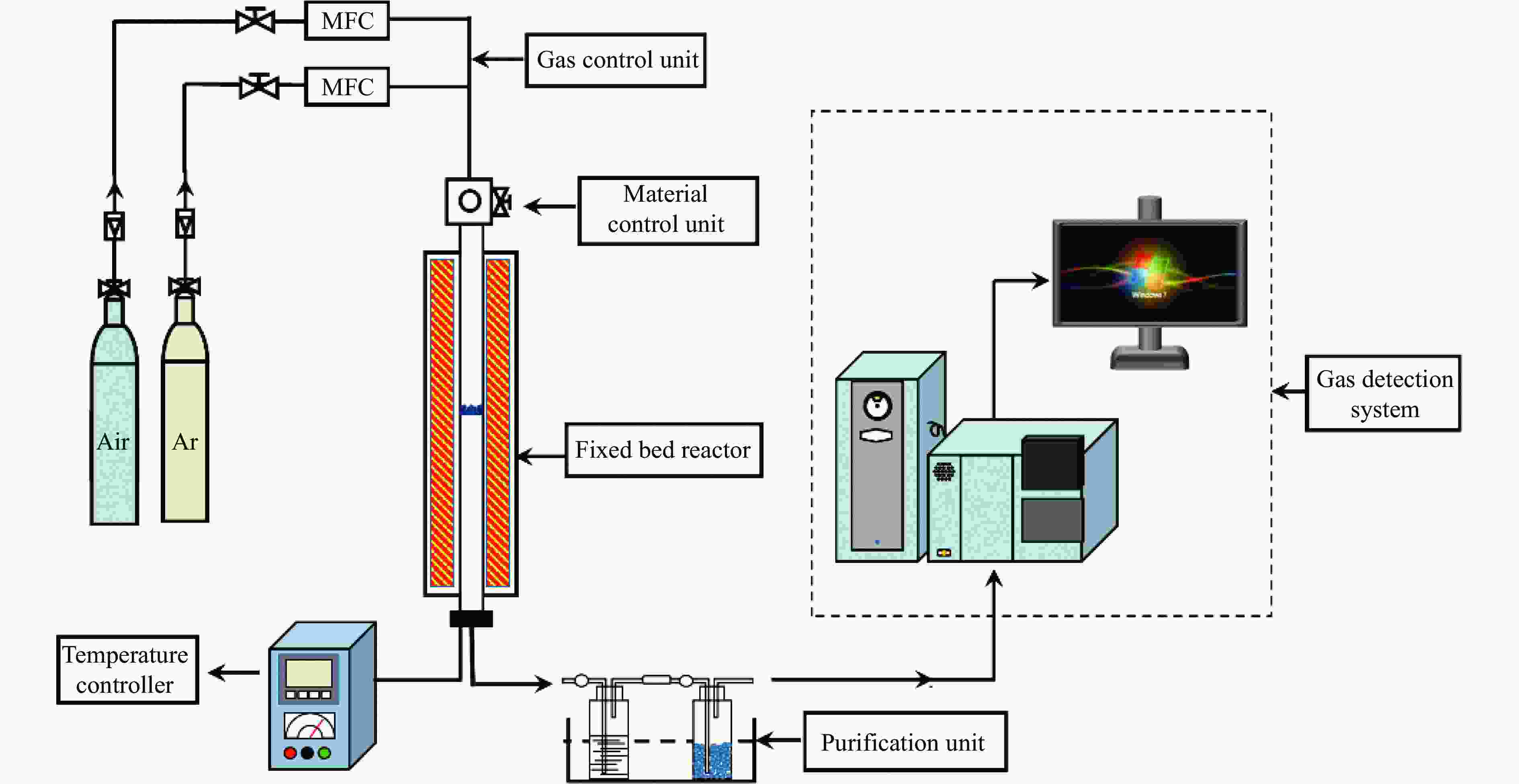
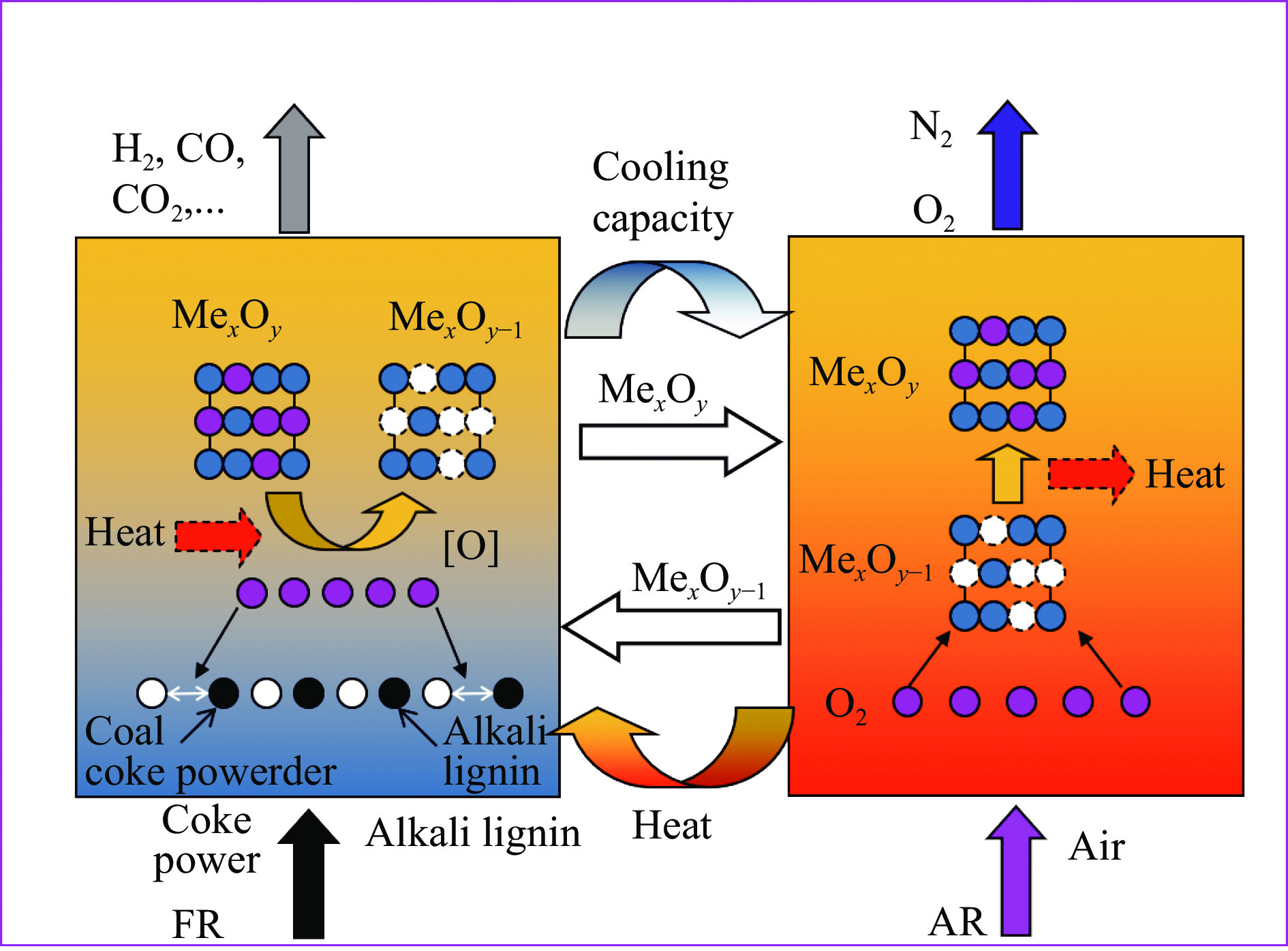
Experimental study on alkali lignin enhanced chemical looping gasification of pulverized coal char
-
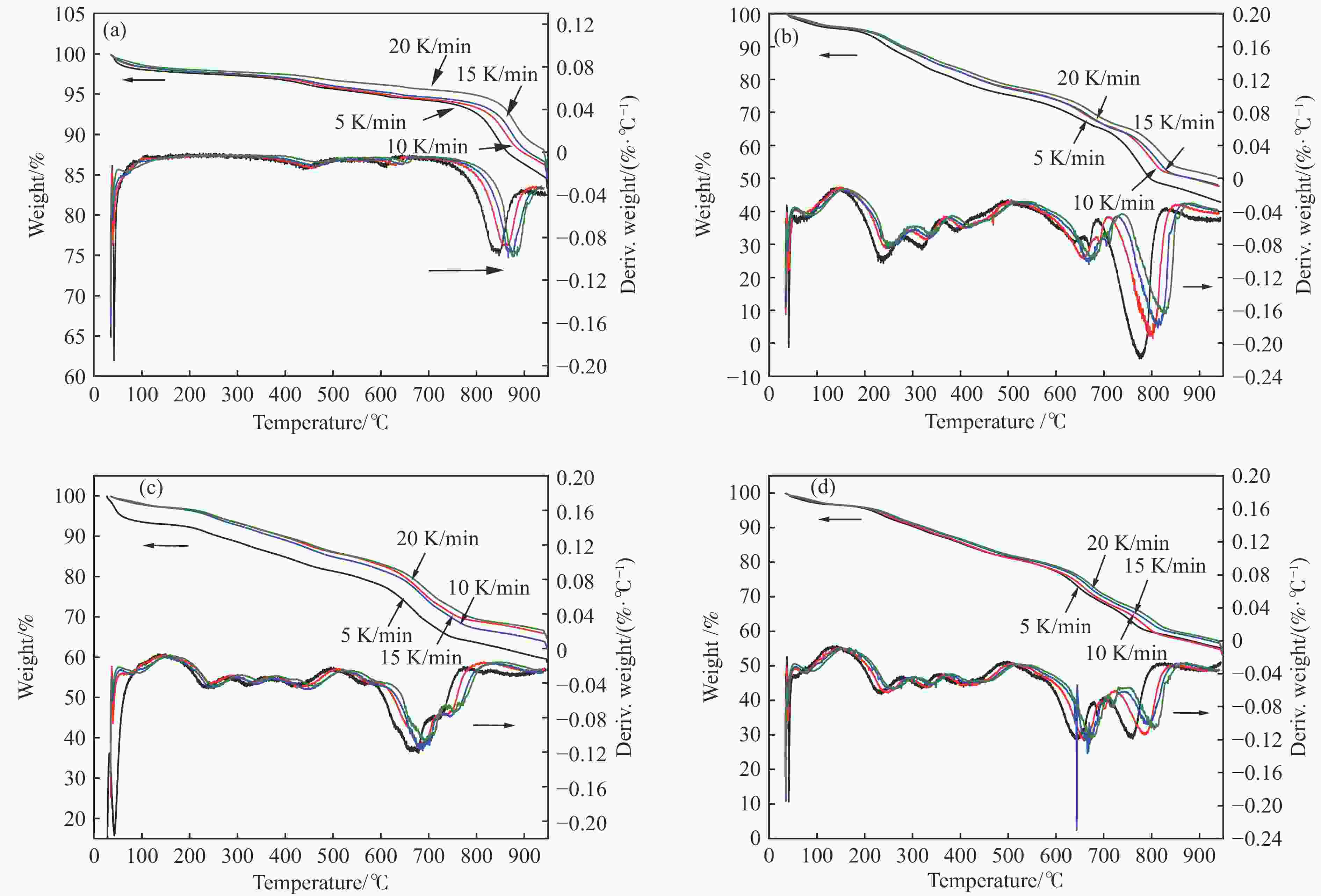
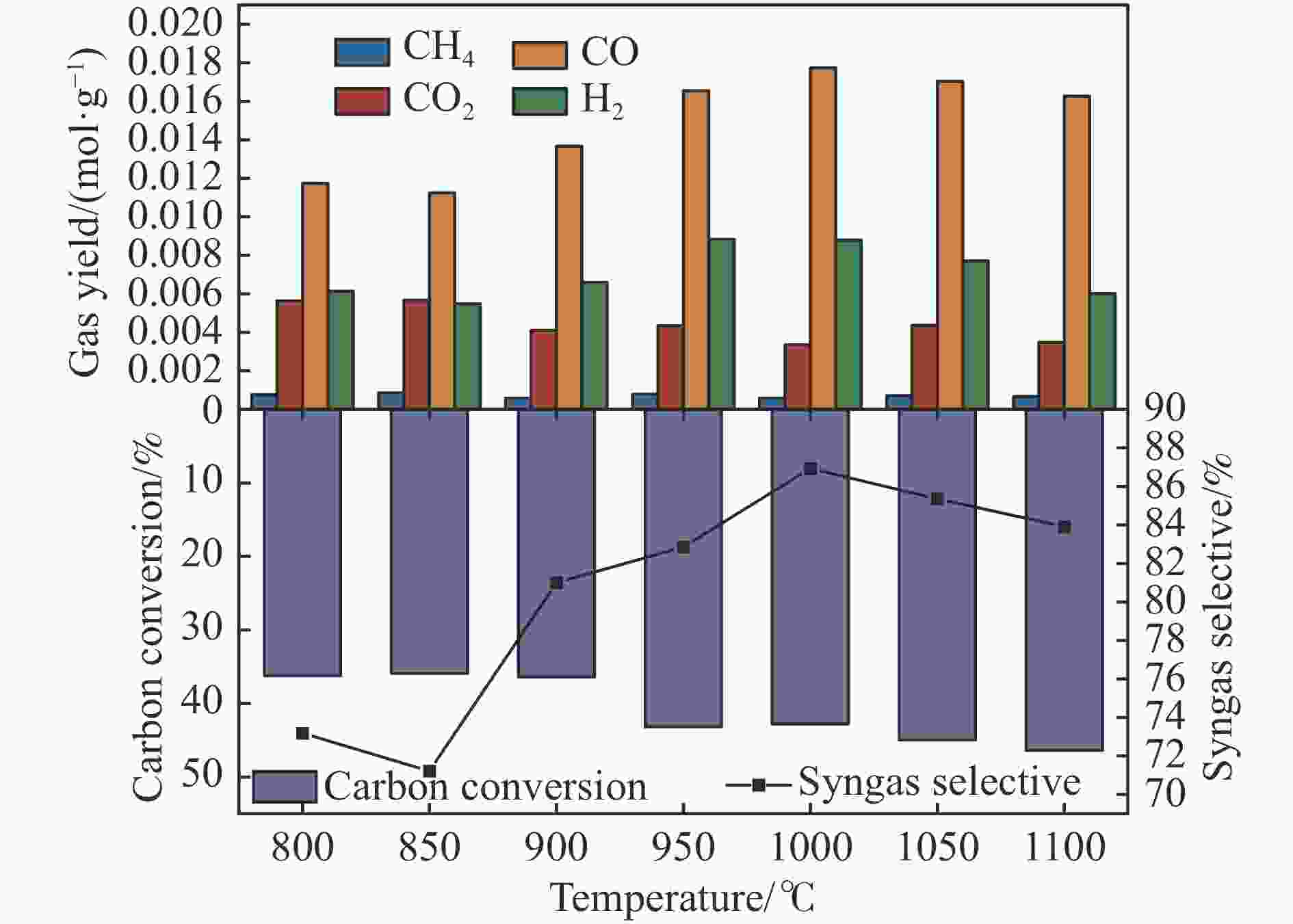
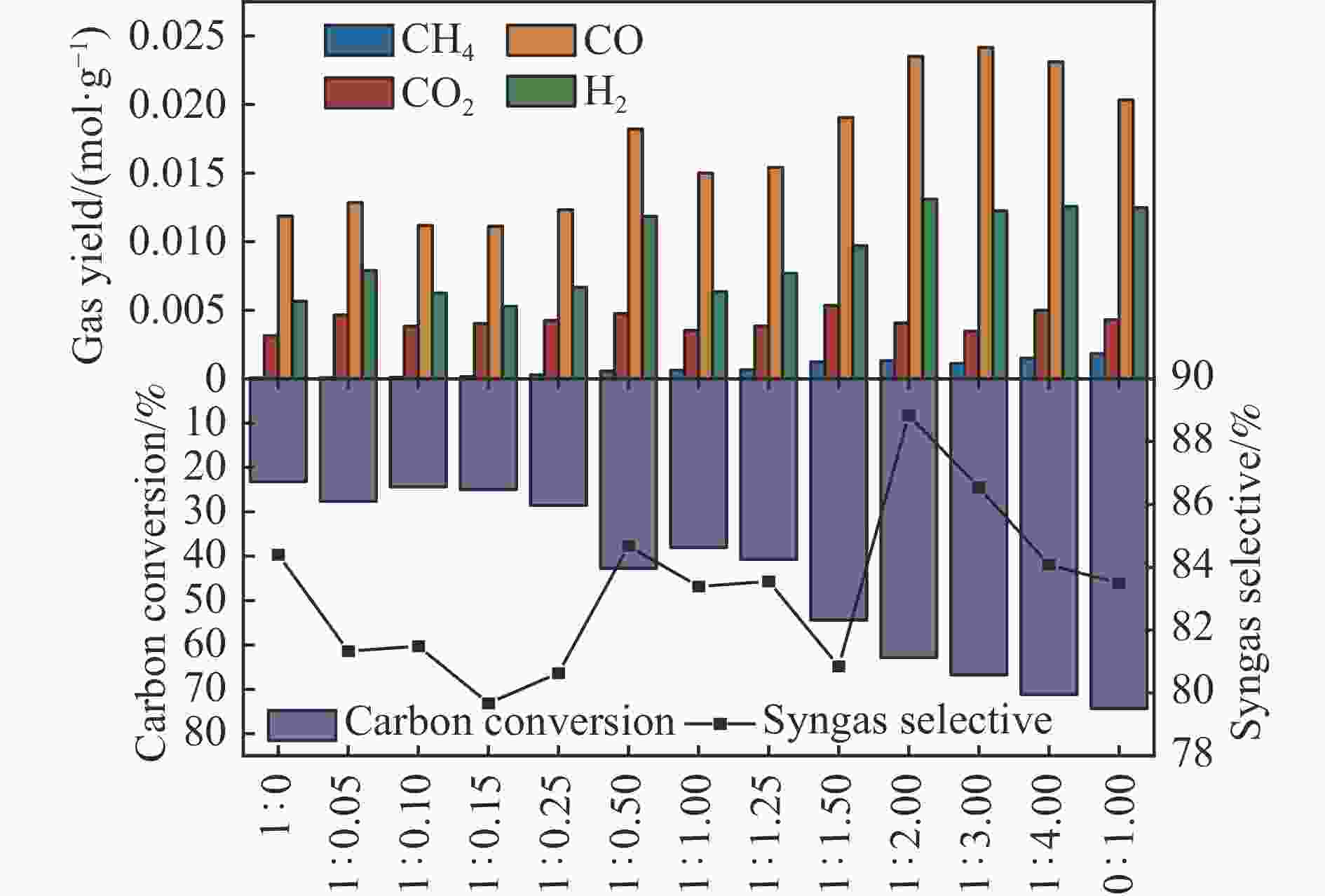
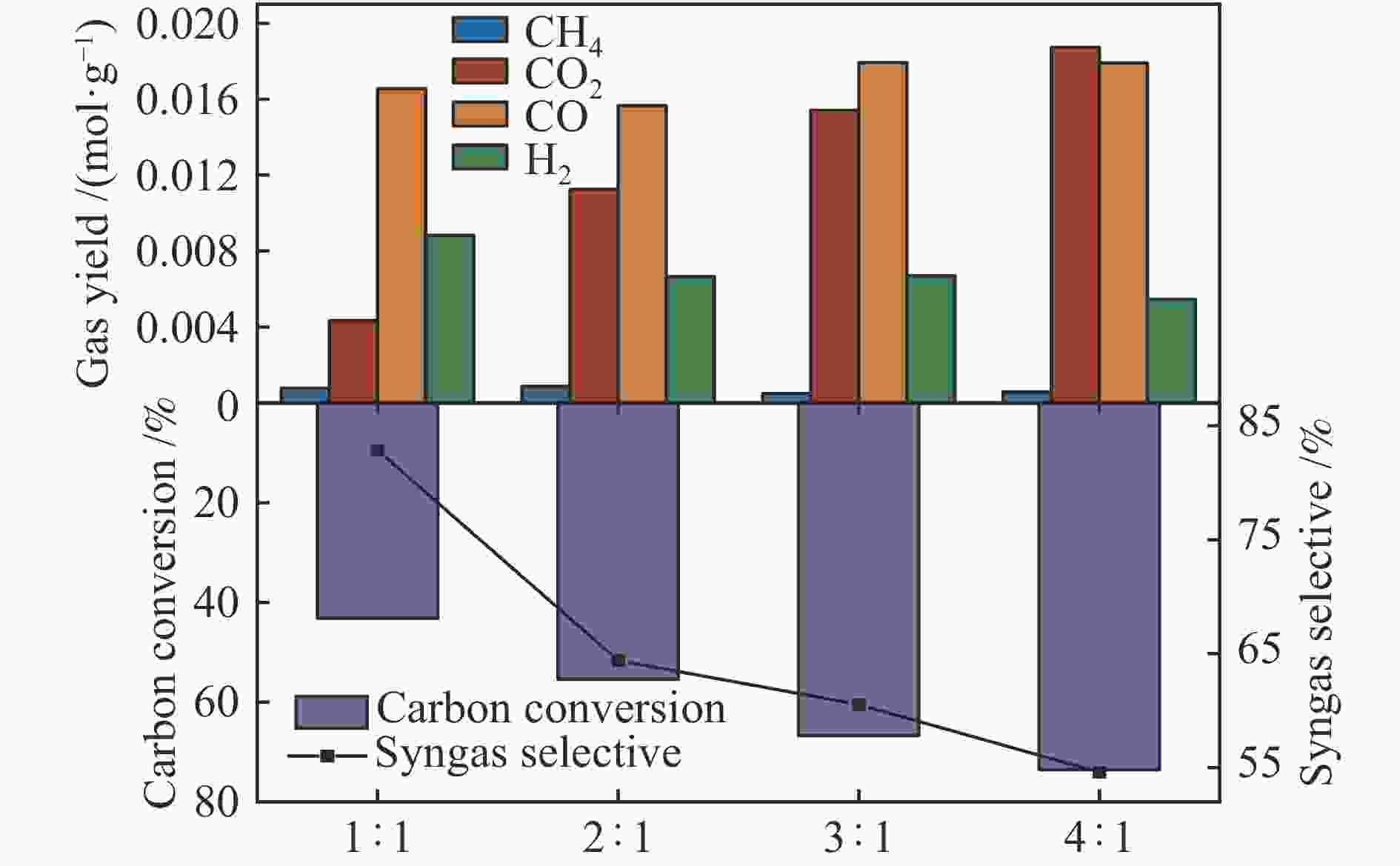
摘要: 中国煤炼焦工业副产物煤焦粉产量大、活性低,难以被直接回收利用,常规热化学利用方式反应条件苛刻、催化剂易失活且存在动力学限制。本研究通过造纸副产物碱木质素作为可弃型催化剂,构建碱木质素强化化学链气化的方式来处理煤焦粉,实现工业副产物协同资源化利用。热转化实验和动力学分析研究表明,碱木质素可强化煤焦粉化学链气化过程,促进煤焦粉热解峰向低温方向移动。当煤焦粉与碱木质素质量比为1∶3时,反应活化能比单独煤焦粉反应降低87.56%。固定床实验证实气化温度提高、碱木质素以及载氧体赋存量增加,可以有效提高燃料碳转化率及合成气产物择性,促进气化反应进行,但氧载体过量会导致合成气转化为终端产物,降低合成气选择性。在气化温度为950 ℃,煤焦粉与碱木质素质量比为1∶2, 氧载体与煤焦粉/碱木质素混合体系质量比为1∶1的最佳反应条件下,基于NiFe2O3的碱木质素/煤焦粉化学链气化合成气选择性高达82.85%。该研究为碱木质素与煤焦粉的资源化利用提供科学依据。Abstract: The pulverized coal char from the byproduct of China's coal coking industry has high yield and low activity, which is difficult to be directly recycled. The conventional thermochemical utilization method has harsh reaction conditions, catalyst deactivation and kinetic limitations. By using the alkali lignin from paper-making as a disposable catalyst, an alkali lignin enhanced chemical looping gasification method was constructed to treat coal coke powder, which can realize the collaborative resource utilization of industrial by-products. In this study, the reaction process of alkali lignin and coal char powder was studied by thermogravimetry and kinetic analysis. The thermal transformation experiment and kinetic analysis showed that alkali lignin could strengthen the chemical looping gasification process of pulverized coal char and promote the pyrolysis peak to move to low temperature. When the mass ratio of pulverized coal char to alkali lignin was 1∶3, the activation energy was 87.56% lower than that of coal coke powder alone, indicating that there was a synergistic effect between the two in the co pyrolysis process. The experiments in fixed bed reactor verified that the carbon conversion rate and the selectivity of syngas increased as the increasing of temperature and the content of alkali lignin and oxygen carrier, which effectively promoted the gasification reaction. However, excessive oxygen loading led to combustion reaction between syngas and lattice oxygen and reduce syngas selectivity. Under the optimal reaction conditions of 950 ℃, the mass ratio of coal char powder to alkali lignin is 1∶2, and the mass ratio of oxygen carrier to pulverized coal char/alkali lignin is 1∶1, the selectivity of syngas of alkali lignin/coal char powder in chemical looping gasification was 82.85%. This study provides a scientific basis for the resource utilization of alkali lignin and coal char powder.
-
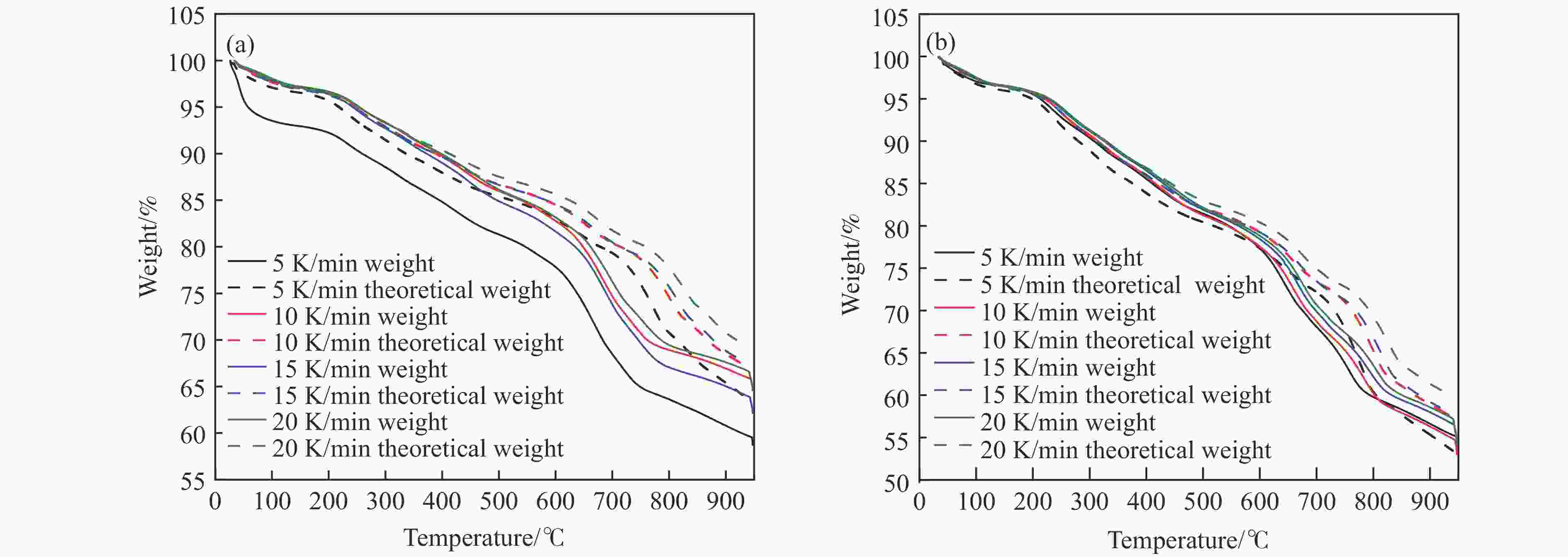
图 4 不同升温速率下煤焦碱木质素混合体系的理论失重量与实际失重量对比图
Figure 4 presents a comparative analysis between the theoretical and experimental weight loss of a coal-coke-lignin composite system at various heating rate
(a): Pulverized coal char/alkali lignin (1∶1) with oxygen carrier; (b): Pulverized coal char/alkai lignin (1∶3) with oxygen carrier.
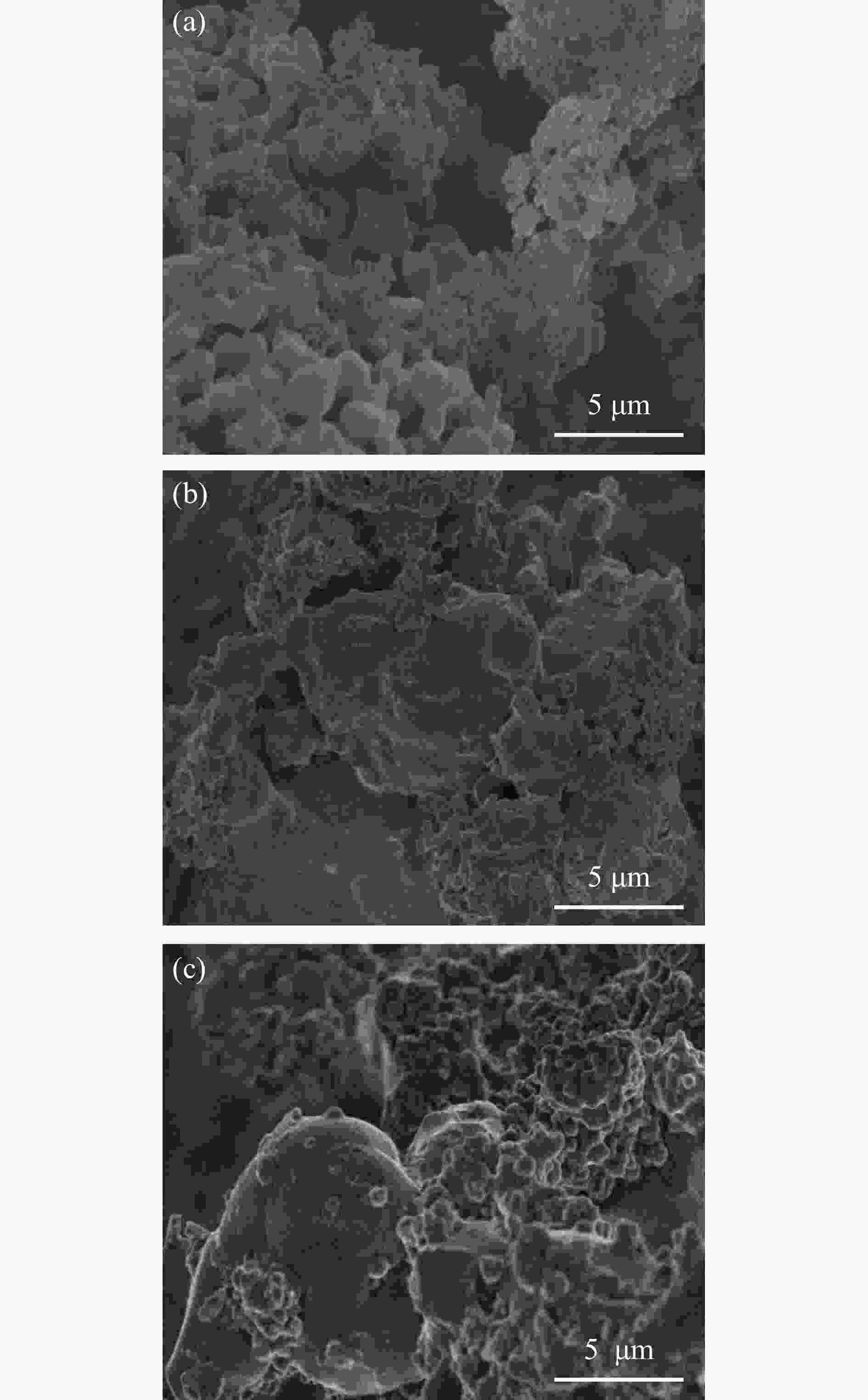
图 10 氧载体与煤焦粉和氧载体与煤焦粉和碱木质素两者混合物反应后的SEM照片
Figure 10 The SEM images of the oxygen carrier, the mixture of oxygen carrier and coal char, and the mixture of oxygen carrier with coal char and alkaline lignin after reaction
(a): Pulverized coal char (b): Pulverized coal char: alkali lignin =1∶2 (c): Pulverized coal char: alkali lignin =1∶3.
表 1 碱木质素和云南褐煤的工业分析和元素分析
Table 1 Proximate and ultimate analysis of lignin alkaline and coal char
Sample Ultimate analysis wdry/% Proximate analysis wdry/% AAEMs/(mg·kg−1) C N H S O* V M A FC Na K Mg Ca Lignin alkaline 59.4 0.09 5.30 3.17 32.0 41.49 34.39 19.10 5.02 93110 14193 76 292 Pulverized coal char 69.77 0.61 3.91 1.06 12.97 40.30 10.22 11.68 37.8 − − − − O*: by difference. 表 2 常用固相动力学模型
Table 2 Common solid-state kinetic models
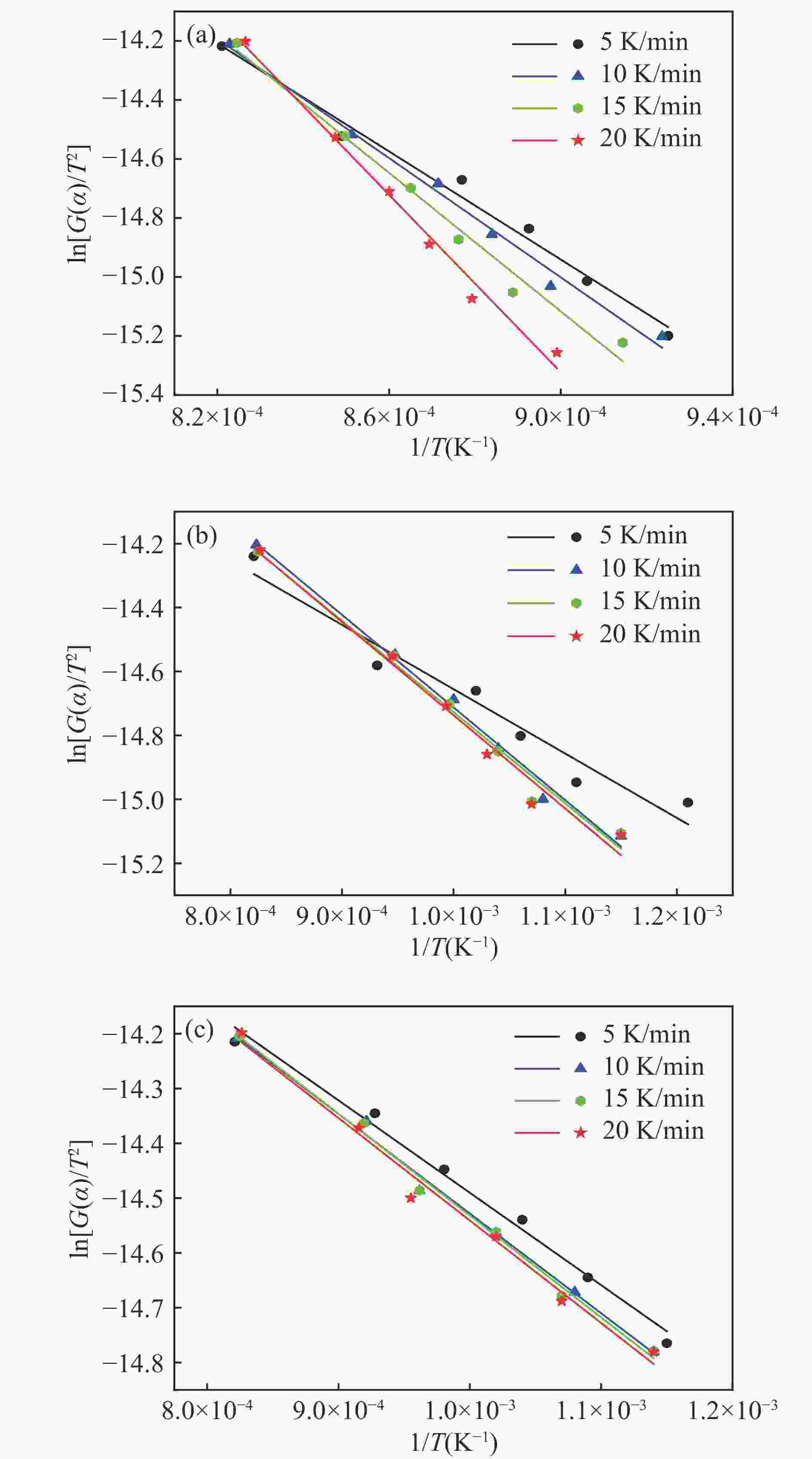
Symbol f (α) G (α) D1 $ {1/2\alpha} $ $ {{\alpha }}^{2} $ D2 ${\text{[}-\text{ln}\left(1-\alpha\right)\text{]} }^{-\text{1} }$ $ {\alpha + }\left(1-\alpha\right){\ln(1-\alpha)} $ D3 $ \left({3}/{2}\right){\text{[}{\left(1-\alpha\right)}^{-{1}/{3}}-{1]}}^{-{1}} $ $ 1-(2/3)\alpha -{(1-\alpha )}^{2/3} $ D4 $ \text{(}{3}/{2)}{\left(1-\alpha\right)}^{{2}/{3}}{\text{[1}-{\left(1-\alpha\right)}^{{2}/{3}}\text{]}}^{-{1}} $ $ {\text{[1}-{\left(1-\alpha\right)}^{{1}/{3}}\text{]}}^{{2}} $ A1 $ 1-{\alpha } $ $ -\mathrm{l}\mathrm{n}(1-{\alpha }) $ A2 $ \text{2}\left(1-\alpha\right){\text{[}-\text{ln}\left(1-\alpha\right)\text{]}}^{\text{1}/\text{2}} $ $ {\text{[}-\text{ln}\left(1-\alpha\right)\text{]}}^{{1}/{2}} $ A3 3/(1−α)[−ln(1−α)]2/3 $ {\text{[}-\text{ln}\left(1-\alpha\right)\text{]}}^{\text{1}/\text{3}} $ R1 $ 2{(1-{\alpha })}^{1/2} $ $ 1-{(1-{\alpha })}^{1/2} $ R2 $ 3{(1-{\alpha })}^{2/3} $ $ 1-{(1-{\alpha })}^{1/3} $ C $ {(1-{\alpha })}^{2} $ $ {(1-{\alpha })}^{-1}-1 $ 表 3 不同固相动力学模型下的Pearson相关系数和残差平方和
Table 3 Pearson correlation coefficients and sum of squares of residuals for different solid-state kinetic models
Symbol Pulverized coal char Pulverized coal char: alkali lignin=1∶1 Pulverized coal char: alkali lignin=1∶3 Pearson RSS Pearson RSS Pearson RSS D1 −0.95783 0.07751 −0.88223 0.07730 −0.90771 0.07487 D2 −0.98287 0.06168 −0.96501 0.06173 −0.97443 0.05194 D3 −0.99219 0.03960 −0.98555 0.04032 −0.99325 0.02115 D4 −0.99239 0.07720 −0.98712 0.08862 −0.99641 0.02670 A1 −0.9522 0.34100 −0.91697 0.40541 −0.93641 0.34202 A2 −0.94155 0.08440 −0.81679 0.09102 −0.87767 0.07790 A3 −0.92689 0.03717 −0.39472 0.03616 −0.67311 0.03140 R1 −0.99327 0.00832 −0.96960 0.00874 −0.99472 0.00211 R2 −0.99097 0.01910 −0.97629 0.01853 −0.99519 0.00465 C −0.85667 16.7274 −0.82857 22.3155 −0.84368 21.2446 表 4 不同条件下热解过程的活化能
Table 4 Activation Energies of Pyrolysis Processes under Different Conditions
Sample Heating
rate/(K·min−1)Activation energy
E/(kJ·mol−1)Pulverized coal char 5 76.17875 10 84.09264 15 97.56443 20 125.00554 Pulverized coal char:Alkali lignin =1∶1 5 16.74055 10 24.11223 15 23.7565 20 24.28225 Pulverized coal char:Alkali lignin =1∶3 5 14.01977 10 15.12626 15 15.46651 20 15.55402 表 5 氧载体与煤焦粉、煤焦粉/碱木质素混合体系反应后比表面积与粒径分布
Table 5 BET specific area and particle size distribution after the reaction of the oxygen carrier with pulverized coal char, pulverized coal char/alkali lignin mixture system
Oxygen carrier BET specific surface area/(m2·g−1) Particle size distribution/μm d10% d50% d90% NiFe2O4/C 2.38 5.8 108.9 904.8 NiFe2O4/CA 3.28 60.3 267.8 868.2 -
[1] 《中国统计年鉴−2017》, 中华人民共和国国家统计局编, 中国统计出版社,http://www.stats.gov.cn/tjsj/ndsj/2017/indexch.htm. [2] 栗艳平. 除尘焦粉回配炼焦的技术研究及应用[J]. 化工管理,2016,(26):208.JIA Yanping. Study on the comprehensive utilization of high-ash waste carbon powder for the preparation of carbonaceous reductants[J]. Chem Enterprise Management,2016,(26):208. [3] 宁哲. 综合利用高灰分废弃焦粉制备碳质还原剂的研究[D]. 昆明: 昆明理工大学, 2013.NING Zhe. Research on the comprehensive utilization of high-ash waste coke fines for the production of carbonaceous reductants[D]. Kunming: Kunming University of Science and Technology, 2013. [4] 林金良. 焦粉回配技术在焦化厂的应用探讨[J]. 山东工业技术,2017,(6):2.LIN Jinliang. Exploration of the application of coke fines recycling technology in coking plants[J]. Shandong Ind Technol,2017,(6):2. [5] 冯家俊, 袁本雄. 除尘焦粉配煤炼焦生产高炉用焦的新工艺应用[J]. 煤质技术,2017,(S1):33−36.FENG Jiajun, YUAN Benxiong. New technology application of coal blending coking using dust removal coke powder for production of blast furnace coke[J]. Coal Technol,2017,(S1):33−36. [6] 武海燕, 贾超雄, 马国顺. 粉焦成型制造气化用焦的研究[J]. 煤气与热力,2001,21(6):492−493.WU Haiyan , JIA Chaoxiong , MA Guoshun. Study on gasification coke made of coke powders[J]. Gas Heat,2001,21(6):492−493. [7] 郑明东, 晏善成, 何孝军. 焦粉的高附加值利用[J]. 燃料与化工,2007,38(2):21−23.ZHENG Mingdong, YAN Shangcheng, HE Xiaojun. High value-added-value utilization of coke fine[J]. Fuel Chem Proc,2007,38(2):21−23. [8] 张云, 许凯, 苏胜, 等. K-/Ca-/Fe-化合物对煤焦催化气化作用特性[J]. 煤炭学报,2007,38(11):2668−2673.ZHANG Yun, XU Kai, SU Sheng, et al. Characteristics of catalytic and gasification of coal by K-/Ca-/Fe-compunds[J]. J China Coal Soc,2007,38(11):2668−2673. [9] XIAO Y, XU S, TURSUN Y, et al. Catalytic steam gasification of lignite for hydrogen-rich gas production in a decoupled triple bed reaction system[J]. Fuel,2017,189:57−65. doi: 10.1016/j.fuel.2016.10.078 [10] 李海宾, 韩敏芳. 拜耳法赤泥催化煤焦-O2/CO2反应[J]. 煤炭技术,2014,33:309−311.(LI Haibin, HAN Minfang. Coal Char-O2/CO2 reaction catalyzed by bayer process red mud[J]. Coal Technol,2014,33:309−311. [11] 占均志. 造纸浓缩黑液木质素基木材胶粘剂的研究[D]. 南宁: 广西大学; 2013.ZHAN Junzhi. Research on lignin-based wood adhesive from papermaking concentrated black liquor[D]. Nanning: Guangxi University, 2013. [12] 王晓红, 赵谦. 造纸黑液中木质素在农业领域的应用[J]. 生物质化学工程,2004,(2):36−40.WANG Xiaohong, ZHAO Qian. Appliction of Linin from Pilping Black Liquor in Agriculture[J]. Biomass Chem Eng,2004,(2):36−40. [13] HANG Z, HE F, ZHAO K, et al. Natural iron ore as an oxygen carrier for biomass chemical looping gasification in a fluidized bed reactor[J]. J Therm Anal Calorim,2014,116:1315−24. doi: 10.1007/s10973-013-3630-1 [14] 孙云娟, 蒋剑春, 王燕杰, 等. Coats-Redfern积分法研究生物质与煤单独热解和共热解动力学特性[J]. 林产化学与工业,2014,34(5):8−14.SUN Yunjuan, JIANG Jianchun,WANG Yanjie, et al. Kinetic analysis of biomass and coal mono-pyrolysis as well as Co-pyrolysis by Coats-Redfern[J]. Chem Ind For Prod,2014,34(5):8−14. [15] 谢华清, 于庆波, 秦勤, 等. 生物质热解过程两种动力学分析方法的比较[J]. 东北大学学报(自然科学版),2013,34(6):845−848.XIE Huaqing, YU Qingbo, QIN Qin, et al. Comparison of two kinetic analysis methods for biomass pyrolysis processes[J]. J Northeastern Univ (Nat Sci),2013,34(6):845−848. [16] MALEK J, SESTAK J, ROUQUEROL F, et al. Possibilities of two non-isothermal procedures (temperature or rate-controlled) for kinetical studies[J]. J Therm Anal,1992,38(1/2):71−87. [17] 李少华, 柏静儒, 孙佰仲, 等. 升温速率对油页岩热解特性的影响[J]. 化学工程,2007,35(1):64−67. doi: 10.3969/j.issn.1005-9954.2007.01.017LI Shaohua, BAI Jingru, SUN Baizhong, et al. Effect of heating rate on the pyrolysis characteristics of oil shales[J]. Chem Eng,2007,35(1):64−67. doi: 10.3969/j.issn.1005-9954.2007.01.017 [18] 廖艳芬, 张横锦, 吴宇婷等. 碱金属对生物质化学链气化过程的影响[J]. 华南理工大学学报(自然科学版),2018,46(4):67−74.LIAO Yanfen, ZHANG Hengjin, WU Yuting, et al. Investigation of the influence of potassium on biomass chemical looping gasification[J]. J South China Univ Technol (Nat Sci Ed),2018,46(4):67−74. [19] MA Z, ZHANG S, XIAO R. Insights into the relationship between microstructural evolution and deactivation of Al2O3 supported Fe2O3 oxygen carrier in chemical looping combustion[J]. Energy Convers Manag,2019,188:429−437. [20] SHEN L, WU J, GAO Z, et al. Reactivity deterioration of NiO/Al2O3 oxygen carrier for chemical looping combustion of coal in a 10 kWth reactor[J]. Combust Flame,2009,156:1377−1385. [21] YU Z, LI C, FANG Y, et al. Reduction rate enhancements for coal direct chemical looping combustion with an iron oxide oxygen carrier[J]. Energy Fuels,2012,26:2505−2511. [22] 卫俊涛, 丁路, 周志杰等. 负载碳酸钾煤焦-CO2催化气化反应特性的原位研究[J]. 燃料化学学报,2015,43(11):1311−1319.WEI Juntao, DING Lu, ZHOU Zhijie, et al. In-situ analysis of catalytic gasification reaction characteristics of coal char-CO2 with K2CO3 additive[J]. J Fuel Chem Technol,2015,43(11):1311−1319. [23] 肖振华. K及Ca等碱金属对煤气化过程的产物调控研究[J]. 煤炭与化工,2017,40(5):72−74.XIAO Zhenhua. Product control research on K, Ca and other alkali metal on coal gasification process[J]. Coal Chem Ind,2017,40(5):72−74. [24] 周玉飞, 沈来宏, 顾海明等. 生物质灰对铁矿石载氧体性能的影响[J]. 东南大学学报(自然科学版),2015,45(3):503−508. doi: 10.3969/j.issn.1001-0505.2015.03.016ZHOU Yufei, SHEN Laihong, GU Haiming, et al. Effect of biomass ash on performance of iron ore as oxygen carrier in chemical looping combustion[J]. J Southeast Univ (Nat Sci Ed),2015,45(3):503−508. doi: 10.3969/j.issn.1001-0505.2015.03.016 [25] 黄祥能. 生物质化学链重整气化受碱(土)金属作用的机制研究[D]. 广州: 华南农业大学, 2023.HUANG Xiangneng. Study on mechanism of biomass chemical looping reforming and gasification by alkali metal and alkaline earth metal[D]. Guangzhou: South China Agricultural University. 2023. [26] XIANG D, JIN T, LEI X, et al. The high efficient synthesis of natural gas from a joint-feedstock of coke-oven gas and pulverized coke via a chemical looping combustion scheme[J]. Appl Energy,2018,212:944−954. doi: 10.1016/j.apenergy.2017.12.095 -




 下载:
下载: