Research progress of molecular dynamics simulation on adsorption mechanisms of dispersants/surfactants on the surface of coal particles
-
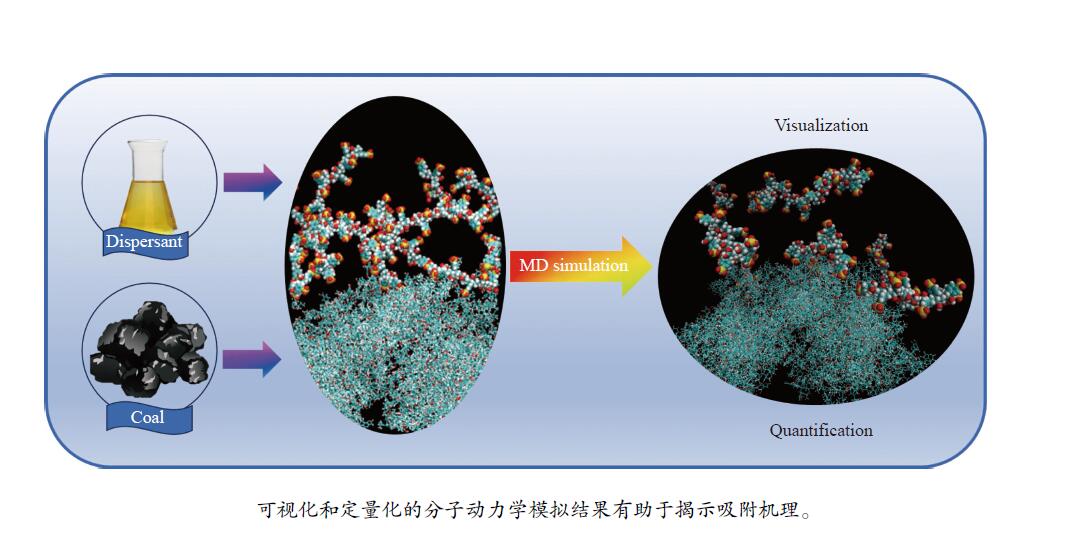
摘要: 本研究从力场、几何优化、牛顿运动方程、周期性边界条件、系宗、控温控压方法、步长步数等方面简述了分子动力学模拟的基本原理。目前,煤大分子的构建方法有三种:经典模型、自主构建模型和含氧官能团修饰的石墨烯层模型。分子动力学模拟中,吸附构型的图像信息可以直接观察分散剂/表面活性剂在煤颗粒表面的吸附情况及吸附历程,而密度分布曲线、均方根位移、吸附能等定量结果可以揭示分散剂/表面活性剂的吸附机理。分子动力学模拟与实验方法相结合可以从微观和宏观两个角度揭示分散剂/表面活性剂在煤颗粒表面吸附模式,将为分散剂和浮选剂的开发和应用提供重要的理论支撑。Abstract: Molecular dynamics simulation (MD) has become an indispensable means to study the adsorption mechanism of dispersants/surfactants on the surface of coal particles. In this paper, the basic principle of MD is described in terms of force field, geometry optimization, Newton equation of motion, periodic boundary condition, ensemble, temperature and pressure control method, step size and step number. At present, there are three methods for constructing coal macromolecules: classical model, self-constructing model, the graphene layer modified by oxygen-containing functional groups. In the results of MD, the image information of adsorption configuration can directly observe the adsorption status and adsorption process, and the quantitative results, including density distribution curve, root mean square displacement of water, and adsorption energy, can reveal the adsorption mode of dispersant/surfactant. MD combined with experimental methods can shed light on the adsorption mechanism of dispersants/surfactants on the surface of coal particles from both microscopic and macroscopic perspectives, which will provide important theoretical support for the development and application of the dispersant and flotation agent.
-
Key words:
- molecular dynamics simulation /
- dispersant /
- surfactant /
- coal particle /
- adsorption mechanism
-
图 3 G14、NSF、G14+NSF在LQ石油焦颗粒表面的吸附构型图[46]
Figure 3 The stable configuration of the three adsorption systems (a) G14 on LQ by the lateral horizontal adsorption; (b) cation-π interaction between naphthalene nucleus of NSF and -N(CH3)${}_4^+ $ of G14; (c) electrostatic interaction between −${\rm{SO}}_3^- $ of NSF and -N (CH3)${}_4^+ $ of G14 on LQ[46]
(with permission from Elsevier)
表 1 不同体系吸附能数据对比
Table 1 Comparison of adsorption energy data of different systems
-
[1] STILLINGER F H, RAHMAN A. Improved simulation of liquid water by molecular dynamics[J]. J Chem Phys,1974,60(4):1545−1557. doi: 10.1063/1.1681229 [2] KREMER K, GREST G S. Dynamics of entangled linear polymer melts: A molecular-dynamics simulation[J]. J Chem Phys,1990,92(8):5057−5086. doi: 10.1063/1.458541 [3] XIAO F, YAN B Q, ZOU X Y, et al. Study on ionic liquid modified montmorillonite and molecular dynamics simulation[J]. Colloid Surface A,2020,587:124311. doi: 10.1016/j.colsurfa.2019.124311 [4] WANG L, HU Y H, LIU J P, et al. Flotation and adsorption of muscovite using mixed cationic-nonionic surfactants as collector[J]. Powder Technol,2015,276:26−33. doi: 10.1016/j.powtec.2015.02.019 [5] XIE L Y, SHAO Y J, ZHONG W Q, et al. Molecular dynamic simulation on the oxidation process of coal tar pitch[J]. Fuel,2019,42:50−61. [6] LYU X J, YOU X F, HE M, et al. Adsorption and molecular dynamics simulations of nonionic surfactant on the low rank coal surface[J]. Fuel,2018,211:529−534. doi: 10.1016/j.fuel.2017.09.091 [7] TEOH G H, OOI B S, JAWAD Z A, et al. Impacts of PVDF polymorphism and surface printing micro-roughness on superhydrophobic membrane to desalinate high saline water[J]. J Environ Chem Eng,2021,9(4):105418. doi: 10.1016/j.jece.2021.105418 [8] LIU Z Q, ZHOU G, LI S L, et al. Molecular dynamics simulation and experimental characterization of anionic surfactant: Influence on wettability of low-rank coal[J]. Fuel,2020,279(1/2):118323. [9] LI L, MA C D, LIN M Y, et al. Study of sodium lignosulfonate prepare low-rank coal-water slurry: Experiments and simulations[J]. Chin J Chem Eng,2021,29:344−353. doi: 10.1016/j.cjche.2020.07.064 [10] JIN H, ZHANG Y S, CHEN K, et al. Preparation and characterization of a composite dust suppressant for coal mines[J]. Polymers,2020,12(12):2942. doi: 10.3390/polym12122942 [11] 王诗生, 张梦梦, 盛广宏, 等. 基于密度泛函理论和实验研究磁性生物炭吸附水中甲基橙的作用机制[J]. 中国环境科学, https://doi.org/10.19674/j.cnki.issn1000-6923.20230626.00523WANG Shisheng, ZHANG Mengmeng, SHENG Guanghong, et al. The mechanism of magnetic biochar adsorption of methyl orange in water was studied based on density functional theory and experiment[J]. Chin Environ Sci, https://doi.org/10.19674/j.cnki.issn1000-6923.20230626.00523 [12] 姚晨牧, 冯丽, 安亚雄, 等. 碳材料的分子模型及模拟方法在吸附中应用的研究进展[J]. 天然气化工,2021,46(1):1−9.YAO Chenmu, FENG Li, AN Yaxiong, et al. Research progress of molecular models of carbon materials and application of simulation methods in adsorption[J]. Nat Gas Ind,2021,46(1):1−9. [13] LEACH A R. Molecular Modelling Principles and Applications[M]. Harlow: Pearson Education Limited, 2001. [14] 钟璟, 邵伟, 夏庆, 等. 有机蒸气/氮气混合物透过炭膜的非平衡分子动力学模拟[J]. 天然气化工,2008,33(24):24−28.ZHONG Jing, SHAO Wei, XIA Qing, et al. Non-equilibrium molecular dynamics simulation of organic vapor/nitrogen mixture through carbon film[J]. Nat Gas Ind,2008,33(24):24−28. [15] REZAEE F, YOUSEFI F, KHOEINI F. Heat transfer in strained twin graphene: A non-equilibrium molecular dynamics simulation[J]. Physica A,2021,564:125542. doi: 10.1016/j.physa.2020.125542 [16] ASAI P, JIN J, DEO M, et al. Non-equilibrium molecular dynamics simulation to evaluate the effect of confinement on fluid flow in silica nanopores[J]. Fuel.,2022,317:123373. doi: 10.1016/j.fuel.2022.123373 [17] ZHANG Q, ZHU H Q, KANG R X, et al. Insights into influence of CH4 on CO2/N2 injection into bituminous coal matrix with biaxial compression strain in molecular terms|[J]. J Nat Gas Sci Eng,2023,117:205077. doi: 10.1016/j.jgsce.2023.205077 [18] GAO D M, HONG L, WANG J, et al. Molecular simulation of gas adsorption characteristics and diffusion in micropores of lignite[J]. Fuel,2020,269:1174431. [19] 许江涛. 基于分子模拟方法的温度对软硬无烟煤吸附甲烷特性的影响研究[J]. 煤矿安全,2002,53(7):158−165. doi: 10.13347/j.cnki.mkaq.2022.07.026XU Jiangtao. Effect of temperature on methane adsorption characteristics of soft and hard anthracite based on molecular simulation method[J]. Safety Coal Mines,2002,53(7):158−165. doi: 10.13347/j.cnki.mkaq.2022.07.026 [20] JIN H, ZHANG Y S, DONG H T, et al. Molecular dynamics simulations and experimental study of the effects of an ionic surfactant on the wettability of low-rank coal[J]. Fuel,2022,320:123951. doi: 10.1016/j.fuel.2022.123951 [21] LI B, GUO J Y, LIU S Y, et al. Molecular insight into the mechanism of benzene ring in nonionic surfactants on low-rank coal floatability[J]. J Mol Liq,2020,302:112563. doi: 10.1016/j.molliq.2020.112563 [22] LIU Z C, REN H R, YANG Z, et al. Effect of mixed collector addition sequence on the adsorption behavior of low-rank coal surface: Experimental and molecular dynamics simulation study[J]. Powder Technol,2022,397:117119. doi: 10.1016/j.powtec.2022.117119 [23] 周晓平, 田壮壮, 薛迪杰. 力场在分子动力学模拟中的应用[J]. 科学导报,2016,000(9):280−281.ZHOU Xiaoping, TIAN Zhuangzhuang, XUE Dijie. Application of force field in molecular dynamics simulation[J]. Sci Guide,2016,000(9):280−281. [24] 赵立峰. 分子力场方法及其在材料科学中的若干应用问题[D]. 上海: 上海交通大学, 2008.ZHAO Lifeng. Force field methods and representative applications in material science[D]. Shanghai: Shanghai Jiao Tong University, 2008. [25] 梁太宁, 杨小震. 分子力场——COMPASS简介[J]. 化学通报, 2001, 网络版.LIANG Taining, YANG Xiaozhen. Introduction to COMPASS Forcefield[J]. Chem online, 2001, Chem Online. [26] 王艺峰, 程时远, 王世敏, 等. 高分子材料模拟中的分子力学法和力场[J]. 高分子材料科学与工程,2003,19(1):10−14. doi: 10.3321/j.issn:1000-7555.2003.01.003WANG Yifeng, CHENG Shiyuan, WANG Shimin, et al. Molecular dynamics method and force field in polymer materials simulation[J]. Highpolymer Mater Sci Eng,2003,19(1):10−14. doi: 10.3321/j.issn:1000-7555.2003.01.003 [27] LI J J, YAN G C, KONG S Q, et al. Molecular mechanism study on the effect of microstructural differences of octylphenol polyoxyethylene ether (opeo) surfactants on the wettability of anthracite[J]. Molecules,2023,28(12):4748. doi: 10.3390/molecules28124748 [28] CHEN X L, LIU J S, YAN G C, et al. Molecular mechanism of hydrophobic tail chain saturation in nonionic surfactants changing the wettability of anthracite[J]. J Mol Liq,2022,397:117119. [29] 李煌, 鲁红权, 陈钦煌, 等. 分子动力学模拟基本原理及其应用[J]. 科技视界,2018,(5):25−26. doi: 10.3969/j.issn.2095-2457.2018.05.010LI Huang, LU Hongquan, CHEN Qinhuang, et al. The basic principle of molecular dynamics simulation and its application[J]. Sci Technol V,2018,(5):25−26. doi: 10.3969/j.issn.2095-2457.2018.05.010 [30] 陈正隆, 徐为人, 汤立达. 分子模拟的理论与实践[M]. 北京: 化学工业出版社, 2007.CHEN Zhenglong, XU Weiren, TANG Lida. Theory and Practice of Molecular Simulation [M]. Beijing: Chemical Industry Press, 2007. [31] 高玉凤. 典型固液界面热力学与动力学性质的分子动力学研究[D]. 上海: 华东师范大学, 2011.GAO Yufeng. Typical thermodynamic and kinetic properties of solid-liquid interface: A molecular dynamics simulations[D]. Shanghai: East China Normal University, 2011. [32] HATCHER P G. Chemical structural models for coalified wood (vitrinite) in low rank coal[J]. Org Geochem,1990,16:959−968. doi: 10.1016/0146-6380(90)90132-J [33] WENDER. Catalytic synthesis of chemicals from coal[J]. Catal Rev,1976,14(1):97−129. doi: 10.1080/03602457608073408 [34] WISER W H. Conversion of bituminous coal to liquids and gases: Chemistry and representative processes[J]. Magn Reason,1984,124:325−350. [35] YOU X F, HE M, ZHANG W, et al. Molecular dynamics simulations of nonylphenol ethoxylate on the Hatcher model of subbituminous coal surface[J]. Powder Technol,2018,332:323−330. doi: 10.1016/j.powtec.2018.04.004 [36] ZHANG L, LI B, XIA Y C, et al. Wettability modification of Wender lignite by adsorption of dodecyl poly ethoxylated surfactants with different degree of ethoxylation: A molecular dynamics simulation study[J]. J Mol Graph Model,2017,76:106−117. doi: 10.1016/j.jmgm.2017.06.028 [37] SUN L Y, GE S C, JING D J, et al. Wetting mechanism and experimental study of synergistic wetting of bituminous coal with SDS and APG1214[J]. ACS Omega,2022,7(1):780−785. doi: 10.1021/acsomega.1c05422 [38] ZHANG Z Q, WANG C L, YAN K F. Adsorption of collectors on model surface of Wiser bituminous coal: A molecular dynamics simulation study[J]. Min Eng,2015,79:31−39. doi: 10.1016/j.mineng.2015.05.009 [39] 蔺华林, 李克健, 章序文, 等. 煤液化沥青分析表征及结构模型[J]. 燃料化学学报,2014,42(7):779−784.LIN Hualin, LI Kejian, ZHANG Xuwen, et al. Analysis and structural model of coal liquefaction asphaltene[J]. J Fuel Chem Technol,2014,42(7):779−784. [40] ZHANG K, HOU Y, YE Z, et al. Interactions of coal pitch with amphoteric polycarboxylate dispersant in coal pitch–water slurry: Experiments and simulations[J]. Fuel,2022,318:123608. doi: 10.1016/j.fuel.2022.123608 [41] 崔馨, 严煌, 赵培涛. 煤分子结构模型构建及分析方法综述[J]. 中国矿业大学学报,2019,48(4):704−717. doi: 10.13247/j.cnki.jcumt.001026CUI Xin, YAN Huang, ZHAO Peitao. A review on the model construction and analytical methods of coal molecular structure[J]. J China Univ Min Technol,2019,48(4):704−717. doi: 10.13247/j.cnki.jcumt.001026 [42] LU H Y, LI X F, ZHANG C Q, et al. Experiments and molecular dynamics simulations on the adsorption of naphthalenesulfonic formaldehyde condensates at the coal-water interface[J]. Fuel,2020,264:116838. doi: 10.1016/j.fuel.2019.116838 [43] MENG X L, ZHANG T H, WU G G, et al. Investigation on the interaction between the lipophilic structure of anionic dispersants and Shenhua non-caking coal in coal water slurry[J]. Colloid Surfa A,2022,643:128812. doi: 10.1016/j.colsurfa.2022.128812 [44] LI L, LI Z H, MA C D, et al. Molecular dynamics simulations of nonionic surfactant adsorbed on subbituminous coal model surface based on XPS analysis[J]. Mol Simulat,2019,45(9):736−742. doi: 10.1080/08927022.2019.1582773 [45] LIU Z C, XIA Y C, LAI Q T, et al. Adsorption behavior of mixed dodecane/n-valeric acid collectors on low-rank coal surface: Experimental and molecular dynamics simulation study[J]. Colloid Surface A,2019,583:123840. doi: 10.1016/j.colsurfa.2019.123840 [46] LV D M, YAN H, WU H J, et al. Effect of modification by Gemini cationic surfactant on the properties of slurries prepared with petroleum coke: Experiments and molecular dynamics simulation[J]. Fuel,2022,317:123541. doi: 10.1016/j.fuel.2022.123541 [47] VU T, CHAFFEE A, YAROVSKY I. Investigation of lignin-water interactions by molecular simulation[J]. Mol Simul,2002,28(10/11):981−991. doi: 10.1080/089270204000002610 [48] MENG Z Y, YANG Z Y, YIN Z Q, et al. Interaction between dispersant and coal slime added in semi-coke water slurry: An experimental and DFT study[J]. Appl Surf Sci,2021,540:148327. doi: 10.1016/j.apsusc.2020.148327 -





 下载:
下载: