Preparation of carbon nitride nanosheets with wide spectral response range and photocatalytic hydrogen production properties
-
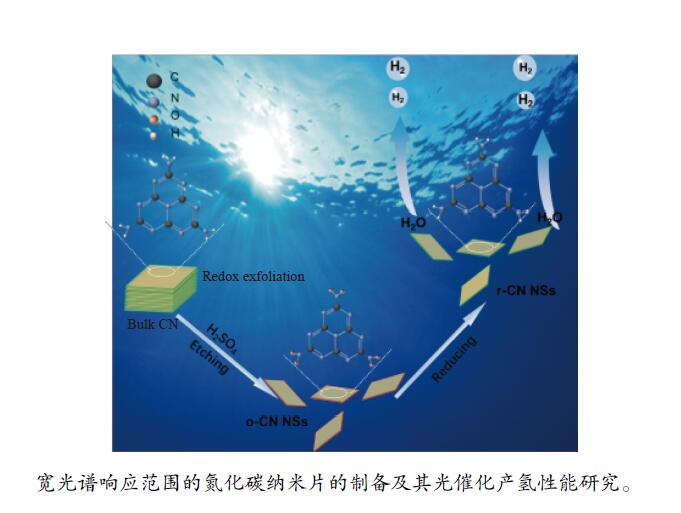
摘要: 以块体氮化碳 (CN) 为前驱物,采用氧化剥离制备氧化型氮化碳纳米片 (o-CN NSs),将o-CN NSs还原制得了还原型氮化碳纳米片 (r-CN NSs)。o-CN NSs和r-CN NSs厚度均约2 nm,且都保留了纯CN的庚嗪环骨架结构;相比于o-CN NSs,r-CN NSs具有更小的禁带宽度 (2.62 eV)、更宽的光响应范围 (485 nm) 和更高的产氢速率((1700 μmol/(g·h));r-CN NSs的光催化产氢速率是CN的8.5倍、o-CN NSs的2.1倍。经过20 h的循环测试,r-CN NSs的光催化产氢速率没有衰减,具备良好的光催化稳定性。实验和理论分析表明,r-CN NSs是边缘基团为氨基的纳米片结构,氨基的引入改善了纳米片的结晶性,提高了电子和空穴的分离效率、拓宽了纳米片的光响应范围,从而导致光催化性能增强。Abstract: Oxidized carbon nitride nanosheets (o-CN NSs) was prepared by oxidative stripping with bulk carbon nitride (CN) as the precursor, and then reduced carbon nitride nanosheet (r-CN NSs) was prepared via reduced o-CN NSs. The thickness of o-CN NSs and r-CN NSs are both about 2 nm and retain the heptazine ring skeleton structure of pure CN. Compared with o-CN NSs, r-CN NSs has a smaller band gap (2.62 eV), wider photoresponse range (485 nm) and higher H2 evolution rates (1700 μmol/(g·h)). The H2 evolution rates of r-CN NSs is 8.5 times of CN and 2.1 times of o-CN NSs. After a 20 h cycle test, the photocatalytic hydrogen production efficiency of r-CN NSs has no attenuation, has well photocatalytic stability. Experimental and theoretical analyses reveal that r-CN NSs is nanosheet structure with amino group at the edge. The introduction of amino group improves the crystallinity of nanosheets, improves the separation efficiency of electrons and holes, broadens the photoresponse range of nanosheets, thus resulting in the enhanced photocatalytic performance.
-
Key words:
- carbon nitride /
- nanosheet structure /
- photocatalysis /
- photoresponse range /
- hydrogen evolution
-
图 5 (a) 光催化产氢时间曲线图;(b) 光催化产氢速率图;(c) r-CN NSs光催化产氢稳定性测试;(d) r-CN NSs的光催化产氢在有光和无光环境中测试
Figure 5 (a) Time course of photocatalytic H2 evolution; (b) Photocatalytic H2 evolution rates of obtained samples; (c) Photostability test of r-CN NSs for photocatalytic H2 evolution; (d) Photocatalytic H2 evolution of r-CN NSs was tested in light and without light
表 1 单一氮化碳纳米片制备方法和性能对比
Table 1 Comparison of preparation methods and properties of naked carbon nitride nanosheets
No. Exfloiated strategies and
preparation methodsThickness/
nmLight source/nm Band gap/eV H2 evolution
rates/(μmol·g–1·h–1)Ref. Year
published1. Strong acid oxidation etching, then reduction 2 485 2.62 1700 this work 2024 2. Physical grinding+Thermal oxidation etching 6 <460 2.90 1254.75 [15] 2022 3. Thermal oxidation etching − 430 2.70 495 [16] 2021 4. Chemical exfoliation+Thermal oxidation etching 0.8−1.4 435 3.10 115.5 [18] 2019 5. High-temperature calcination in ammonia atmosphere − 430 2.51 <10 [21] 2022 6. Ultrasound-assisted method − 450 2.86 20 [31] 2023 7. Thermal oxidation etching <0.8 440 2.67 241.2 [32] 2021 -
[1] SHIH C F, ZHANG T, LI J H, et al. Powering the future with liquid sunshine[J]. Joule,2018,2(10):1925−1949. doi: 10.1016/j.joule.2018.08.016 [2] DALLE K E, WARNAN J, LEUNG J J, et al. Electro-and solar-driven fuel synthesis with first row transition metal complexes[J]. Chem Rev,2019,119(4):2752−2857. doi: 10.1021/acs.chemrev.8b00392 [3] WEI J D, LUO D, SHI M M, et al. Ultrathin carbon nitride nanosheets exfoliated and In situ modified with a nickel bis (Chelate) complex for boosting photocatalytic performances[J]. Inorg Chem,2023,62(28):10973−10983. doi: 10.1021/acs.inorgchem.3c00952 [4] VU N N, KALIAGUINE S, DO T O. Critical aspects and recent advances in structural engineering of photocatalysts for sunlight -driven photocatalytic reduction of CO2 into fuels[J]. Adv Funct Mater,2019,29(31):1901825. doi: 10.1002/adfm.201901825 [5] SHARMA R, ALMASI M, NEHRA S P, et al. Photocatalytic hydrogen production using graphitic carbon nitride (GCN): A precise review[J]. Renewable Sustainable Energy Rev,2022,168:112776. doi: 10.1016/j.rser.2022.112776 [6] LAM S S, NUYEN V H, DINH M T N, et al. Mainstream avenues for boosting graphitic carbon nitride efficiency: Towards enhanced solar light-driven photocatalytic hydrogen production and environmental remediation[J]. J Mater Chema,2020,8(21):10571−10603. doi: 10.1039/D0TA02582H [7] YUAN Y J, CHEN D Q, XIONG M, et al. Bandgap engineering of (AgIn)xZn2 (1–x) S2 quantum dot photosensitizers for photocatalytic H2 generation[J]. Appl Catal B: Environ,2017,204:58−66. doi: 10.1016/j.apcatb.2016.11.024 [8] ZHANG P, GUAN B Y, YU L, et al. Facile synthesis of multi-shelled ZnS-CdS cages with enhanced photoelectron chemical performance for solar energy conversion[J]. Chem,2018,4(1):162−173. doi: 10.1016/j.chempr.2017.10.018 [9] YANG B, LI X L, ZHANG Q, et al. Ultrathin porous carbon nitride nanosheets with well-tuned band structures via carbon vacancies and oxygen doping for significantly boosting H2 production[J]. Appl Catal B: Environ,2022,314:121521. doi: 10.1016/j.apcatb.2022.121521 [10] 孙有为, 王曦, 周峰, 等. CoNi 双金属改性石墨相氮化碳的制备及光催化性能的研究[J]. 燃料化学学报,2022,50(11):1449−1457.SUN Youwei, WANG Xi, ZHOU Feng, et al. CoNi bimetallic co-catalyst decorated graphitic-phase carbon nitride preparation and photocatalytic properties[J]. J Fuel Chem Technol,2022,50(11):1449−1457. [11] JIANG W S, ZHAO Y J, ZONG X P, et al. Photocatalyst for high-performance H2 production: Ga-doped polymeric carbon nitride[J]. Angew Chem Int Ed,2021,60(11):6124−6129. doi: 10.1002/anie.202015779 [12] WANG Y Y, ZHANG X, DING X, et al. Enhanced thermal conductivity of carbon nitride-doped graphene/polyimide composite film via a “deciduous-like” strategy[J]. Compost Sci Technol,2021,205:108693. doi: 10.1016/j.compscitech.2021.108693 [13] WU Y, XIONG P, WU J, et al. Band engineering and morphology control of oxygen-incorporated graphitic carbon nitride porous nanosheets for highly efficient photocatalytic hydrogen evolution[J]. Nano-micro Let,2021,13:47−59. doi: 10.1007/s40820-020-00572-5 [14] YUAN Y J, SHEN Z K, WU S T, et al. Liquid exfoliation of g-C3N4 nanosheets to construct 2D-2D MoS2/g-C3N4 photocatalyst for enhanced photocatalytic H2 production activity[J]. Appl Catal B: Environ,2019,246:120−128. doi: 10.1016/j.apcatb.2019.01.043 [15] CHEN L, LIANG X, WANG H X, et al. Ultra-thin carbon nitride nanosheets for efficient photocatalytic hydrogen evolution[J]. Chem Eng J,2022,442:136115. doi: 10.1016/j.cej.2022.136115 [16] MAHVELATI-SHAMSABADI T, FATTAHIMOGHADDAM H, LEE B K, et al. Caesium sites coordinated in Boron-doped porous and wrinkled graphitic carbon nitride nanosheets for efficient charge carrier separation and transfer: Photocatalytic H2 and H2O2 production[J]. Chem Eng J,2021,423:130067. doi: 10.1016/j.cej.2021.130067 [17] REN Y M, YU C M, CHEN Z H, et al. Two-dimensional polymer nanosheets for efficient energy storage and conversion[J]. Nano Res,2021,14:2023−2036. doi: 10.1007/s12274-020-2976-5 [18] GAO X C, FENG J, SU D W, et al. In-situ exfoliation of porous carbon nitride nanosheets for enhanced hydrogen evolution[J]. Nano Energy,2019,59:598−609. doi: 10.1016/j.nanoen.2019.03.016 [19] ZHOU X B, LI Y F, XING Y, et al. Effects of the preparation method of Pt/g-C3N4 photocatalysts on their efficiency for visible-light hydrogen production[J]. Dalton Trans,2019,48:15068−15073. doi: 10.1039/C9DT02938A [20] MALIK R, TOMER V K. State-of-the-art review of morphological advancements in graphitic carbon nitride (g-CN) for sustainable hydrogen production[J]. Renewable Sustainable Energy Rev,2021,135:1−14. [21] LIN Z, ZHANG Z Q, WANG Y Q, et al. Anchoring single nickel atoms on carbon-vacant carbon nitride nanosheets for efficient photocatalytic hydrogen evolution[J]. Chem Res Chin Univ,2022,38:1243−1250. doi: 10.1007/s40242-022-2194-7 [22] OU H H, YANG P J, LIN L H, et al. Carbon nitride aerogels for the photoredox conversion of water[J]. Angew Chem Int Ed,2017,129(36):10905−10910. [23] YANG H, ZHOU Q, FANG Z Z, et al. Carbon nitride of five-membered rings with low optical bandgap for photoelectrochemical biosensing[J]. Chem,2021,7(10):2708−2721. doi: 10.1016/j.chempr.2021.06.010 [24] NIU P, ZHANG L, LIU G, et al. Graphene-like carbon nitride nanosheets for improved photocatalytic activities[J]. Adv Funct Mater,2012,22(22):4763−4770. doi: 10.1002/adfm.201200922 [25] LOTSCH B V, DOBLINGER M, SEHNERT J, et al. Unmasking melon by a complementary approach employing electron diffraction, solid-state NMR spectroscopy, and theoretical calculations-structural characterization of a carbon nitride polymer[J]. Chem-Eur J,2007,13(17):4969−4980. doi: 10.1002/chem.200601759 [26] REDDY N R, BHARGAV U, KUMARI M M, et al. Review on the interface engineering in the carbonaceous titania for the improved photocatalytic hydrogen production[J]. Int J Hydrogen Energy,2020,45(13):7584−7615. doi: 10.1016/j.ijhydene.2019.09.041 [27] VOIRY D, YANG J, KUPFERBERG J, et al. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science, 2016, 353(6306): 1413-1416. [28] LI D, MVLLER M B, GILJE S, et al. Processable aqueous dispersions of graphene nanosheets[J]. Nat Nanotechnol,2008,3(2):101−105. doi: 10.1038/nnano.2007.451 [29] STANKOVICH S, DUKIN D A, PINER R D, et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide[J]. Carbon,2007,45(7):1558−1565. doi: 10.1016/j.carbon.2007.02.034 [30] RAHMAN M Z, KIBRIA M G, MULLINS C B. Metal-free photocatalysts for hydrogen evolution[J]. Chem Soc Rev,2020,49(6):1887−1931. doi: 10.1039/C9CS00313D [31] WEI J D, ZHAO R Q, LUO D, et al. Atomically precise Ni6 (SC2H4Ph)12 nanoclusters on graphitic carbon nitride nanosheets for boosting photocatalytic hydrogen evolution[J]. J Colloid Interf Sci,2023,631:212−221. doi: 10.1016/j.jcis.2022.11.010 [32] LUO L, GONG Z, MA J, et al. Ultrathin sulfur-doped holey carbon nitride nanosheets with superior photocatalytic hydrogen production from water[J]. Appl Catal B: Environ,2021,284:119742. doi: 10.1016/j.apcatb.2020.119742 -





 下载:
下载: