surface of metal oxide-doped nitrogen-rich biochar was carried out, which is of great significance for the multi-component synergistic adsorption and removal of biomass-based adsorbents. In this study, the adsorption mechanism of CO2 and C3H6O (CO2&C3H6O) on the surface of different metal oxide-coupled pyrrole biochar (CN5@MOx, MOx=ZnO, CaO, Na2O) was explored by comparing the adsorption capacity, adsorption energy, state density and charge differential density analysis. Firstly, the adsorption capacity and adsorption energy of CO2/C3H6O single component were calculated from the CN5@MOx surface, and the calculation results show that at 333 K and 100 kPa, the adsorption capacity of CO2/C3H6O on the surface of the CN5@Na2O is 3.65 and 15.34 mmol/g, and the adsorption energy is −145.86 and −132.47 kJ/mol, respectively, which are higher than that of CO2/C3H6O on the surface of CN5@CaO and CN5@ZnO. It was concluded that Na2O-doped pyrrole-nitrogen biochar had the best adsorption effect on CO2/C3H6O one-component adsorption. The common/competitive adsorption of CO2&C3H6O on the CN5@MOx surface was further studied. The calculation results show that there are critical temperatures for the adsorption of CO2&C3H6O on the surface of CN5@Na2O, CN5@CaO and CN5@ZnO (333, 353 and 393 K, respectively), and the adsorption capacity of CO2&C3H6O coexistence system on the CN5@MOx surface is higher than that of CO2/C3H6O single component after the critical temperature. The adsorption energy of CO2&C3H6O on the surface of CN5@Na2O, CN5@CaO and CN5@ZnO was at least 141.59, 112.77 and 31.75 kJ/mol higher than that of CO2 or C3H6O single-component adsorption, respectively, and the adsorption of CO2 and C3H6O on the CN5@MOx surface showed a synergistic promotion effect, and the CN5@Na2O had the best co-adsorption effect on CO2&C3H6O. Finally, the electron density difference and density of state were used to analyze the mechanism of synergistic adsorption of CO2&C3H6O on the CN5@MOx surface, and it was concluded that the adsorption force of CO2 was generated by the indirect interaction between C3H6O and CO2, and the electron cloud of Na and C3H6O in Na2O overlapped, and charge transfer occurred, which enhanced the interaction force between the two. The binding energy of the main formant of C3H6O and CN5 in the p orbital on the CN5@Na2O surface is 3.43 eV lower than that of CN5@ZnO, making the most stable adsorption of C3H6O on the CN5@Na2O surface.
-
Key words:
- pyrrole functional biochar /
- metal oxides /
- CO2 /
- C3H6O
-
表 1 CN5、CN5@MOx构型比表面积和孔容
Table 1 CN5, CN5@MOx configuration specific surface area (SBET) and pore volume (vTotal)
Sample SBET/(m2·g−1) vTotal/(cm3·g−1) CN5 1803.37 0.70 CN5@ZnO 1912.31 0.83 CN5@CaO 1576.90 0.69 CN5@Na2O 1678.84 0.68 表 2 CN5与ZnO不同耦合位点优化平衡能量
Table 2 CN5 and ZnO have different coupling sites to optimize the balance energy
Coupling site Balance energy/eV Top −74207.0723 Bridge −74207.1353 Hollow −74207.2676 表 3 CN5与ZnO不同耦合位点对CO2吸附能
Table 3 CN5 and ZnO have different coupling sites for CO2 adsorption energy
Coupling site Eads/eV Eads/(kJ·mol−1) Top −1.23 −119.05 Bridge −1.20 −116.19 Hollow −1.15 −111.05 表 4 C3H6O的吸附能
Table 4 Calculation results of adsorption energy of C3H6O
Structure Eads/eV Eads/(kJ·mol−1) CN5@ZnO −0.14944 −14.42 CN5@CaO −0.50754 −48.97 CN5@Na2O −1.37294 −132.47 表 5 CO2/C3H6O吸附能
Table 5 Calculation results of CO2/C3H6O adsorption energy
Structure Eads/(kJ·mol−1) CO2 C3H6O CO2&C3H6O CO2//C3H6O C3H6O⊥CO2 CO2⊥C3H6O CN5@ZnO −120.88 −14.42 −138.63 −140.21 −152.63 CN5@CaO −133.04 −48.97 −161.99 −245.81 −168.58 CN5@Na2O −145.86 −132.47 −242.82 −285.86 −287.45 -
[1] BORHAN A, YUSUP S, LIM J. W, et al. Characterization and modelling studies of activated carbon produced from rubber-seed shell using KOH for CO2 adsorption[J]. Processes,2019,7(11):855. doi: 10.3390/pr7110855 [2] GE C, LIAN D, CUI S, et al. Highly selective CO2 capture on waste polyurethane foam-based activated carbon[J]. Processes,2019,7(9):592. doi: 10.3390/pr7090592 [3] BIRCH E L. A review of “climate change 2014: Impacts, adaptation, and vulnerability” and “climate change 2014: mitigation of climate change[J]. J Am Plan Assoc,2014,80:184−185. doi: 10.1080/01944363.2014.954464 [4] KOU J, SUN L. Nitrogen-doped porous carbons derived from carbonization of a nitrogen-containing polymer: Efficient adsorbents for selective CO2 capture[J]. Ind Eng Chem Res,2016,55(41):10916−10925. [5] JIANG Q, RENTSCHLER J, SETHIA G. Synthesis of T-type zeolite nanoparticles for the separation of CO2/N2 and CO2/CH4 by adsorption process[J]. Chem Eng J,2013,230(16):380−388. [6] SUMIDA K, ROGOW D, MASON J, et al. Carbon dioxide capture in metal–organic frameworks[J]. Chem Rev,2012,112(2):724−781. doi: 10.1021/cr2003272 [7] SUN L, KANG Y, SHI Y. Highly selective capture of the greenhouse gas CO2 in polymers[J]. ACS Sustainable Chem Eng,2015,3(12):3077−3085. doi: 10.1021/acssuschemeng.5b00544 [8] XIN H, RADOSZ M, CYCHOSZ K, et al. CO2-filling capacity and selectivity of carbon nanopores: Synthesis, texture, and pore-size distribution from quenched-solid density functional theory(QSDFT)[J]. Environ Sci Technol,2011,45(16):7068−7074. doi: 10.1021/es200782s [9] JIMENEZ V, RAMIREZ-LUCAS A, DÍAZ J-A, et al. CO2 capture in different carbon materials[J]. Environ Sci Technol,2012,46(13):7407−7414. doi: 10.1021/es2046553 [10] 卢立栋, 王浩, 郑娟等. 关中地区炼焦行业VOCs排放特征及潜势影响研究[J]. 环境科技,2021,34(4):11−16. doi: 10.3969/j.issn.1674-4829.2021.04.004LU Lidong, WANG Hao, ZHENG Juan, et al. Study on VOCs emission characteristics and potential impact of coking industry in Guanzhong area[J]. Environ Sci Technol,2021,34(4):11−16. doi: 10.3969/j.issn.1674-4829.2021.04.004 [11] XIONG Z, JING W, YANG H, et al. Preparation of nitrogen-doped microporous modified biochar by high temperature CO2-NH3 treatment for CO2 adsorption: Effects of temperature[J]. RSC Adv,2016,6:98157−98166. doi: 10.1039/C6RA23748G [12] GONZ´ALEZ A S, PLAZA M G, RUBIERA F, et al. Sustainable biomass-based carbon adsorbents for post-combustion CO2 capture[J]. Chem Eng J,2013,230:456−465. doi: 10.1016/j.cej.2013.06.118 [13] PALLAR´ES J, GONZ´ALEZ-CENCERRADO A, ARAUZO I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam[J]. Biomass Bioenergy,2018,115:64−73. doi: 10.1016/j.biombioe.2018.04.015 [14] LIU W J, JIANG H, TIAN K, et al. Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture[J]. Environ Sci Technol,2013,47:9397−9403. doi: 10.1021/es401286p [15] CHATTERJEE R, SAJJADI B, MATTERN D, et al. Ultrasound cavitation intensified amine functionalization: A feasible strategy for enhancing CO2 capture capacity of biochar[J]. Fuel,2018,225:287−298. doi: 10.1016/j.fuel.2018.03.145 [16] CHATTERJEE R, SAJJADI B, CHEN W, et al. Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption[J]. Front Energy Res,2020,8:1−18. doi: 10.3389/fenrg.2020.00001 [17] XING W, LIU C, ZHOU Z, et al. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction[J]. Energy Environ Sci,2012,5(6):7323−7327. doi: 10.1039/c2ee21653a [18] MA X, CAO M, HU C. Bifunctional HNO3 catalytic synthesis of N-doped porous carbons for CO2 capture[J]. J Mater Chem A,2012,1(3):913−918. [19] QI J, LI Y, WEI G, et al. Nitrogen doped porous hollow carbon spheres for enhanced benzene removal[J]. Sep Purif Technol,2017,188:112−118. doi: 10.1016/j.seppur.2017.07.021 [20] KIM K-J, KANG C-S, YOU Y-J, et al. Adsorption-desorption characteristics of VOCs over impregnated activated carbons[J]. Catal Today,2006,111:223−228. doi: 10.1016/j.cattod.2005.10.030 [21] ZHOU K, MA W, ZENG Z, et al. Waste biomass-derived oxygen and nitrogen co-doped porous carbon/MgO composites as superior acetone adsorbent: Experimental and DFT study on the adsorption behavior[J]. Chem Eng J,2020,387:124173. doi: 10.1016/j.cej.2020.124173 [22] YUE L, XIA Q, WANG L. CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell[J]. J Colloid Interf Sci,2018,511:259−267. doi: 10.1016/j.jcis.2017.09.040 [23] SETHIA G, SAYARI A. Comprehensive study of ultra-microporous nitrogen-doped activated carbon for CO2 capture[J]. Carbon,2015,93:68−80. doi: 10.1016/j.carbon.2015.05.017 [24] 陈伟. 生物质富氮热解过程中氮的迁移转化及含氮目标产物调控研究[D]. 武汉: 华中科技大学, 2018.CHEN WEI. Research on nitrogen migration and transformation and regulation of nitrogen-containing target products during nitrogen-rich pyrolysis of biomass[D]. Wuhan: Huazhong University of Science and Technology, 2018. [25] SANCHEZ Á, SUAREZ-GARCIA F, MARTINEZ-ALONSO A, et al. Influence of porous texture and surface chemistry on the CO adsorption capacity of porous carbons: Acidic and basic site interactions.[J]. ACS Appl Mater Inter,2014,6(23):21237−21247. doi: 10.1021/am506176e [26] WANG Y, HU X, HAO J, et al. Nitrogen and oxygen codoped porous carbon with superior CO2 adsorption performance: A combined experimental and DFT calculation study[J]. Ind Eng Chem Res,2019,58(29):13390−13400. doi: 10.1021/acs.iecr.9b01454 [27] LI L, MA X, CHEN R, et al. Nitrogen-containing functional groups-facilitated acetone adsorption by ZIF-8-derived porous carbon[J]. Materials,2018,11(1):159. doi: 10.3390/ma11010159 [28] XU X, GUO Y, SHI R, et al. Natural honeycomb-like structure cork carbon with hierarchical micro-mesopores and N-containing functional groups for VOCs adsorption[J]. Appl Surf Sci,2021,565:150550. doi: 10.1016/j.apsusc.2021.150550 [29] SUN H, WU C, SHEN B, ZHANG X, ZHANG Y, HUANG J. Progress in the development and application of CaO-based adsorbents for CO2 capture—a review, Mater[J]. Today Sustainable,2018,(1/2):1−27. [30] CHEN Z, SONG H, PORTILLO M, et al. Long-term calcination/carbonation cycling and thermal pretreatment for CO2 capture by limestone and dolomite[J]. Energy Fuels,2009,23(3):1437−1444. doi: 10.1021/ef800779k [31] ROUZITALAB Z, MOHAMMADY MAKLAVANY D, RASHIDI A, et al. Synthesis of N-doped nanoporous carbon from walnut shell for enhancing CO2 adsorption capacity and separation[J]. J Environ Chem Eng,2018,6:6653−6663. doi: 10.1016/j.jece.2018.10.035 [32] LAHIJANI P, MOHAMMADI M, MOHAMED A R. Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition[J]. J CO2 Util,2018,26:281−293. doi: 10.1016/j.jcou.2018.05.018 [33] ZUBBRI N A, MOHAMED A R, KAMIUCHI N, et al. Enhancement of CO2 adsorption on biochar sorbent modified by metal incorporation[J]. Environ Sci Pollut Res,2020,27:11809−11829. doi: 10.1007/s11356-020-07734-3 [34] ZHOU K, LI D, ZHOU C. Metal heteroatom (Mg, Cu and Co) and porous carbon co-doped MIL-101 composites with superior acetone capture capacity[J]. Chem Eng J,2022,430:132656. doi: 10.1016/j.cej.2021.132656 [35] FANG M, WU K, MA X. Alkali metals modified porous carbon for enhanced methanol and acetone selective adsorption: A theoretical study[J]. Appl Surf Sci,2022,602:154271. doi: 10.1016/j.apsusc.2022.154271 [36] ZHOU S, LI S, GUO C, et al. Edge-functionalized nanoporous carbons for high adsorption capacity and selectivity of CO2 over N2[J]. Appl Surf Sci,2017,410:259−266. doi: 10.1016/j.apsusc.2017.03.136 [37] SHAFAWIi A N, MOHAMED A R, LAHIJANI P, et al. Recent advances in developing engineered biochar for CO2 capture: An insight into the biochar modification approaches[J]. J Environ Chem Eng,2021,9(6):106869. doi: 10.1016/j.jece.2021.106869 [38] PERRY S T, HAMBLY E M, FLETCHER T H, et al. Solid-state 13C NMR characterization of matched tars and chars from rapid coal devolatilization[J]. Proc Combust Inst,2000,28(2):2313−2319. doi: 10.1016/S0082-0784(00)80642-6 [39] ZHANG X, XIE M, WU H, et al. DFT study of the effect of Ca on NO heterogeneous reduction by char[J]. Fuel,2020,265:116995. doi: 10.1016/j.fuel.2019.116995 [40] ZHANG X, LV X, WU H, et al. Microscopic mechanism for effect of sodium on NO heterogeneous reduction by char[J]. J Fuel Chem Technol,2020,48(6):663−673. doi: 10.1016/S1872-5813(20)30050-5 [41] ZHANG H, JIANG X, LIU J, et al. Application of density functional theory to the nitric oxide heterogeneous reduction mechanism in the presence of hydroxyl and carbonyl groups[J]. Energy Convers Manage,2014,83:167−176. doi: 10.1016/j.enconman.2014.03.067 [42] FENG K, HU Y, CAO T. Mechanism of fuel gas denitration on the KOH-activated biochar surface[J]. J Phys Chem A,2022,126(2):296−305. doi: 10.1021/acs.jpca.1c09518 [43] YU X, LIU S, LIN G, et al. Insight into the significant roles of microstructures and functional groups on carbonaceous surfaces for acetone adsorption[J]. RSC Adv,2018,8(38):21541−21550. doi: 10.1039/C8RA03099E [44] 汪辉春, 顾明言, 陈萍, 等. 金属氧化物耦合含吡咯氮生物质炭吸附CO2的机理研究[J]. 燃料化学学报(中英文),2023,51(8):1182−1192.WANG Huichun, GU Mingyan, CHEN Ping, et al. Study on the mechanism of CO2 adsorption by metal oxide coupled with pyrrole nitrogen biochar[J]. J Fuel Chem Technol,2023,51(8):1182−1192. -





 下载:
下载: