Study on the impact of using decarbonized gasification slag for CO2 mineralization and storage to prepare calcium carbonate
-
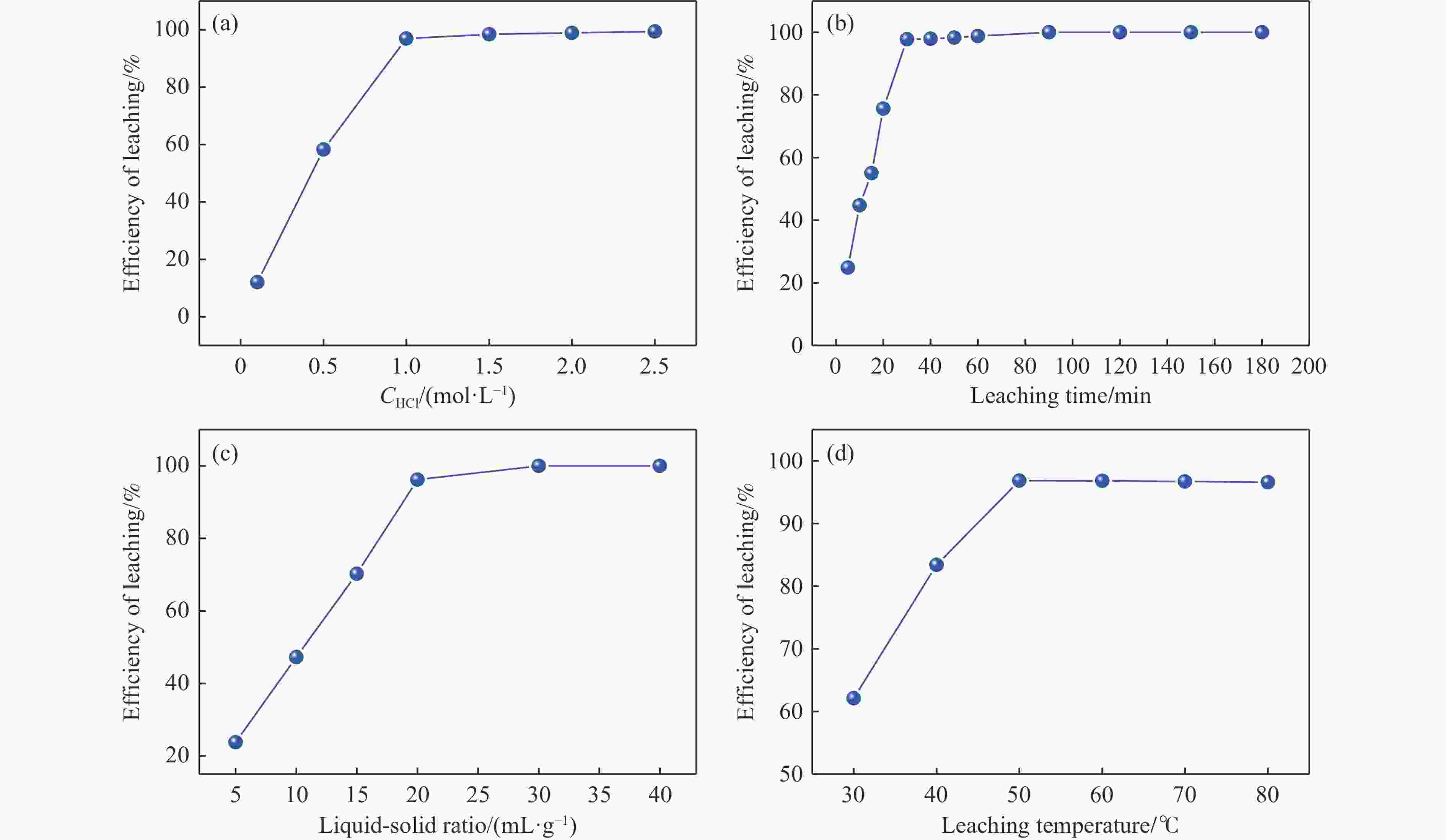
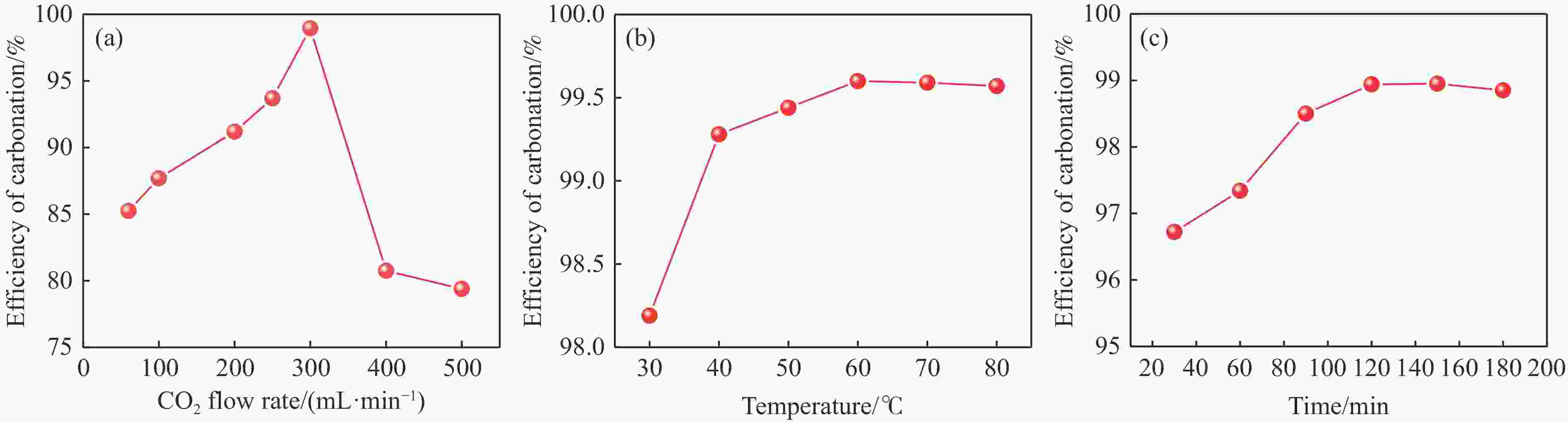
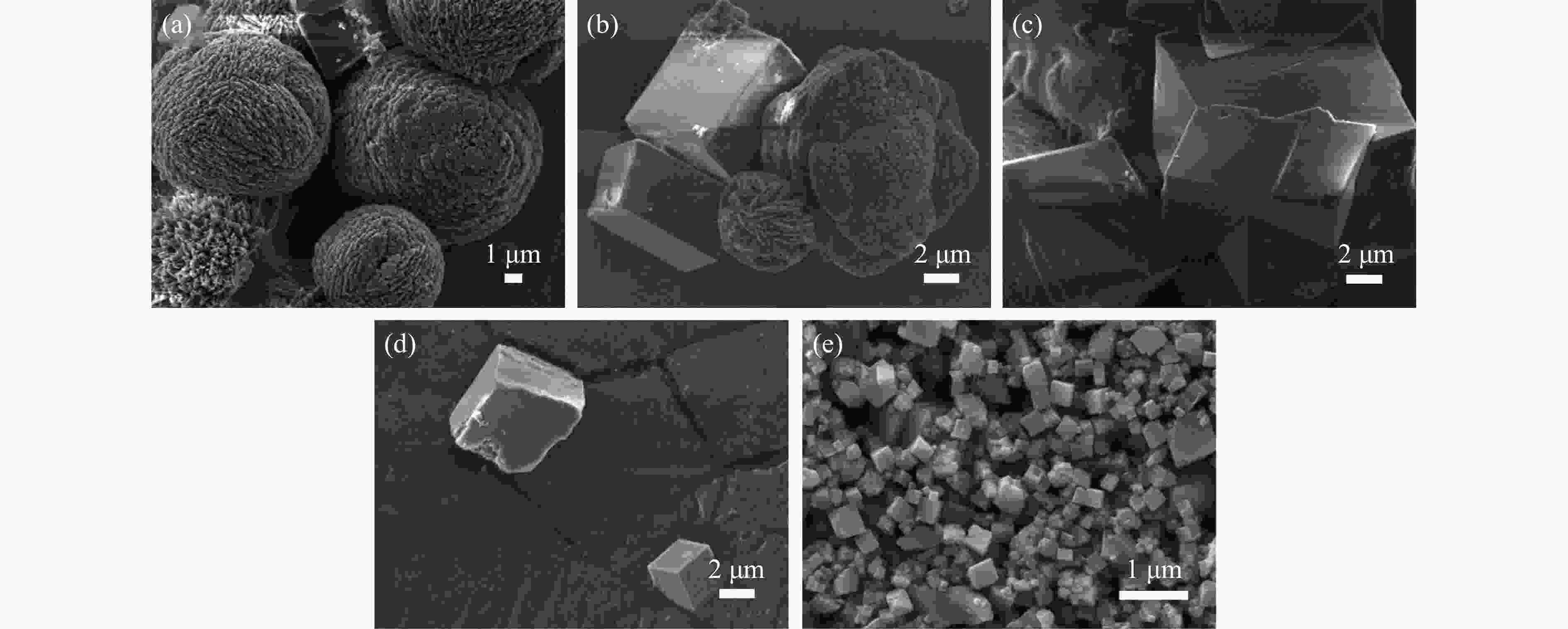
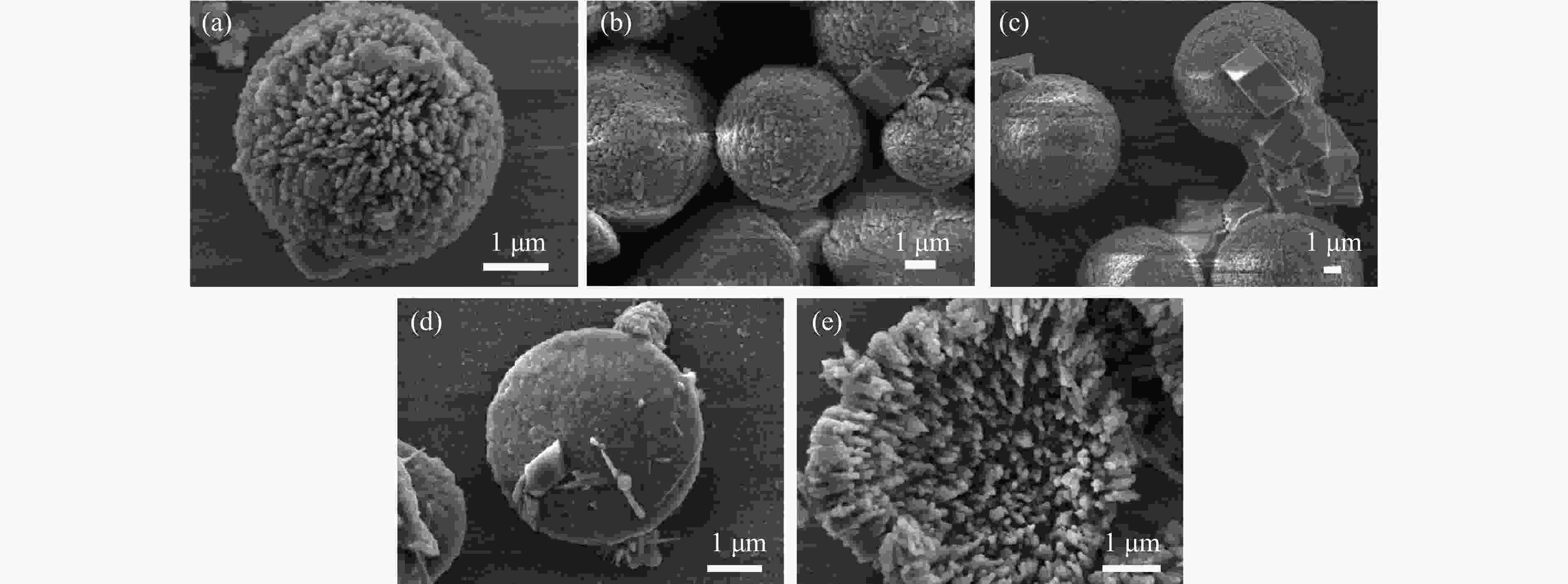
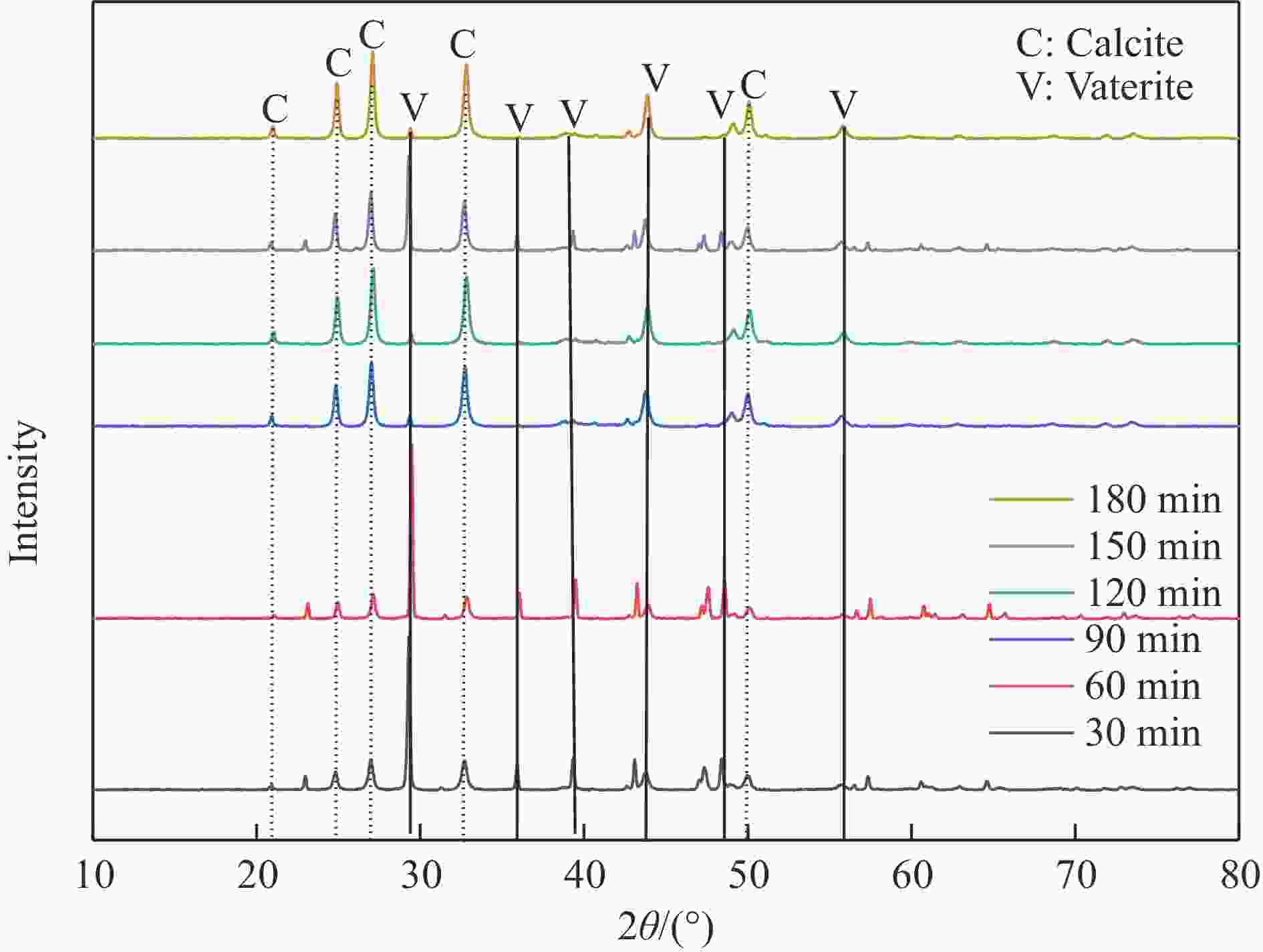
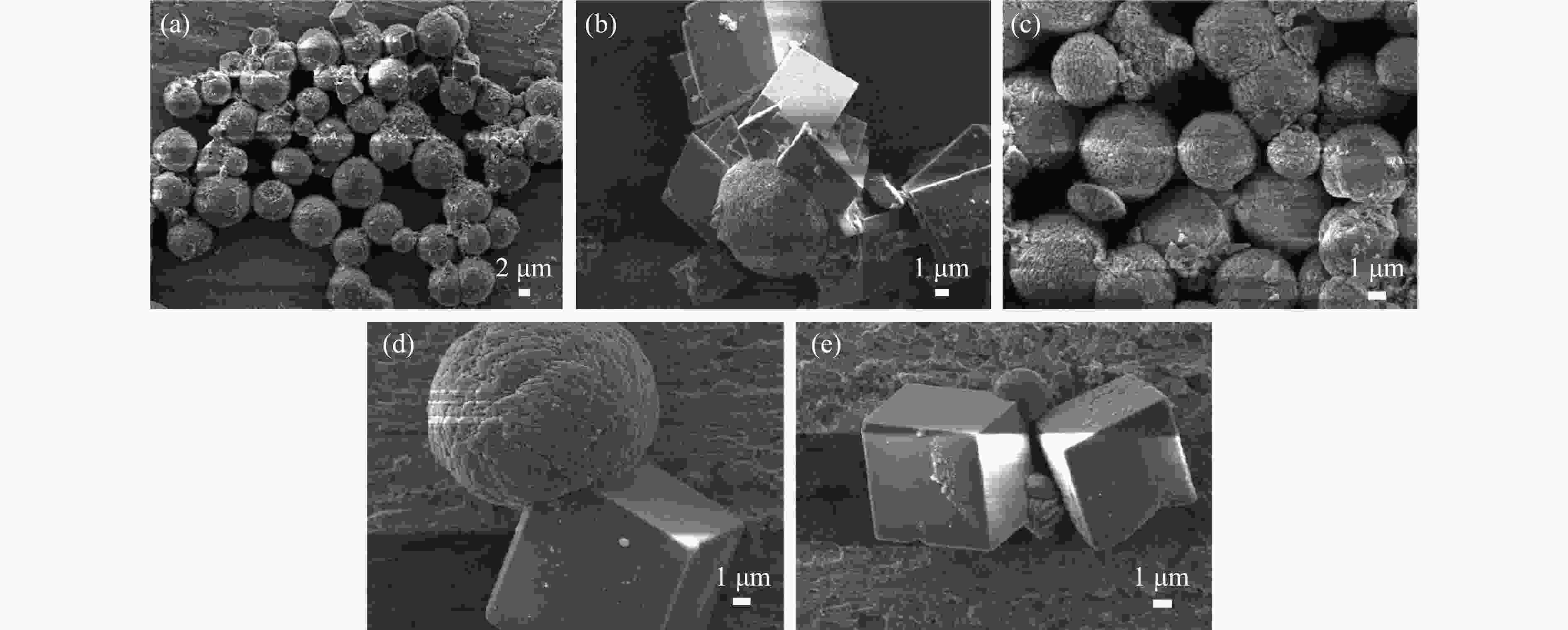
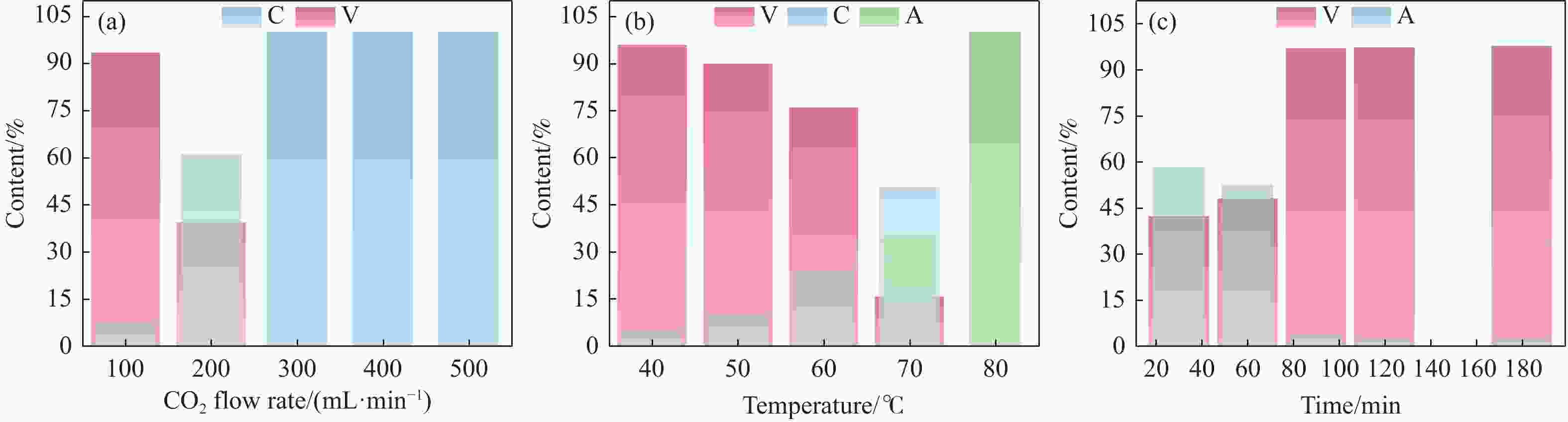
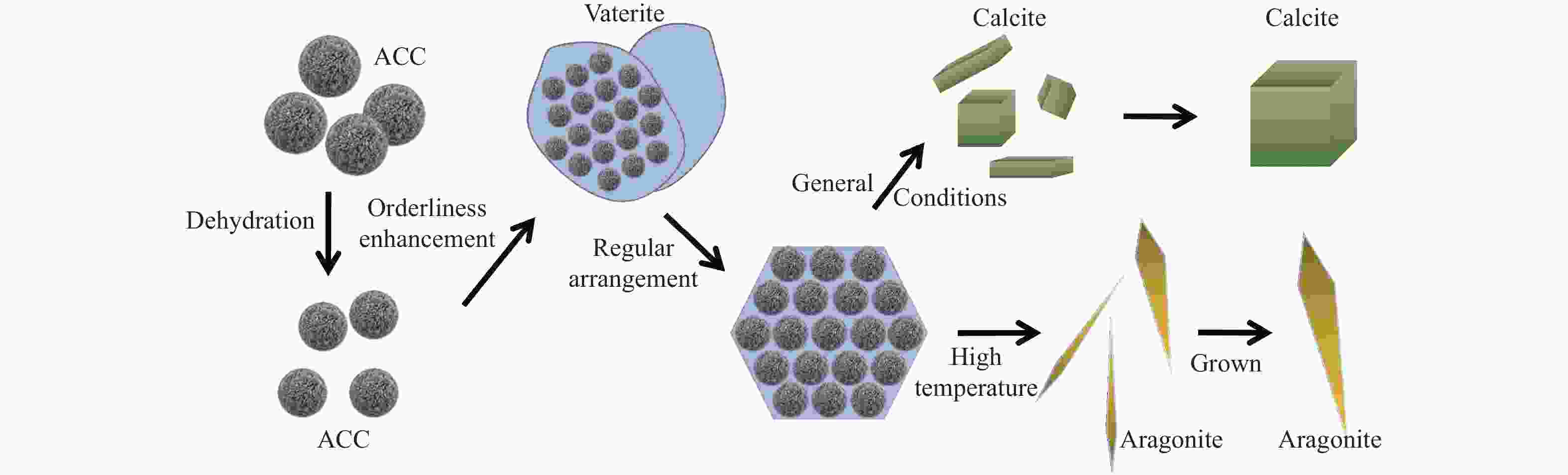
摘要: 本实验详细研究了浸出剂种类、浓度、反应时间、温度和液固比等对脱碳气化渣中钙浸出率的影响,并讨论了CO2流量、温度、碳酸化时间对碳酸化效率和生成的沉淀碳酸钙(PCC)晶型结构的影响规律。结果表明,在2 mol/L 盐酸、液固比为20 mL/g、反应温度为50 ℃、反应时间为90 min的浸出条件下,钙浸出率最高,为98.79%。在碳酸化阶段,CO2流量主要影响碳酸化效率,通过优化碳酸化反应条件,最高碳酸化效率可达99.59%。而反应温度和时间则会对碳酸钙晶型和形貌产生显著影响,降低反应温度和缩短反应时间更有利于球霰石型碳酸钙的生成。Abstract: The gasification slag after carbon separation is difficult to realize effective utilization because of its high content of water and the presence of a small amount of residual carbon. To address these problems, a mineralization based on indirect carbonation to sequester CO2 and recycle calcium extraction to prepare nano-calcium carbonate process is proposed. The gasification slag after carbon separation mainly consists of CaO, Al2O3, Fe2O3, MgO, as well as some non-metallic components such as SiO2. Most of the metal components exist in amorphous form. After preliminary screening of acidic leaching agents, it was found that hydrochloric acid can effectively destroy the structure of gasification slag and dissolve the metal components in gasification slag. In this paper, the effects of leaching agent type, concentration, reaction time, temperature and liquid-solid ratio on the leaching rate of calcium from decarbonized gasification slag were investigated in detail. The results showed that the highest calcium leaching rate of 98.79% was achieved under the leaching conditions of 2 mol/L HCl, liquid-to-solid ratio of 20 mL/g, reaction temperature of 50 ℃ and reaction time of 90 min. Meanwhile, the effects of CO2 flow, temperature and time on the carbonation efficiency and precipitated calcium carbonate (PCC) crystal structure were investigated. In the carbonation stage, the main factor affecting carbonation efficiency is CO2 flow. This is because excessive CO2 will cause carbonic acid to form in the solution and partially dissolve the precipitated CaCO3, resulting in a sharp decrease in carbonation efficiency. And the carbonation efficiency gradually increases with the increase of reaction temperature. Generally speaking, increasing the temperature is beneficial for chemical reactions. However, owing to the exothermic nature of the carbonation reaction, the positive promotion effect of high temperature on the reaction process is weakened, and the solubility of CO2 in water is reduced, resulting in a slow decrease in carbonation efficiency. The effect of reaction time on carbonation process has the same trend as the change in reaction temperature. The highest carbonation efficiency could reach 99.59% by optimizing the carbonation reaction conditions. In addition, the reaction temperature and time significantly affected the calcium carbonate crystal structure and micromorphology. The formation of calcium carbonate crystals mainly goes through three stages. In the first stage, as the reaction time prolongs, disordered amorphous calcium carbonate rapidly dehydrates to form ordered calcium carbonate crystal structure. At high supersaturation, vaterite begins to nucleate and undergoes spherical growth through nucleation at the growth front. Gradually, the solubility of amorphous calcium carbonate gradually decreases, and vaterite continues to grow into polycrystalline spheres composed of roughly equal sized crystals. In the second stage, vaterite is formed under equilibrium conditions, and its crystal size almost no longer increases, leaving part of the remaining amorphous calcium carbonate dissolution and crystallization process. In the third stage, vaterite begins to decompose and forms calcite or aragonite through dissolution-recrystallization process. Experiments result have shown that vaterite and calcite are formed at low temperatures, and aragonite is formed when heated to a certain temperature. As the reaction time increases, the particle size of calcium carbonate gradually increases. Therefore, lowering the reaction temperature and time is more favorable to the formation of vaterite type calcium carbonate.
-
表 1 脱碳气化渣的灰成分分析和烧失量
Table 1 Ash composition analysis and burn loss of decarburized gasification slag
Composition Al2O3 Fe2O3 CaO MgO SiO2 SO3 Others Loss Content/% 19.13 23.65 8.82 5.00 25.94 13.64 3.82 8.30 表 2 不同浸出剂条件下滤液中的主要金属离子浓度对比
Table 2 Comparison of major metal ion concentrations in filtrates under different leaching agent conditions
Leaching agent $I_{\mathrm{C}}^+ $/(mg·L−1) $I_{\mathrm{C}}^- $/(mg·L−1) $I_{\mathrm{A}}^+ $/(mg·L−1) $I_{\mathrm{A}}^- $/(mg·L−1) $I_{\mathrm{F}}^+ $/(mg·L−1) $I_{\mathrm{F}}^- $/(mg·L−1) NH4Cl 24.04 1.53 36.22 <0.001 25.33 <0.001 CH3COONH4 0.03 <0.001 <0.001 NH4HSO4 0.30 <0.001 <0.001 (NH4)2CO3 0.01 <0.001 <0.001 CH3COOH 3.61 <0.001 <0.001 HCl 17.70 21.49 24.63 I(C/A/F)+: measured in decarburization gasification slag (mg·L−1); I(C/A/F)−: Measured in leachate (mg/L); C: Ca2+, A: Al3+, F: Fe3+. -
[1] International Energy Agency (IEA). Global energy & CO2 status report [EB/OL]. (2019-03-26)[2020-12-20]. [2] WEE J H. A review on carbon dioxide capture and storage technology using coal fly ash[J]. Appl Energy,2013,106:143−151. doi: 10.1016/j.apenergy.2013.01.062 [3] SEIFRITZ W. CO2 Disposal by means of silicates[J]. Nature,1990,345(7):486−486. [4] ZHANG Z, BORHANI TN, OLABI AG. Status and perspective of CO2 absorption process[J]. Energy,2020,205:1−16. [5] 张建树, 张荣, 毕继诚. CO2 矿化反应基础研究 II. 有机胺介质中碳酸化反应热力学研究[J]. 燃料化学学报(中英文),2011,39(11):871−875.ZHANG Jianshu, ZHANG Rong, BI Jicheng. Fundamental research on CO2 mineralization II. The thermodynamic equilibrium of carbonation in ternary amine[J]. J Fuel Chem Technol, 2,2011,39(11):871−875 [6] REDDY K, JOHN S, WEBER H, et al. Simultaneous capture and mineralization of coal combustion flue gas carbon dioxide (CO2)[J]. Energy Procedia,2011,4:1574−1583. doi: 10.1016/j.egypro.2011.02.027 [7] DILMORE R, LU P, ALLEN D, et al. Sequestration of CO2 in mixtures of bauxite residue and saline wastewater[J]. Energy Fuels.,2007,22:343−353. [8] BERTOS M, Li X, SIMONS S, et al. Investigation of accelerated carbonation for the stabilisation of MSW incinerator ashes and the sequestration of CO2[J]. Green Chem,2004,6(8):428−436. doi: 10.1039/B401872A [9] PARK A, Fan L. CO2 mineral sequestration: physically activated dissolution of serpentine and pH swing process[J]. Chem Eng Sci, 2004. 59(22−23): 5241−5247. [10] SANNA A, DRI M, HALL M, et al. Waste materials for carbon capture and storage by mineralisation (CCSM)–A UK perspective[J]. Appl. Energy, 2012, 99: 545–554. [11] ZEVENHOVEN R, FAGERLUND J, SONGOK J, et al. CO2 mineral sequestration: developments toward large-scale application[J]. Greenhouse Gases:Sci Technol,2011,1(1):48−57. doi: 10.1002/ghg3.7 [12] REN S, ALDAHRI T, LIU W, et al. CO2 mineral sequestration by using blast furnace slag: from batch to continuous experiments[J]. Energy,2021,214:1−10. [13] GAO X, GUO XL, GONG X. Characterization of slag from entrained-flow coal gasification[J]. J East China Univ Sci Technol,2009,35(5):677−683. [14] LIU C, LI Y, SUN R, et al. Cyclic CO2 capture of carbide slag modified by pyroligneous acid in calcium looping cycles[J]. Asia Pac J Chem Eng,2014,9(5):678−685. doi: 10.1002/apj.1799 [15] UKWATTAGE N, RANJITH P, LI X. Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation[J]. Measurement,2017,97:15−22. doi: 10.1016/j.measurement.2016.10.057 [16] MUN M, CHO H. Combustibility analysis of high-carbon fine slags from an entrained flow gasifier[J]. Energy Procedia.,2013,37:6999−7005. doi: 10.1016/j.egypro.2013.06.633 [17] XUAN D, ZHAN B, POON C, et al. Carbon dioxide sequestration of concrete slurry waste and its valorisation in construction products[J]. Constr Build Mater,2016,113:664−672. doi: 10.1016/j.conbuildmat.2016.03.109 [18] YADAV V, PRASAD M, KHAN J, et al. Sequestration of carbon dioxide (CO2) using red mud[J]. J Hazard Mater,2010,176(1-3):1044−1050. doi: 10.1016/j.jhazmat.2009.11.146 [19] YU C, CAI L, JIANG G, et al. Mineral carbonation of CO2 with utilization of coal gasification slags based on chemical looping[J]. Asia-Pac. J Chem Eng,2021,16(4):1−11. [20] 范宁, 张逸群, 樊盼盼, 等. 煤气化渣特性分析及资源化利用研究进展[J]. 洁净煤技术,2022,28(8):145−154.FAN Ning, ZHANG Yiqun, FAN Panpan, et al. Research progress on characteristic analysis and resource utilization of coal gasification slag[J]. Clean Coal Technol,2022,28(8):145−154. [21] WANG H, WANG H, LIU G, et al. In-situ pyrolysis of Taihu blue algae biomass as appealing porous carbon adsorbent for CO2 capture: Role of the intrinsic N[J]. Sci Total Environ,2021,771:1−8. [22] PARK H, KANG J, PARK J, et al. One-pot synthesis of novel porous carbon adsorbents derived from poly vinyl chloride for high methane adsorption uptake[J]. Chem Eng J,2022,440:1−9. [23] 马子涵, 陈娅, 孙睿, 等. 多孔聚合物制备多孔炭及其应用进展[J]. 当代化工研究,2022,22:10−12. doi: 10.3969/j.issn.1672-8114.2022.15.003MA Zihan, CHEN Ya, SUN Rui, et al. Preparation of Porous Carbon by Porous Polymer and Its Application Progress[J]. Modern Chemical Research,2022,22:10−12. doi: 10.3969/j.issn.1672-8114.2022.15.003 [24] 张建树, 张荣, 毕继诚. CO2 矿化反应基础研究 I. 镁橄榄石和蛇纹石盐酸浸出动力学研究[J]. 燃料化学学报(中英文),2011,39(9):706−711.ZHANG Jianshu, ZHANG Rong, BI Jicheng. Fundamental research on CO2 mineralization I: Leaching kinetics of forsterite and serpentine with hydrochloric acid[J]. J Fuel Chem Technol,2011,39(9):706−711. [25] 王中辉, 苏胜, 马智伟, 等. 混合胺溶液耦合 CaO 吸收-矿化 CO2 特性及矿化过程关键影响因素研究[J]. 燃料化学学报(中英文),2022,50(10):1371−1379. doi: 10.1016/S1872-5813(22)60020-3WANG Zhonghui, SU Sheng, MA Zhiwei, et al. Study on CO2 absorption-mineralization characteristics of mixed amine solution coupled with CaO and key influencing factors in mineralization process[J]. J Fuel Chem Technol,2022,50(10):1371−1379. doi: 10.1016/S1872-5813(22)60020-3 [26] CHANG R. , CHOI D, KIM M, et al. Tuning crystal polymorphisms and structural investigation of precipitated calcium carbonates for CO2 mineralization[J]. ACS Sustainable Chem Eng,2017,5(2):1659−1667. doi: 10.1021/acssuschemeng.6b02411 [27] IZUKA A. , FUJII M, YAMASAKI A, et al. Development of a new CO2 sequestration process utilizing the carbonation of waste cement[J]. Ind Eng Chem Res,2004,43(24):7880−7887. doi: 10.1021/ie0496176 [28] SPANOS N, KOUTSOUKOS P. The transformation of vaterite to calcite: effect of the conditions of the solutions in contact with the mineral phase[J]. J Cryst Growth,1998,191(4):783−790. doi: 10.1016/S0022-0248(98)00385-6 [29] OGINO T, SUZUKI T, SAWADA K. The formation and transformation mechanism of calcium carbonate in water[J]. Geochim Cosmochim Acta,1987,51(10):2757−2767. doi: 10.1016/0016-7037(87)90155-4 [30] RODRIGUEZ J, SHAW S, BENNING L. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystal-lization to calcite, via vaterite[J]. Nanoscale,2011,3:265−271. doi: 10.1039/C0NR00589D [31] WEI H, SHEN Q, ZHAO Y, et al. Influence of polyvinylpyrrolidone on the precipitation of calcium carbonate and on the transformation of vaterite to calcite[J]. J Cryst Growth,2003,250(3-4):516−524. doi: 10.1016/S0022-0248(02)02484-3 -





 下载:
下载: