| [1] |
李缔, 李攀, 王贤华, 等. 基于Fe负载的HZSM-5催化热解制备生物油实验研究[J]. 燃料化学学报,2016,44(5):540−547.LI Di, LI Pan, WANG Xianhua, et al. Experimental study on bio-oil from catalytic pyrolysis on Fe modified HZSM-5[J]. J Fuel Chem Technol,2016,44(5):540−547.
|
| [2] |
魏珺楠, 唐兴, 孙勇, 等. 新型生物质基平台分子γ-戊内酯的应用[J]. 化学进展,2016,28(11):1672−1681.WEI Junnan, TANG Xing, SUN Yong, et al. Applications of novel biomass-derived platform molecule γ-valerolactone[J]. Chem Ind Eng Prog,2016,28(11):1672−1681.
|
| [3] |
海雪清, 谭静静, 何静, 等. CuCo双金属催化剂催化糠醛加氢制备1, 5-戊二醇的研究[J]. 燃料化学学报,2023,51(7):959−969. doi: 10.1016/S1872-5813(23)60334-2HAI Xueqing, TAN Jinging, HE Jing, et al. Hydrogenation of furfural to 1, 5-pentanediol over CuCo bimetallic catalysts[J]. J Fuel Chem Technol,2023,51(7):959−969. doi: 10.1016/S1872-5813(23)60334-2
|
| [4] |
李微, 贡红辉, 史显磊. 催化转移氢化制生物质基2, 5-呋喃二甲醇研究进展[J]. 燃料化学学报,2023,52(x):1−21.LI Wei, GONG Honghui, SHI Xianlei. Recent advances in preparing biomass-based 2, 5-bis(hydroxymethyl)furan by catalytic transfer hydrogenation[J]. J Fuel Chem Technol,2023,52(x):1−21.
|
| [5] |
林鹿, 何北海, 孙润仓, 等. 木质生物质转化高附加值化学品[J]. 化学进展,2007,19(7/8):1206−1216.LIN Lu, HE Beihai, SUN Runcang, et al. High-value chemicals from lignocellulosic biomass[J]. Chem Ind Eng Prog,2007,19(7/8):1206−1216.
|
| [6] |
韩冬, 孙来芝, 陈雷, 等. 生物质与塑料共催化热解制备芳烃化合物研究进展[J]. 燃料化学学报,2023,52(x):1−15.HAN Dong, SUN Laizhi1, CHEN Lei, et al. Review on the progress in the production of aromatic hydrocarbons by Co-catalytic pyrolysis of biomass and plastics[J]. J Fuel Chem Technol,2023,52(x):1−15.
|
| [7] |
张军, 李丹妮, 袁浩然, 等. 生物质基糠醛和5-羟甲基糠醛加氢转化研究进展[J]. 燃料化学学报,2021,49(12):1752−1767. doi: 10.1016/S1872-5813(21)60135-4ZHANG Jun, LI Danni, YUAN Haoran, et al. Advances on the catalytic hydrogenation of biomass-derived furfural and 5- hydroxymethylfurfural[J]. J Fuel Chem Technol,2021,49(12):1752−1767. doi: 10.1016/S1872-5813(21)60135-4
|
| [8] |
LI K M, ZHANG Q, XU Z M, et al. Tunable mono- and di-methylation of amines with methanol over bimetallic CuCo nanoparticle catalysts. Green Chem, 2022, 24(15): 5965-5977.
|
| [9] |
CHEN J Y, HUANG Y B, HU B, et al. A bio-based click reaction leading to the dihydropyridazinone platform for nitrogen-containing scaffolds. Green Chem, 2023, 25(7): 2672-2680.
|
| [10] |
LI H, GUO H X, Fang Z, et al. Cycloamination strategies for renewable N-heterocycles[J]. Green Chem,2020,22(3):582−611. doi: 10.1039/C9GC03655E
|
| [11] |
HULSEY M J, YANG H Y, YAN N. Sustainable routes for the synthesis of renewable heteroatom-containing chemicals[J]. ACS Sustain Chem Eng,2018,6(5):5694−5707. doi: 10.1021/acssuschemeng.8b00612
|
| [12] |
代金杭, 蔡雅洁. 由甲壳素生物质合成含氮化学品研究进展[J]. 化学研究与应用,2020,32(7):1111−1116. doi: 10.3969/j.issn.1004-1656.2020.07.002DAI Jinhang, CAI Yajie. Research progressof nitrogen-containing chemicals production employing chitin biomass as substrate[J]. Chemical research and application,2020,32(7):1111−1116. doi: 10.3969/j.issn.1004-1656.2020.07.002
|
| [13] |
苏文韬, 李昌志. 木质素制备含氮杂环芳香化合物研究进展[J]. 湖南师范大学自然科学学报,2023,46(4):1−13.SU Wentao, LI Changzhi. Progress on the production of nitrogen-containing heterocyclic aromatic compounds from lignin[J]. Journal of Natural Science of Hunan Normal University,2023,46(4):1−13.
|
| [14] |
ZHANG X, XU S Q, LI Q F, et al. Recent advances in the conversion of furfural into bio-chemicals through chemo- and bio-catalysis[J]. RSC Adv,2021,11(43):27042−27058. doi: 10.1039/D1RA04633K
|
| [15] |
谭静静, 苏以豪, 高宽, 等. 糠醛及其衍生物选择性加氢制备戊二醇的研究进展[J]. 燃料化学学报,2021,49(6):780−790. doi: 10.1016/S1872-5813(21)60036-1TAN Jingjing, SU Yihao, GAO Kuan, et al. Recent advances in the selective hydrogenation of furfural and its derivatives to pentanediol[J]. J Fuel Chem Technol,2021,49(6):780−790. doi: 10.1016/S1872-5813(21)60036-1
|
| [16] |
QI H F, YANG J, LIU F, et al. Highly selective and robust single-atom catalyst Ru1/NC for reductive amination of aldehydes/ketones[J]. Nat Commun,2021,12(1):3295. doi: 10.1038/s41467-021-23429-w
|
| [17] |
GAO Z X, CAI L Y, MA H R, et al. Dual scale hydrogen transfer bridge construction for biomass tandem reductive amination[J]. ACS Catal,2023,13(19):12835−12847. doi: 10.1021/acscatal.3c03486
|
| [18] |
CHATTERJEE M, ISHIZAKA T, KAWANAMI H. Reductive amination of furfural to furfurylamine using aqueous ammonia solution and molecular hydrogen: an environmentally friendly approach[J]. Green Chem,2016,18(2):487−496. doi: 10.1039/C5GC01352F
|
| [19] |
SONG W J, WAN Y J, LI Y F, et al. Electronic Ni–N interaction enhanced reductive amination on an N-doped porous carbon supported Ni catalyst[J]. Catal Sci Technol,2022,12(23):7208−7218. doi: 10.1039/D2CY01551J
|
| [20] |
ZOU H T, CHEN J Z. Efficient and selective approach to biomass-based amine by reductive amination of furfural using Ru catalyst[J]. Appl Catal B:Environ,2022,309:121262. doi: 10.1016/j.apcatb.2022.121262
|
| [21] |
JIANG S, RAMDANI W, MULLER E, et al. Direct catalytic conversion of furfural to furan-derived amines in the presence of Ru-based catalyst[J]. ChemSusChem,2020,13(7):1699−1704. doi: 10.1002/cssc.202000003
|
| [22] |
JIANG S, MA C R, MULLER E, et al. Selective synthesis of THF-derived amines from biomass-derived carbonyl compounds[J]. ACS Catal,2019,9(10):8893−8902. doi: 10.1021/acscatal.9b03413
|
| [23] |
LIN C C, ZHOU J M, ZHENG Z F, et al. An efficient approach to biomass-based tertiary amines by direct and consecutive reductive amination of furfural[J]. J Catal,2022,410:164−179. doi: 10.1016/j.jcat.2022.04.016
|
| [24] |
DAS A K, NANDY S, BHAR S. Cu(OAc)2 catalysed aerobic oxidation of aldehydes to nitriles under ligand-free conditions[J]. RSC Adv,2022,12(8):4605−4614. doi: 10.1039/D1RA07701E
|
| [25] |
CHEN H, SUN S J, XI H Y, et al. Catalytic oxidative conversion of aldehydes into nitriles using NH3·H2O/FeCl2/NaI/Na2S2O8: A practical approach to febuxostat[J]. Tetrahedron Lett,2019,60(21):1434−1436. doi: 10.1016/j.tetlet.2019.04.043
|
| [26] |
HUA M L, SONG J L, HUANG X, et al. Highly efficient oxidative cyanation of aldehydes to nitriles over Se, S, N- tri-doped hierarchically porous carbon nanosheets[J]. Angew Chem Int Ed Engl,2021,60(39):21479−21485. doi: 10.1002/anie.202107996
|
| [27] |
PAN L M, FU W Q, ZHANG L, et al. Highly dispersed Co species in N-doped carbon enhanced the aldehydes ammoxidation reaction activity[J]. Mol Catal,2022,518:112087. doi: 10.1016/j.mcat.2021.112087
|
| [28] |
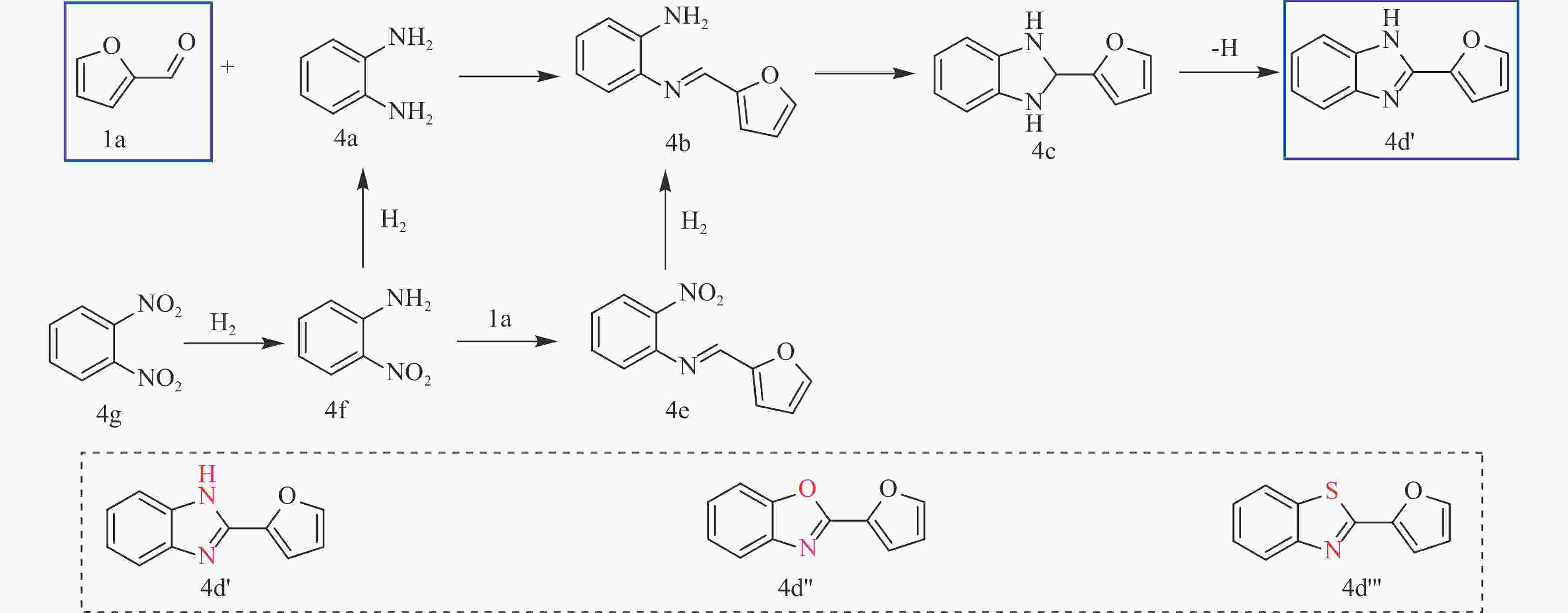
YANG S W, CHEN J Z. Kinetic analysis of consecutive/parallel transformation of furfural to biomass-based primary amide by using a “concentration–time” integral[J]. ACS Catal,2022,13(1):113−131.
|
| [29] |
KIDWAI M, BANSAL V, SAXENA A, et al. Cu-nanoparticles: efficient catalysts for the oxidative cyclization of Schiffs’ bases[J]. Tetrahedron Lett,2006,47(46):8049−8053. doi: 10.1016/j.tetlet.2006.09.066
|
| [30] |
ZHOU Y K, LIU W, LIU Y C, et al. Oxidative NHC catalysis for base-free synthesis of benzoxazinones and benzoazoles by thermal activated NHCs precursor ionic liquid catalyst using air as oxidant[J]. Mol Catal,2020,492:111013. doi: 10.1016/j.mcat.2020.111013
|
| [31] |
CHARI M A, SHOBHA D, SASAKI T. Room temperature synthesis of benzimidazole derivatives using reusable cobalt hydroxide (II) and cobalt oxide (II) as efficient solid catalysts[J]. Tetrahedron Lett,2011,52(43):5575−5580. doi: 10.1016/j.tetlet.2011.08.047
|
| [32] |
LIN C C, WAN W H, WEI X T, et al. H2 activation with Co nanoparticles encapsulated in N-doped carbon nanotubes for green synthesis of benzimidazoles[J]. ChemSusChem,2021,14(2):709−720. doi: 10.1002/cssc.202002344
|
| [33] |
TANAKA S, ASHIDA K, TATSUTA G, et al. Preparation of fluorescent materials from biomass-derived furfural and natural amino acid cysteine through cross-coupling reactions for extended π-conjugation[J]. Synlett,2015,26(11):1496−1500. doi: 10.1055/s-0034-1380460
|
| [34] |
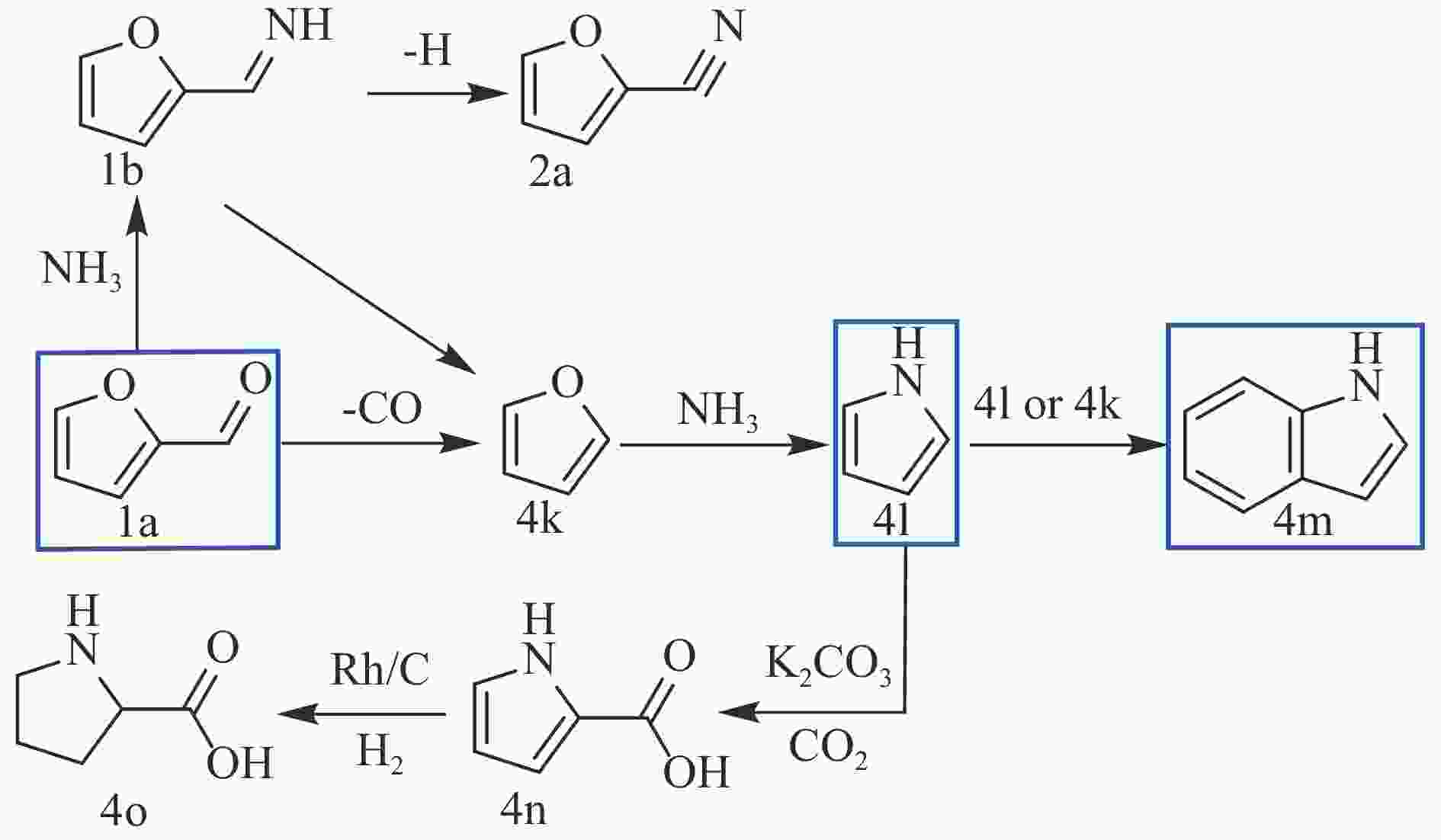
SONG S, YUEN V F K, DI L, et al. Integrating biomass into the organonitrogen chemical supply chain: production of pyrrole and D-proline from furfural[J]. Angew Chem Int Ed Engl,2020,59(45):19846−19850. doi: 10.1002/anie.202006315
|
| [35] |
YAO Q, Xu L J, Han Z, et al. Production of indoles via thermo-catalytic conversion and ammonization of bio-derived furfural[J]. Chem Eng J,2015,280:74−81. doi: 10.1016/j.cej.2015.05.094
|
| [36] |
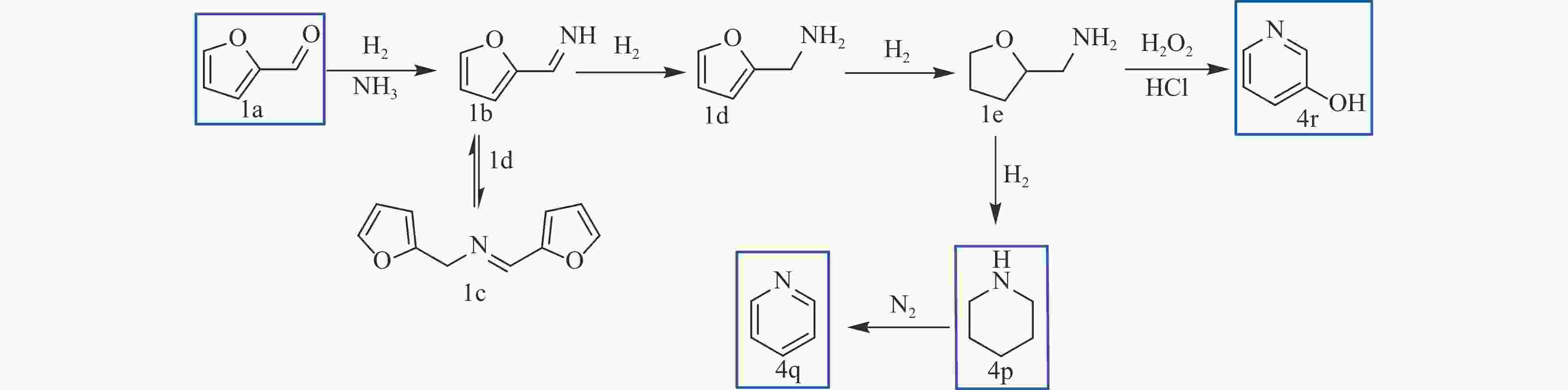
QI H F, LI Y R, ZHOU Z T, et al. Synthesis of piperidines and pyridine from furfural over a surface single-atom alloy Ru1CoNP catalyst[J]. Nat Commun,2023,14(1):6329. doi: 10.1038/s41467-023-42043-6
|
| [37] |
MULLER C, DIEHL V, LICHTENTHALER F W. Building blocks from sugars: Part. 23. : Hydrophilic 3-pyridinols from fructose and isomaltulose[J]. Tetrahedron,1998,54(36):10703−10712. doi: 10.1016/S0040-4020(98)00634-6
|





 下载:
下载: