α-MnO2 as an advanced bifunctional ORR/IOR electrocatalyst for Zn-air battery
-
摘要: 析氧反应(oxygen evolution reaction, OER)和氧还原反应(oxygen reduction reaction, ORR)是可充电锌空电池(rechargeable Zn-air batteries, RZABs)重要的两个反应。其中,析氧反应具有较高的热力学平衡电位和复杂的反应路径,实际应用中需要高的充电电压驱动其发生,这将带来一系列问题并且限制了RZABs的商业化应用。基于此,本研究构造α-MnO2并作为ORR/IOR双功能催化剂。在碱性体系中引入反应改性剂KI,α-MnO2对碘离子氧化反应(iodide oxidation reaction, IOR)具有更低的阳极氧化电位和更快的催化动力学。当1.0 mol/L KOH电解液中添加0.5 mol/L KI时,相比于OER(1.709 V @10 mA/cm2),α-MnO2在IOR过程中电流密度达到10 mA/cm2时阳极电位减小了398 mV(1.311 V vs. RHE),且表现出低至57.5 mV/dec塔菲尔斜率。相对于与Pt/C,在含有KI的KOH电解液中,α-MnO2表现出与Pt/C相媲美的ORR活性。此外,以α-MnO2为空气电极组装成RZAB后,该电池也表现出了优异的充电活性和良好的循环寿命,在5 mA/cm2电流密度下,充放电电压间隙由0.97 V缩减为0.61 V,能量转换效率由54.9%提升至66.2%。Abstract: Oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) are important reactions for rechargeable Zinc-air batteries (RZABs). Unfortunatly, OER holds a high thermodynamic equilibrium potential and complex reaction path, which require an large votage to derive this reaction and greatly hinder its commercial application. Herein, α-MnO2 was successfully achieved and as the bifunctional ORR/iodide oxidation reaction (IOR) electrocatalyst. In alkline media, α-MnO2 exhibits fast kinetics and low oxidation potential for IOR. Expectedly, α-MnO2 exhibits remarkable IOR activity in 1.0 mol/L KOH with 0.5 mol/L KI. Compared with potential at 10 mA/cm2 for OER (1.709 V vs. RHE), the potential at 10 mA/cm2 reduce 398 mV (1.311 V vs. RHE) for α-MnO2 during IOR process. α-MnO2 also provides small Tafel slope of 57.5 mV/dec. Additionly, α-MnO2 represents outsanding ORR performances with respect to Pt/C. As an air electrode for RZAB, the fabricated RZAB delivers excellent performances. To be specific, at 5 mA/cm2, the voltage gap between charging and discharging reduces from 0.97 V to 0.61 V, energy efficiency increses from 54.9% to 66.2%. This work provide an unique strategy to construct bifunction ORR/IOR electrocatalysts and promote the commercialization of RZABs.
-
图 3 (a)α-MnO2、RuO2和Pt/C在1.0 mol/L KOH中的OER曲线,(b)α-MnO2在不同浓度KI的电解液中的LSV曲线,(c)α-MnO2、RuO2和Pt/C在1.0 mol/L KOH+0.5 mol/L KI中的LSV曲线和(d)相应的塔菲尔斜率
Figure 3 (a) The LSV curves of α-MnO2, RuO2 and Pt/C in 1.0 mol/L KOH, (b) the LSV curves of α-MnO2 in KOH containing different concentration of KI, (c) the LSV curves of α-MnO2, RuO2 and Pt/C in 1.0 mol/L KOH containing 0.5 mol/L KI and (d) corresponding Tafel slopes
图 4 (a)Pt/C在含有不同浓度的KI的1.0 mol/L KOH电解液中的LSV曲线,(b)α-MnO2和Pt/C在含有不同浓度的KI的1.0 mol/L KOH电解液中达到10 mA/cm2电流密度时的电位柱状图和(c)α-MnO2的V-t曲线
Figure 4 (a) LSV curves of Pt/C in KOH with different concentrations of KI, (b) potentials of α-MnO2 and Pt/C at 10 mA/cm2 in KOH with different concentrations of KI and (c) V-t curve of α-MnO2
图 5 α-MnO2在不同含量KI的0.1 mol/L KOH中(a)CV曲线(实线为O2饱和溶液,虚线为N2饱和溶液)和(b)LSV曲线(1600 r/min),Pt/C催化剂在不同含量KI的0.1 mol/L KOH中(c)CV曲线(实线为O2饱和溶液,虚线为N2饱和溶液)和(d)LSV曲线(1600 r/min)
Figure 5 (a) CV curves (solid line is O2 saturated solution, dotted line is N2 saturated solution) and (b) LSV curves of α-MnO2 (1600 r/min) in 0.1 mol/L KOH solution containing different concentration of KI, and (c) CV curves (solid line is O2 saturated solution, dotted line is N2 saturated solution) and (d) LSV curves of Pt/C (1600 r/min) in 0.1 mol/L KOH solution containing different concentration of KI
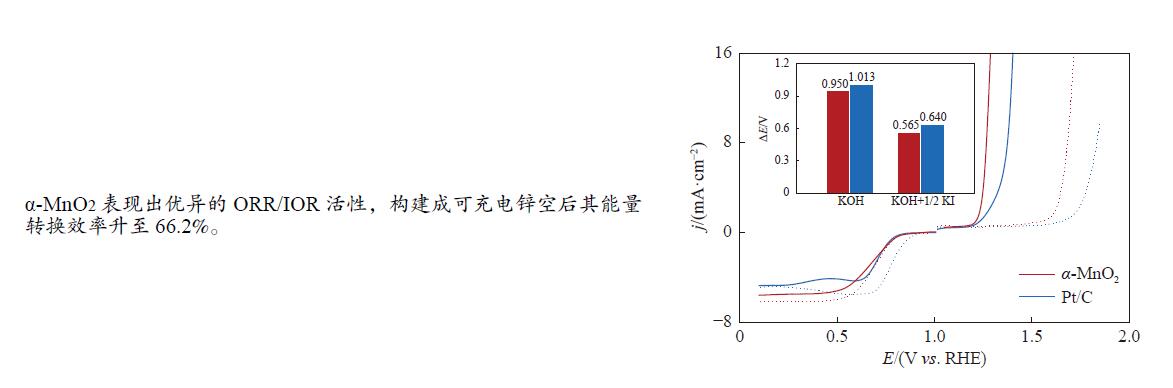
图 6 α-MnO2在0.1 mol/L KOH+0.05 mol/L KI中(a)不同扫速下的LSV曲线和(b)相应的K-L图;α-MnO2与商业Pt/C在不同电解液中(c)α-MnO2和Pt/C的LSV曲线(实线为KOH+1/2 KI,虚线为纯KOH)和(d)相应的ΔE(ΔE=Ej=10 – E1/2)对比
Figure 6 (a) LSV curves of α-MnO2 in 0.1 mol/L KOH with 0.05 mol/L KI under different scan rate and (b) corresponding K-L plots; (c) LSV curves of α-MnO2 and Pt/C (the solid line is KOH+1/2 KI, dasded line is KOH); (d) the ΔE (ΔE=Ej=10 – E1/2) value of α-MnO2 and Pt/C
图 7 α-MnO2在1.0 mol/L KOH+0.5 mol/L KI溶液中IOR测试后的(a)XRD谱图;(b)TEM;(c)高分辨TEM图片和(d)Mn 2p的高分辨XPS光谱谱图;α-MnO2在0.1 mol/L KOH+0.05 mol/L KI溶液中ORR测试后的(e)XRD谱图;(f)TEM;(g)高分辨TEM图片和(h)Mn 2p的高分辨XPS光谱谱图
Figure 7 (a) XRD pattern; (b) TEM; (c) high resolution TEM image and (d) high-resolution XPS spectrum of Mn 2p of α-MnO2 after IOR testing in 1.0 mol/L KOH+0.5 mol/L KI solution; (e) XRD pattern; (f) TEM; (g) high resolution TEM image and (h) high-resolution XPS spectrum of Mn 2p of α-MnO2 after ORR testing in 0.1 mol/L KOH+0.05 mol/L KI solution
表 1 不同催化剂的IOR比较
Table 1 Comparison of IOR of different catalysts
Catalyst Electrolyte IOR potential EOER− EIOR
(@10 mA/cm2)Ref. 20% Pt/C 1.0 mol/L KOH+0.5 mol/L KI 1.24 V @10 mA/cm2 450 mV [5] RuTiO-550 0.1 mol/L KOH+0.1 mol/L NaI 1.29 V @10 mA/cm2 − [30] Ni-Co(OH)2 NSAs 1.0 mol/L KOH+0.33 mol/L KI 1.32 V @50 mA/cm2,
1.33 V @100 mA/cm2320 mV [31] Pt/RuO2/CC 0.1 mol/L KOH+0.033 mol/L KI 1.42 V @10 mA/cm2 310 mV [45] α-MnO2 1.0 mol/L KOH+0.5 mol/L KI 1.311 V @10 mA/cm2 398 mV this work 表 2 不同催化剂的ORR和ΔE比较
Table 2 Comparison of ORR and ΔE of different catalysts
Catalyst E1/2/V Ej=10/V ΔE (Ej=10–E1/2)/V Reference MnO2-IL0.5 0.83 1.624 0.794 [8] 24Co-MnO2 0.787 1.66 0.872 [44] MnO2/NRGO-Urea 0.80 1.69 0.89 [47] MnO2/C 0.67 1.75 1.08 [47] CoO:MnO2@C-CC 0.78 1.45 0.67 [48] MnO-FeCo 0.88 1.501 0.621 [49] α-MnO2/Co3O4 0.76 1.784 1.024 [50] MC@NC-0.3 0.82 1.59 0.77 [51] CoFe@CNT/MnO 0.85 1.423 0.573 [52] α-MnO2 0.746 1.311 V
(IOR)0.565 this
work -
[1] WANG M, CHAO F, LIU Y, et al. Hollow N-doped carbon spheres with anchored single-atom Fe sites for efficient electrocatalytic oxygen reduction[J]. J Fuel Chem Technol,2023,51(5):581−588. doi: 10.1016/S1872-5813(22)60067-7 [2] 徐能能, 乔锦丽. 锌-空气电池双功能催化剂研究进展[J]. 电化学,2020,26(4):531−562. doi: 10.13208/j.electrochem.200524XU Nengneng, QIAO Jinli. Recent progress in bifunctional catalysts for zinc-air batteries[J]. J Electrochem,2020,26(4):531−562. doi: 10.13208/j.electrochem.200524 [3] ZHANG K, ZOU R. Advanced transition metal-based OER electrocatalysts: Current status, opportunities, and challenges[J]. Small,2021,17(37):2100129. doi: 10.1002/smll.202100129 [4] ZHAO C X, LIU J N, WANG J, et al. Recent advances of noble-metal-free bifunctional oxygen reduction and evolution electrocatalysts[J]. Chem Soc Rev,2021,50(13):7745−7778. doi: 10.1039/D1CS00135C [5] ZHAO S, LIU T, DAI Y, et al. Pt/C as a bifunctional ORR/iodide oxidation reaction (IOR) catalyst for Zn-air batteries with unprecedentedly high energy efficiency of 76.5%[J]. Appl Catal B: Environ,2023,320:121992. doi: 10.1016/j.apcatb.2022.121992 [6] LEI H, MA L, WAN Q, et al. Promoting surface reconstruction of NiFe layered double hydroxide for enhanced oxygen evolution[J]. Adv Energy Mater,2022,12(48):2202522. doi: 10.1002/aenm.202202522 [7] XUE H, MENG A, YANG T, et al. Controllable oxygen vacancies and morphology engineering: Ultra-high HER/OER activity under base-acid conditions and outstanding antibacterial properties[J]. J Energy Chem,2022,71:639−651. doi: 10.1016/j.jechem.2022.04.052 [8] GU Y, YAN G, LIAN Y, et al. MnIII-enriched α-MnO2 nanowires as efficient bifunctional oxygen catalysts for rechargeable Zn-air batteries[J]. Energy Storage Mater,2019,23:252−260. doi: 10.1016/j.ensm.2019.05.006 [9] WU K, SHI L, WANG Z, et al. A general strategy to generate oxygen vacancies in bimetallic layered double hydroxides for water oxidation[J]. Chem Commun,2023,59(21):3138−3141. doi: 10.1039/D3CC00096F [10] CHANG Y, ZHAI P, HOU J, et al. Excellent HER and OER catalyzing performance of Se-vacancies in defects-engineered PtSe2: From simulation to experiment[J]. Adv Energy Mater,2022,12(1):2102359. doi: 10.1002/aenm.202102359 [11] 宋卓卓, 余宗宝, 武宏大, 等. CoSOH/Co(OH)2复合纳米片的制备及其氧析出催化性能[J]. 燃料化学学报,2021,49(10):1549−1557. doi: 10.1016/S1872-5813(21)60077-4SONG Zhuozhuo, YU Zongbao, WU Hongda, et al. Preparation of CoSOH/Co(OH)2 composite nanosheets and its catalytic performance for oxygen evolution[J]. J Fuel Chem Technol,2021,49(10):1549−1557. doi: 10.1016/S1872-5813(21)60077-4 [12] YANG L, WANG D, LV Y, et al. Nitrogen-doped graphitic carbons with encapsulated CoNi bimetallic nanoparticles as bifunctional electrocatalysts for rechargeable Zn-Air batteries[J]. Carbon,2019,144:8−14. doi: 10.1016/j.carbon.2018.12.008 [13] JIANG B, CHEONG W C, TU R, et al. Regulating the electronic structure of NiFe layered double hydroxide/reduced graphene oxide by Mn incorporation for high-efficiency oxygen evolution reaction[J]. Sci China Mater,2021,64(11):2729−2738. doi: 10.1007/s40843-021-1678-y [14] QIU B, WANG C, ZHANG N, et al. CeO2-induced interfacial Co2+ octahedral sites and oxygen vacancies for water oxidation[J]. ACS Catal,2019,9(7):6484−6490. doi: 10.1021/acscatal.9b01819 [15] DIAZ-MORALES O, LEDEZMA-YANEZ I, KOPER M T M, et al. Guidelines for the rational design of Ni-based double hydroxide electrocatalysts for the oxygen evolution reaction[J]. ACS Catal,2015,5(9):5380−5387. doi: 10.1021/acscatal.5b01638 [16] YANG X, LI Z, JUN Q I N, et al. Preparation of Ni-Fe alloy foam for oxygen evolution reaction[J]. J Fuel Chem Technol,2021,49(6):827−834. doi: 10.1016/S1872-5813(21)60084-1 [17] ZHANG X, SHEN W, LI Z, et al. Carbon-based active support for water oxidation electrocatalyst: Making full use of the available surface area[J]. Carbon,2020,167:548−558. doi: 10.1016/j.carbon.2020.06.022 [18] 赵丹丹, 张楠, 卜令正, 等. 非贵金属电催化析氧催化剂的最新进展[J]. 电化学,2018,24(5):455−465.ZHAO Dandan, ZHANG Nan, BU Liangzheng, et al. Recent advances in non-noble metal nanomaterials for oxygen evolution electrocatalysis[J]. J Electrochem,2018,24(5):455−465. [19] FANG J, ZHANG X, WANG X, et al. A metal and nitrogen doped carbon composite with both oxygen reduction and evolution active sites for rechargeable zinc-air batteries[J]. J Mater Chem A,2020,8(31):15752−15759. doi: 10.1039/D0TA02544E [20] TAN Y, ZHANG Z, LEI Z, et al. Electronic modulation optimizes OH* intermediate adsorption on Co-Nx-C sites via coupling CoNi alloy in hollow carbon nanopolyhedron toward efficient reversible oxygen electrocatalysis[J]. Appl Catal B: Environ,2022,304:121006. doi: 10.1016/j.apcatb.2021.121006 [21] WANG T, CAO X, JIAO L. Progress in hydrogen production coupled with electrochemical oxidation of small molecules[J]. Angew Chem Int Ed, 2022: e202213328. [22] ZHANG M, ZHU J, WAN R, et al. Synergistic effect of nickel oxyhydroxide and tungsten carbide in electrocatalytic alcohol oxidation[J]. Chem Mater,2022,34(3):959−969. doi: 10.1021/acs.chemmater.1c02535 [23] LI Y, WEI X, HAN S, et al. MnO2 electrocatalysts coordinating alcohol oxidation for ultra‐durable hydrogen and chemical productions in acidic solutions[J]. Angew Chem Int Ed,2021,133(39):21634−21642. doi: 10.1002/ange.202107510 [24] CHEN S, DUAN J, VASILEFF A, et al. Size fractionation of two-dimensional sub-nanometer thin manganese dioxide crystals towards superior urea electrocatalytic conversion[J]. Angew Chem Int Ed,2016,55(11):3804−3808. doi: 10.1002/anie.201600387 [25] ZHU Y, LIU C, CUI S, et al. Multistep dissolution of lamellar crystals generates superthin amorphous Ni(OH) 2 catalyst for UOR[J]. Adv Mater, 2023: 2301549. [26] 陈晨欣, 何苏祺, KAMRAN D, 等. 海胆状NiMoO4纳米棒阵列作为高效双功能催化剂用于电催化及光伏驱动尿素电解[J]. 催化学报,2022,43(5):1267−1276. doi: 10.1016/S1872-2067(21)63962-1CHEN Chenxin, HE Suqi, KAMRAN D, et al. Sea urchin like NiMoO4 nanorod arrays as efficient bifunctional catalysts for electrocatalysis and photovoltaic driven urea electrolysis[J]. Chin J Catal,2022,43(5):1267−1276. doi: 10.1016/S1872-2067(21)63962-1 [27] LIN C, ZHANG P, WANG S, et al. Engineered porous Co-Ni alloy on carbon cloth as an efficient bifunctional electrocatalyst for glucose electrolysis in alkaline environment[J]. J Alloy Compd,2020,823:153784. doi: 10.1016/j.jallcom.2020.153784 [28] WANG Y, YAN W, NI M, et al. Surface valence regulation of cobalt-nickel foams for glucose oxidation-assisted water electrolysis[J]. Chem Commun,2023,59(17):2485−2488. doi: 10.1039/D2CC05270A [29] PENG S M, PATIL S B, CHANG C C, et al. Fast charge transfer between iodide ions and a delocalized electron system on the graphite surface for boosting hydrogen production[J]. J Mater Chem A,2022,10(45):23982−23989. doi: 10.1039/D2TA06517G [30] ADAM D B, TSAI M C, AWOKE Y A, et al. Engineering self-supported ruthenium-titanium alloy oxide on 3D web-like titania as iodide oxidation reaction electrocatalyst to boost hydrogen production[J]. Appl Catal B: Environ,2022,316:121608. doi: 10.1016/j.apcatb.2022.121608 [31] HU E, YAO Y, CHEN Y, et al. Boosting hydrogen generation by anodic oxidation of iodide over Ni-Co(OH)2 nanosheet arrays[J]. Nanoscale Adv,2021,3(2):604−610. doi: 10.1039/D0NA00847H [32] ADAM D B, TSAI M C, AWOKE Y A, et al. Iodide oxidation reaction catalyzed by ruthenium-tin surface alloy oxide for efficient production of hydrogen and iodine simultaneously[J]. ACS Sustainable Chem Eng,2021,9(26):8803−8812. doi: 10.1021/acssuschemeng.1c01867 [33] DESSIE T A, HUANG W H, ADAM D B, et al. Efficient H2 evolution coupled with anodic oxidation of iodide over defective carbon-supported single-atom Mo-N4 electrocatalyst[J]. Nano Lett,2022,22(18):7311−7317. doi: 10.1021/acs.nanolett.2c01229 [34] TANG Y J, ZHENG S S, CAO S, et al. Advances in the application of manganese dioxide and its composites as electrocatalysts for the oxygen evolution reaction[J]. J Mater Chem A,2020,8:18492−18514. doi: 10.1039/D0TA05985D [35] 胡文静, 班锦锦, 谢顺利, 等. 氧化锰基电催化材料的设计合成及其铝空气电池应用[J]. 硅酸盐通报,2023,42(2):728−735 + 742. doi: 10.16552/j.cnki.issn1001-1625.2023.02.011HU Wen-jing, BAN Jin-jin, XIE Shun-li, et al. Design and synthesis of manganese oxide based electrocatalytic materials and application of aluminium-air battery[J]. J Chin Ceram Soc,2023,42(2):728−735 + 742. doi: 10.16552/j.cnki.issn1001-1625.2023.02.011 [36] 赵慧, 姜日娟, 张勇, 等. 用于锌-空气电池的MnO2纳米片@Ni-氮掺杂石墨烯气凝胶[J]. 科学通报,2021,66(14):1758−1766. doi: 10.1360/TB-2020-1469ZHAO Hui, JIANG Rijuan, ZHANG Yong, et al. MnO2 nanosheets@Ni nitrogen doped graphene aerogel for zinc air batteries[J]. Chin Sci Bull,2021,66(14):1758−1766. doi: 10.1360/TB-2020-1469 [37] CAO Y L, YANG H X, AI X P, et al. The mechanism of oxygen reduction on MnO2-catalyzed air cathode in alkaline solution[J]. J Electroanal Chem,2003,557:127−134. doi: 10.1016/S0022-0728(03)00355-3 [38] MENG Y, SONG W, HUANG H, et al. Structure-property relationship of bifunctional MnO2 nanostructures: Highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media[J]. J Am Chem Soc,2014,136(32):11452−11464. doi: 10.1021/ja505186m [39] LI P C, HU C C, LEE T C, et al. Synthesis and characterization of carbon black/manganese oxide air cathodes for zinc-air batteries[J]. J Power Sources,2014,269:88−97. doi: 10.1016/j.jpowsour.2014.06.108 [40] LI P C, HU C C, NODA H, et al. Synthesis and characterization of carbon black/manganese oxide air cathodes for zinc-air batteries: effects of the crystalline structure of manganese oxides[J]. J Power Sources,2015,298:102−113. doi: 10.1016/j.jpowsour.2015.08.051 [41] SHAO C, YIN K, LIAO F, et al. Rod-shaped α-MnO2 electrocatalysts with high Mn3+ content for oxygen reduction reaction and Zn-air battery[J]. J Alloy Compd,2021,860:158427. doi: 10.1016/j.jallcom.2020.158427 [42] 黄乐珩, 程高, 赵英霞, 等. 不同晶型MnO2纳米阵列的可控合成及其电催化析氧性能[J]. 无机化学学报,2022,38(2):333−343.HUANG Leheng, CHENG Gao, ZHAO Yingxia, et al. Controllable synthesis of different crystalline MnO2 nanoarrays and their electrocatalytic oxygen evolution performance[J]. J Inorg Chem,2022,38(2):333−343. [43] YIN M, MIAO H, HU R, et al. Manganese dioxides for oxygen electrocatalysis in energy conversion and storage systems over full pH range[J]. J Power Sources,2021,494:229779. doi: 10.1016/j.jpowsour.2021.229779 [44] CHEN B, MIAO H, HU R, et al. Efficiently optimizing the oxygen catalytic properties of the birnessite type manganese dioxide for zinc-air batteries[J]. J Alloy Compd,2021,852:157012. doi: 10.1016/j.jallcom.2020.157012 [45] ZHANG Y, QIN H, ALFRED M, et al. Reaction modifier system enable double-network hydrogel electrolyte for flexible zinc-air batteries with tolerance to extreme cold conditions[J]. Energy Storage Mater,2021,42:88−96. doi: 10.1016/j.ensm.2021.07.026 [46] 任志立, 段磊, 徐守冬, 等. Pt/Co-N-C电催化材料的制备及其碱性ORR性能[J]. 人工晶体学报,2023,52(4):654−662.REN Zhi-li, DUAN Lei, XU Shou-dong, et al. Preparation of Pt/Co-N-C electrocatalytic materials and their alkaline ORR properties[J]. J Synth Cryst,2023,52(4):654−662. [47] MIAO H, CHEN B, LI S, et al. All-solid-state flexible zinc-air battery with polyacrylamide alkaline gel electrolyte[J]. J Power Sources,2020,450:227653. doi: 10.1016/j.jpowsour.2019.227653 [48] ZAMANI-MEYMIAN M R, KHANMOHAMMADI C K, POURZOLFAGHAR H. Designing high-quality electrocatalysts based on CoO: MnO2@C supported on carbon cloth fibers as bifunctional air cathodes for application in rechargeable Zn-Air battery[J]. ACS Appl Mater Interfaces,2022,14(50):55594−55607. doi: 10.1021/acsami.2c16826 [49] YE Y, ZHANG L, ZHU Q, et al. Interface engineering induced charge rearrangement boosting reversible oxygen electrocatalysis activity of heterogeneous FeCo-MnO@N-doped carbon nanobox[J]. J Colloid Interf Sci,2023,650:1350−1360. doi: 10.1016/j.jcis.2023.07.101 [50] HU T, ZHANG W, XIA Z, et al. Growth restriction of Co3O4 nanoparticles by α-MnO2 nanorods as air cathode catalyst for rechargeable aluminum-air battery[J]. Int J Energy Res,2022,46(8):11174−11184. doi: 10.1002/er.7917 [51] PENG L, PENG X, ZHU Z, et al. Efficient MnO and Co nanoparticles coated with N-doped carbon as a bifunctional electrocatalyst for rechargeable Zn-air batteries[J]. Int J Hydrogen Energy,2023,48(50):19126−19136. doi: 10.1016/j.ijhydene.2023.01.263 [52] CHEN S, HUANG Y, LI M, et al. MnOx anchored on N and O co-doped carbon nanotubes encapsulated with FeCo alloy as highly efficient bifunctional electrocatalyst for rechargeable Zinc-Air batteries[J]. J Electroanal Chem,2021,895:115513. doi: 10.1016/j.jelechem.2021.115513 [53] LU J, WANG H, SUN Y, et al. Charge state manipulation induced through cation intercalation into MnO2 sheet arrays for efficient water splitting[J]. Chem Eng J,2021,417:127894. doi: 10.1016/j.cej.2020.127894 -





 下载:
下载: