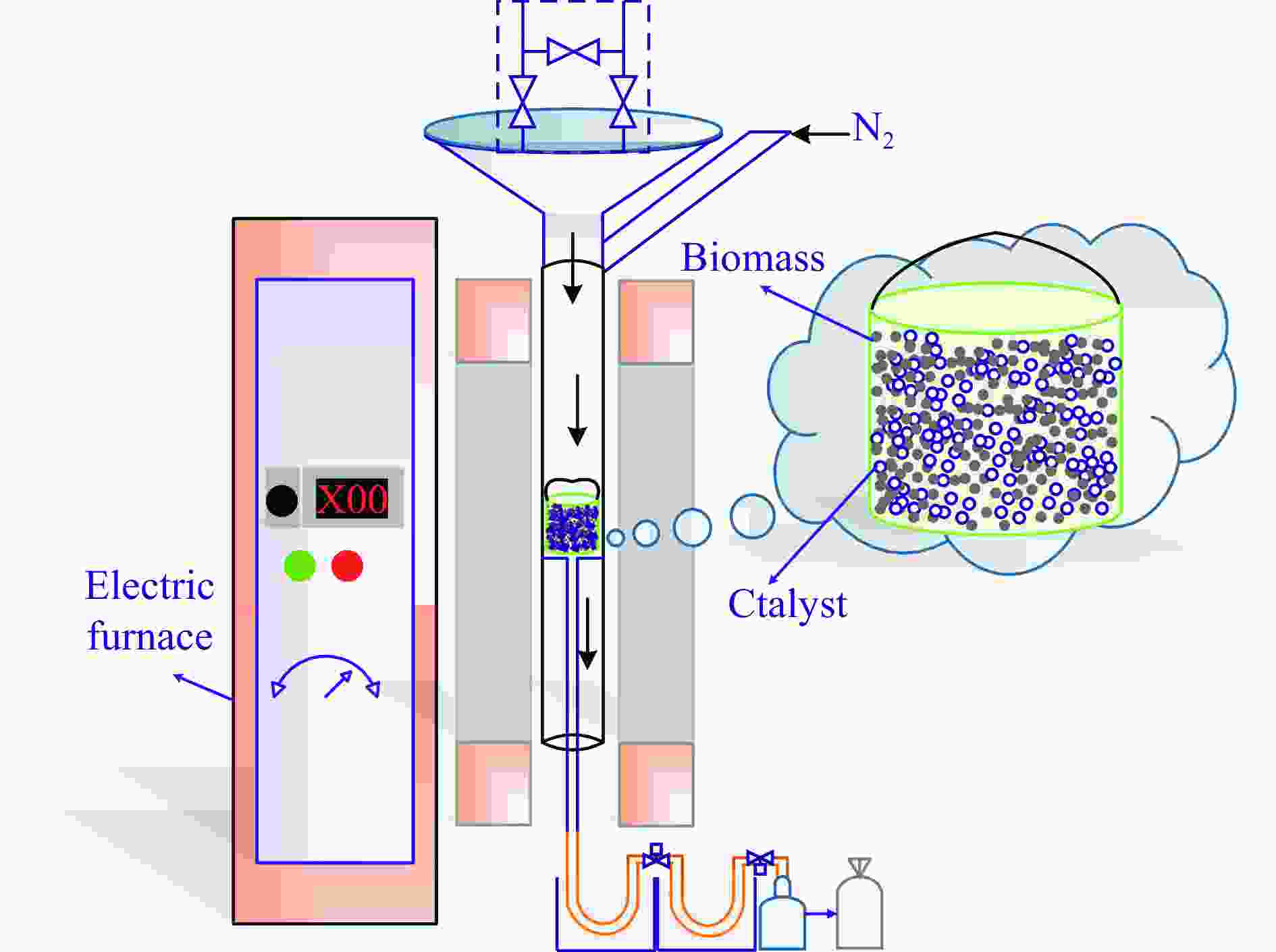
Catalytic pyrolysis of waste biomass to produce hydrogen-rich gas:Influence of catalyst performance
-
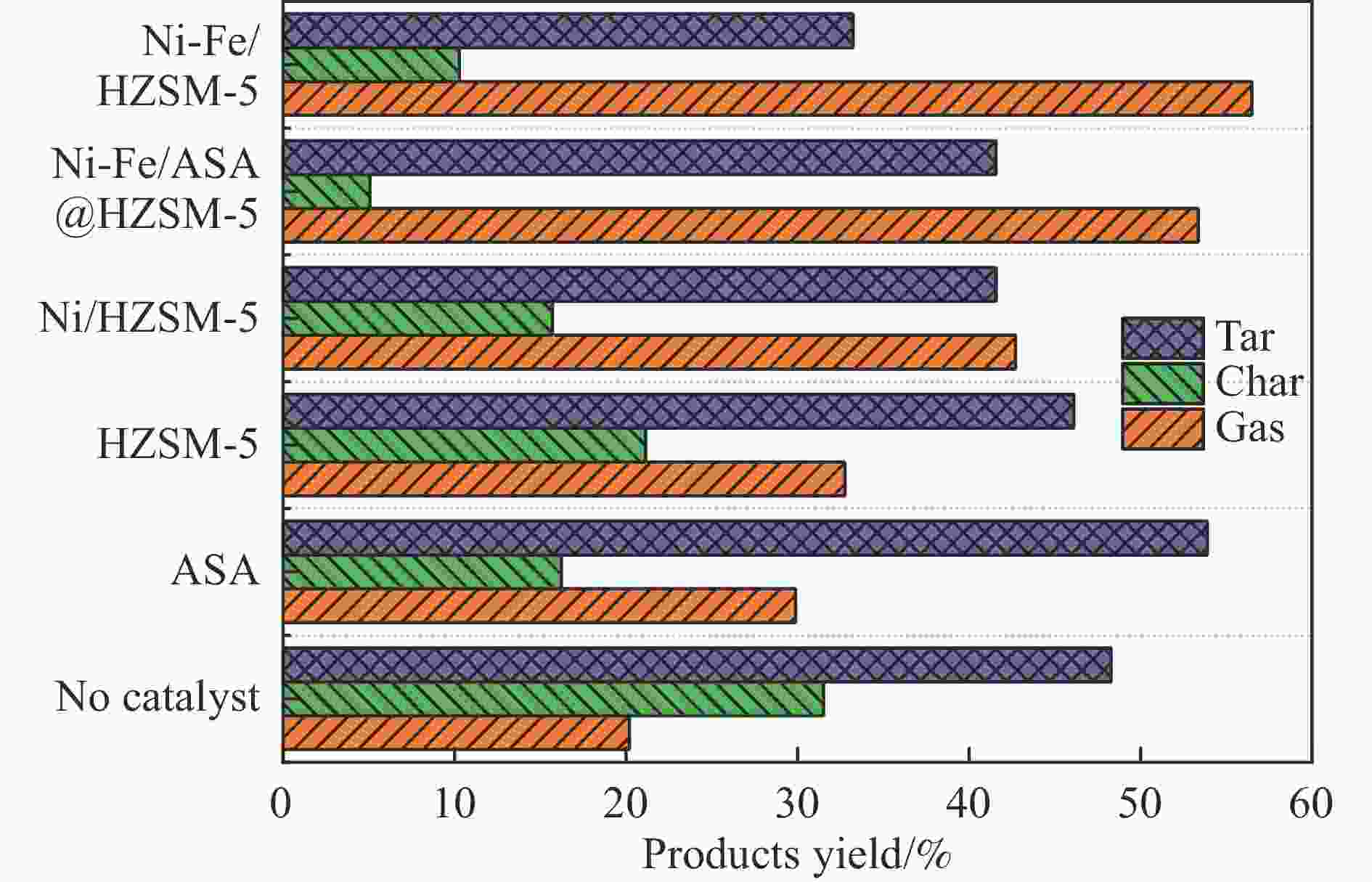
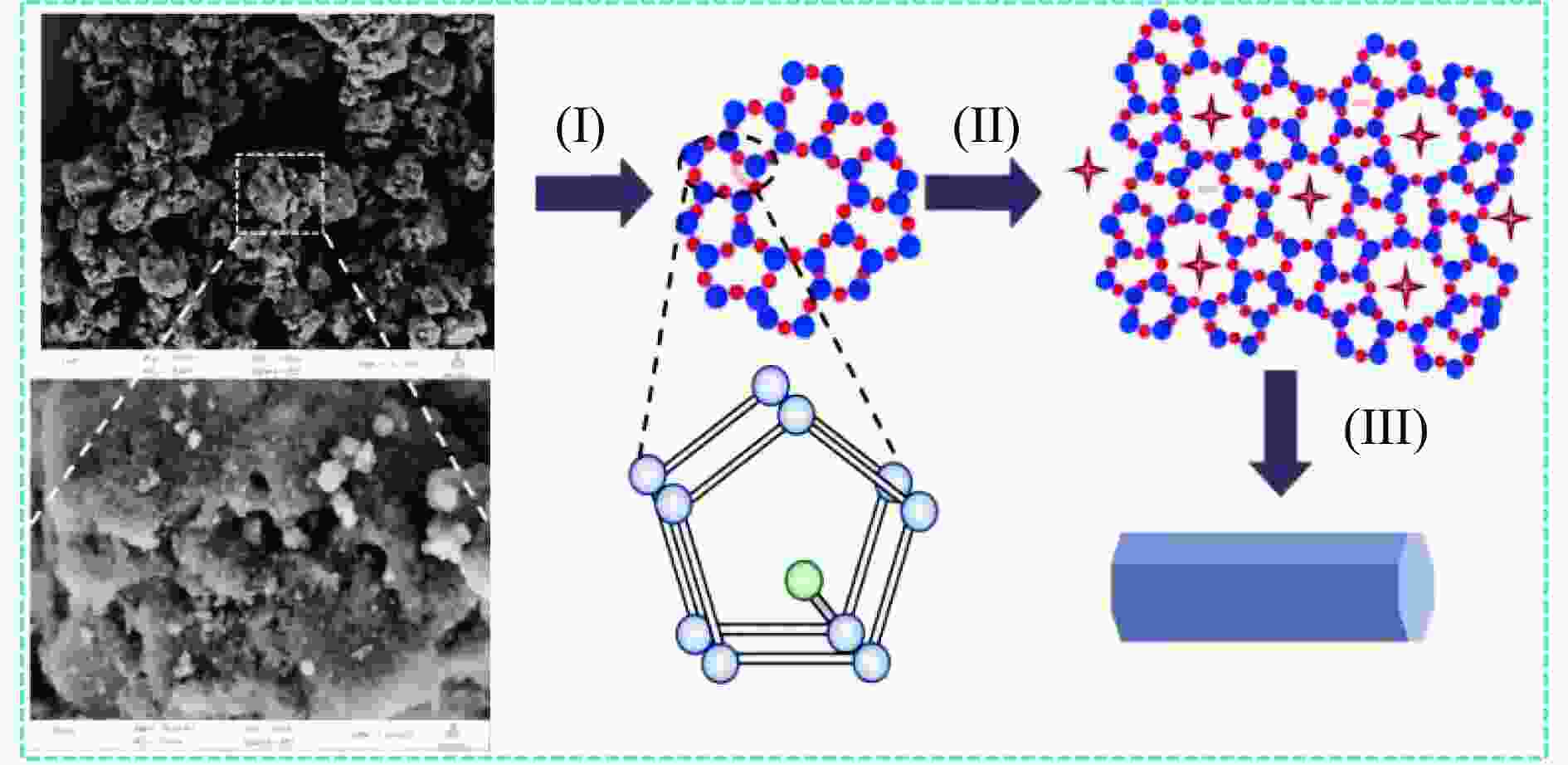
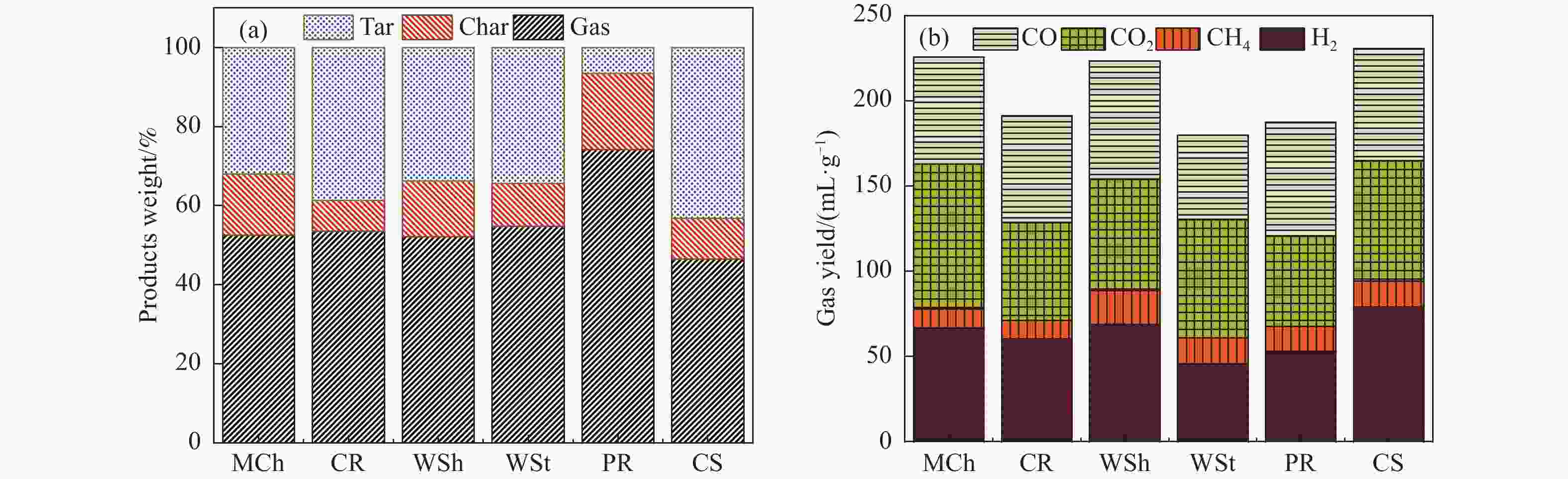
摘要: 本研究通过超声波辅助过量浸渍法将活性组分镍、助剂铁与HZSM-5分子筛结合来提高富氢燃气的产率;进一步以废弃铝灰与HZSM-5分子筛作为共载体制备铝灰与HZSM-5分子筛符合共载镍-铁催化剂并将其用于强化生物质催化热解产富氢燃气的过程。结果表明,在热解温度700 ℃下,Ni-Fe/HZSM-5可使富氢燃气的产率提高到56.49%(约为230.59 mL/g),氢气产率提高到63.12%,产氢效率提高到0.71%,CO得率增加到65.77 mL/g;足够的Ni-Fe/HZSM-5催化剂量强化了生物质热解的产氢路径,促进了积炭气化反应,起到提高H2和CO产率的双重作用。不同种类生物质的组成差异导致催化热解的产物分布也不同,Ni-Fe/HZSM-5催化生物质热解气体产率的顺序为PR(74.21%)>WSt(54.71%)>CR(53.5%)>MCh(52.47%)>WSh(52.10%)>CS(46.49%)。HZSM-5和ASA载体间的协同作用强化了CH4与CO2的重整过程,抑制了逆水汽变换反应,获得了53.37%和41.56%的气体和焦油产率;并加速了积炭气化反应从而减少了积炭量(0.05g/g),获得了5.07%的半焦产率;Ni-Fe/ASA@HZSM-5具有较好的热裂化能力和脱氧能力,有助于促进HZSM-5催化剂上富氢燃气的生成;为开发高温热解气深度净化与高效利用技术提供理论支撑,有效指导多级催化重整的新型双催化床层的开发。Abstract: Catalytic pyrolysis of waste biomass is a promising method for the production of hydrogen-rich gas. HZSM-5 carrier is the premise of ensuring the thermal stability and long life of catalytic materials, and plays a mechanical role in bearing the active component nickel(Ni). At the same time, aluminum ash, as an important waste in the production process of aluminum industry, is mainly composed of Al2O3 and a large number of heavy metal oxides such as Na2O, CaO, MgO, Fe2O3 and so on. In this study, aiming at the technical bottleneck problems such as the low performance of traditional HZSM-5 molecular sieve and the difficulty of resource utilization of aluminum ash, the active component nickel (Ni) and promoter iron (Fe) were combined with HZSM-5 molecular sieve by ultrasonic-assisted excessive impregnation to improve the yield of hydrogen-rich gas. Furthermore, waste aluminum ash (ASA) and HZSM-5 molecular sieve were used as co-carriers to prepare aluminum ash co-supported Ni-Fe catalyst with HZSM-5 molecular sieve, and it was used to enhance the process of hydrogen-rich gas production by the catalytic pyrolysis of biomass. The results showed that the heat transfer efficiency decreased with the increase of heating rate during pyrolysis of biomass. After compensation, the apparent kinetic parameters (E and A) of pyrolysis of different biomass were obtained. At the pyrolysis temperature of 700 ℃, Ni-Fe/HZSM-5 catalyst increases the yield of hydrogen-rich gas to 56.49% (about 230.59 mL/g), hydrogen yield to 63.12%, hydrogen production efficiency to 0.71%, and CO yield to 65.77 mL/g. Sufficient amount of Ni-Fe/HZSM-5 catalyst enhanced the pathway of hydrogen production by the catalytic pyrolysis of biomass, promoted the gasification reaction of carbon deposition, and played a dual role in increasing the yield of H2 and CO. The synergism between HZSM-5 and ASA carriers enhanced the reforming process of CH4 and CO2, inhibited the reverse water vapor shift reaction, obtained 53.37% and 41.56% gas and tar yields. At the same time, the gasification reaction of carbon deposition was also accelerated, reduced the char yield to 5.07%, and obtained the carbon deposition of 0.05 g/g. Ni-Fe/ASA@HZSM-5 has good thermal cracking ability and deoxidization ability, which is helpful to promote the formation of hydrogen-rich gas on HZSM-5 as a base catalyst. From the point of view of proximate analysis and chemical composition of biomass, the composition of different kinds of biomass varies greatly, and the product distribution of catalytic pyrolysis also has a great influence. The order of gas yield of pyrolysis of biomass catalyzed by Ni-Fe/HZSM-5 was PR (74.21%)>WSt (54.71%)>CR (53.5%)>MCh (52.47%)>WSh (52.10%)>CS (46.49%)., which provides theoretical support for the development of deep purification and efficient utilization of high temperature pyrolysis gas, and effectively guides the development of a new double catalytic bed for multi-stage catalytic reforming.
-
Key words:
- HZSM-5 /
- aluminum ash /
- waste biomass /
- catalytic pyrolysis /
- hydrogen-rich gas
-
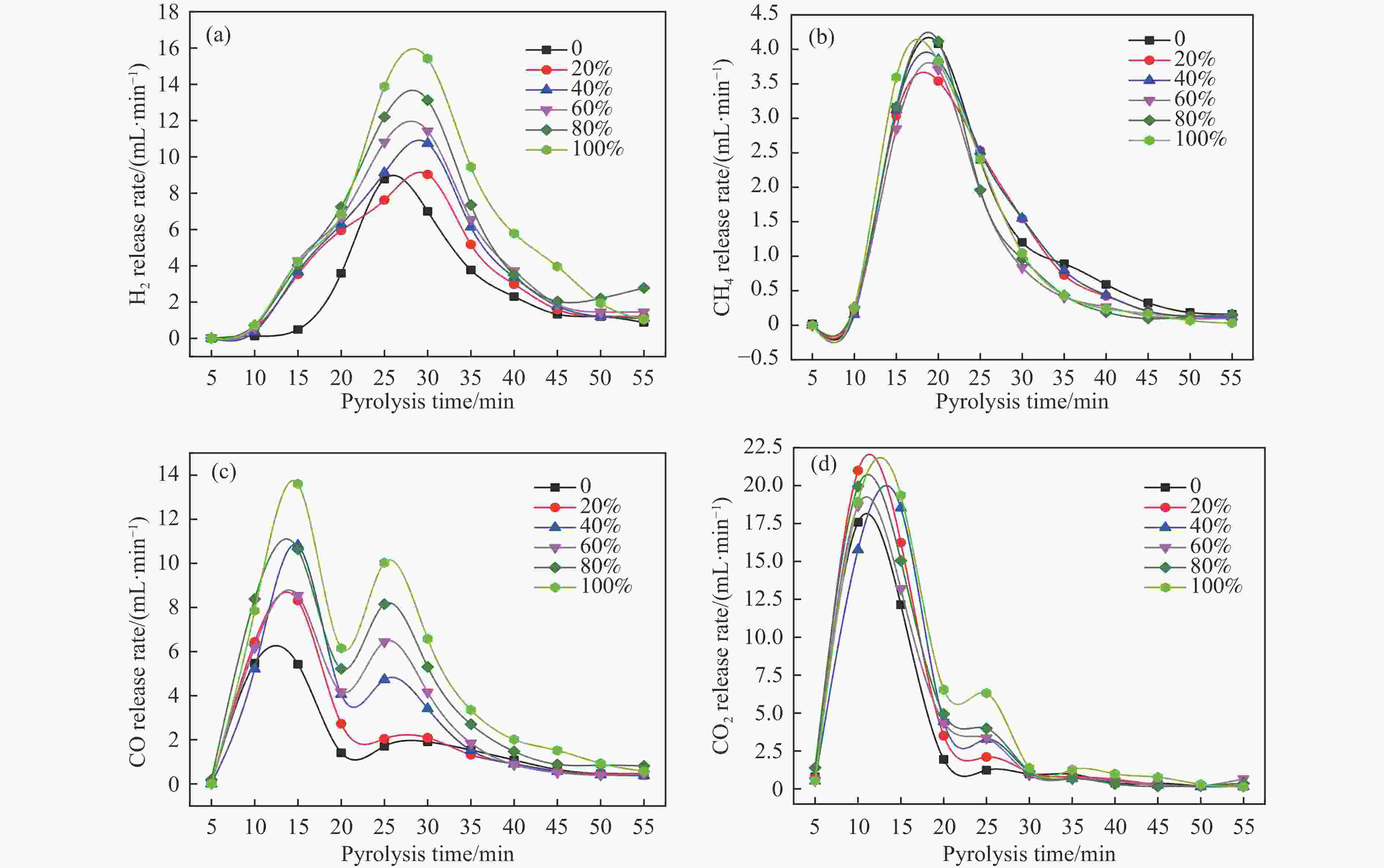
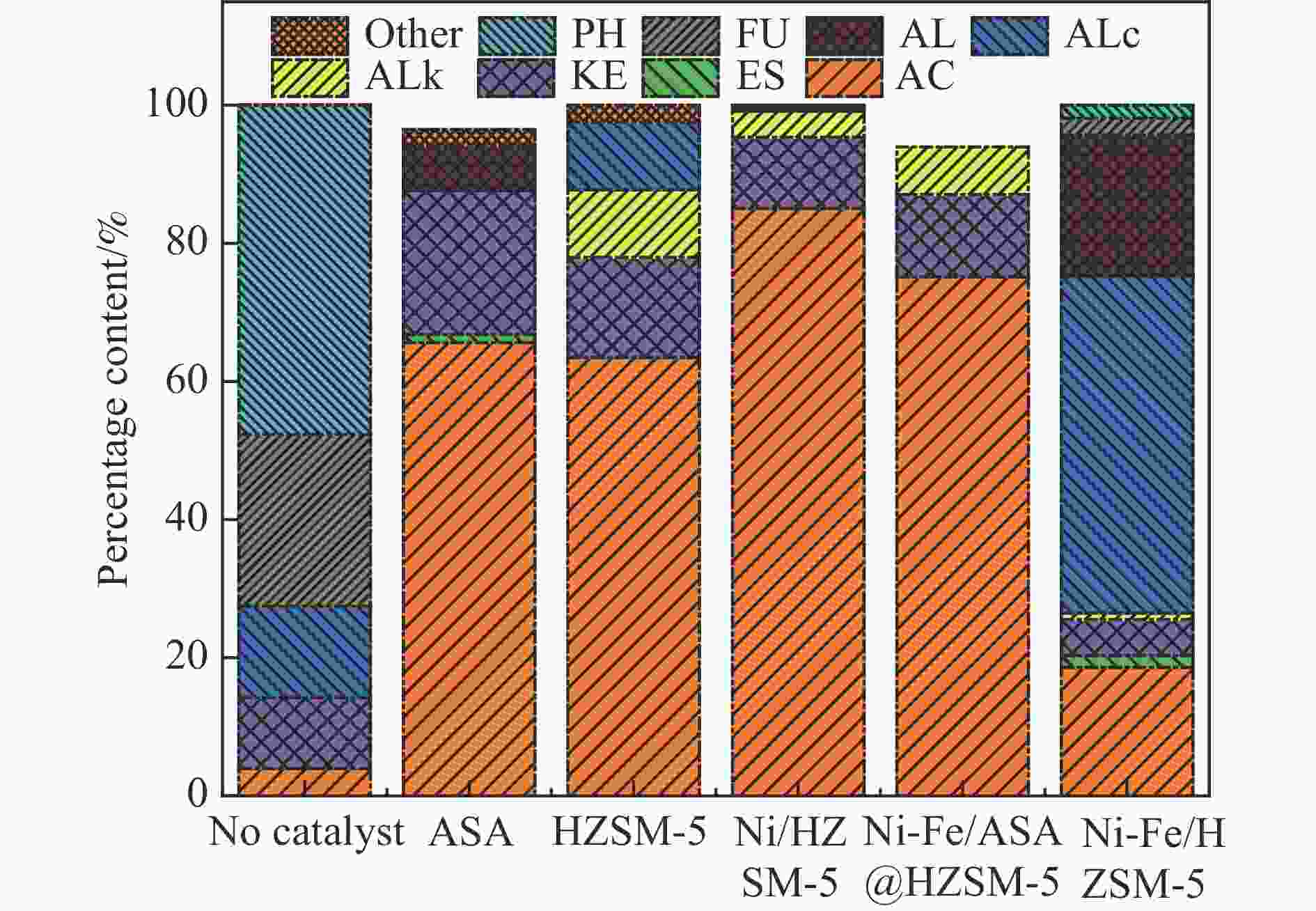
图 6 催化剂用量对产物分布的影响(a)气体产率(b)液体产物分布
注:酸类物质(Acid, AC)、酮类物质(Ketones, KE)、醛类物质(Aldehydes, AL)、醇类物质(Alcohol, ALc)、酚类物质(Phenolic, PH)、呋喃类物质(Furan, FU)、酯类物质(Esters, ES)、烷烃类物质(Alkyl hydrocarbons, ALk)、其他(other)
Figure 6 Effect of catalytic dosage on products distribution (a) Gas yield (b) distribution of liquid products
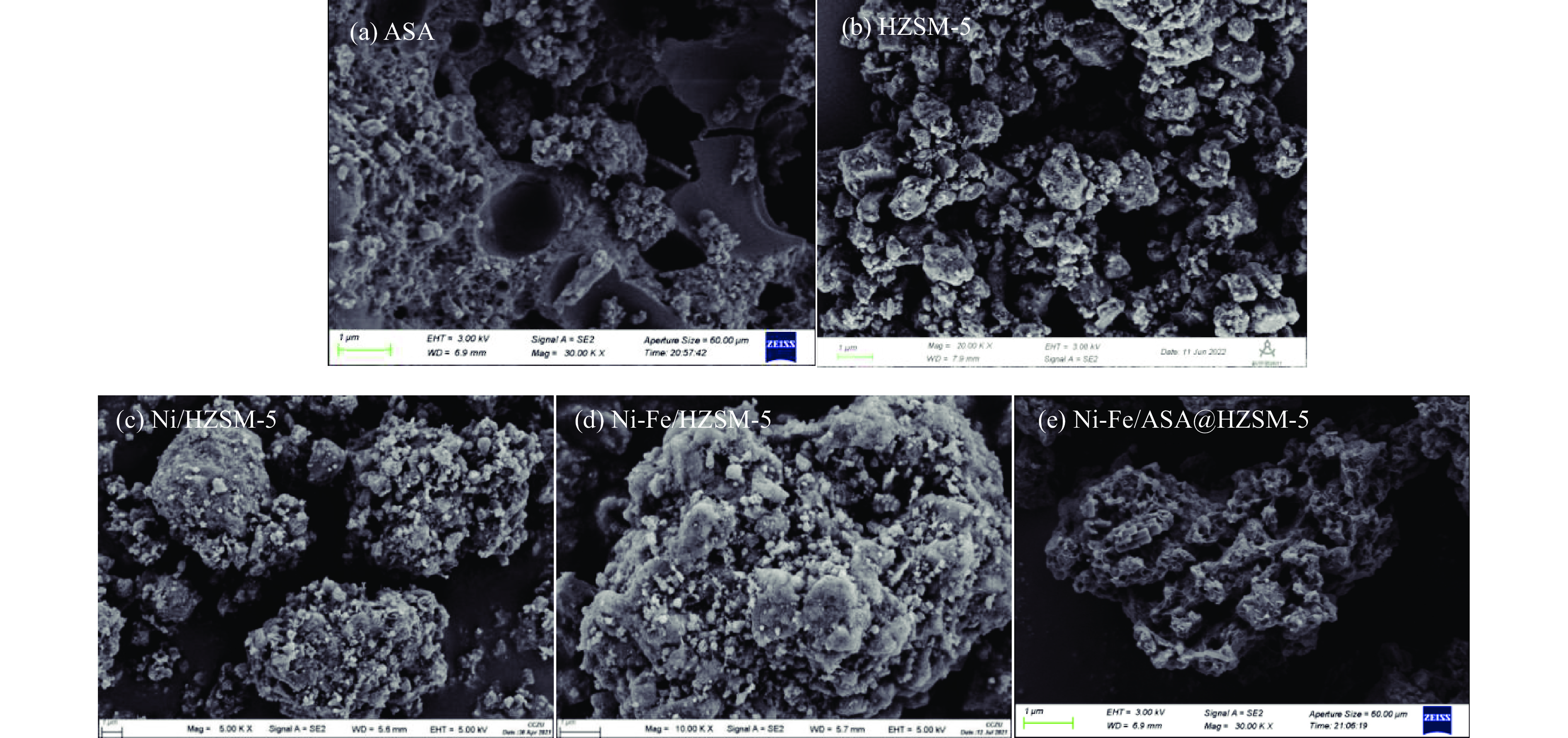
表 1 新鲜催化剂的物理特性及孔道特性
Table 1 Physical properties and pore distribution of fresh catalysts
Fresh catalyst BET surface area/(m2·g−1) t-plot micropore area/(m2·g−1) t-Plot external surface area/(m2·g−1) Total pore
volume/(cm3·g−1)Pore size
/nmASA 262.13 − − 0.44 6.75 HZSM-5 276.21 145.49 130.72 0.23 3.26 Ni/HZSM-5 241.83 155.48 86.35 0.19 3.16 Ni-Fe/HZSM-5 219.50 133.02 84.48 0.17 3.11 Ni-Fe/ASA@HZSM-5 195.90 107.59 88.31 0.16 3.17 表 2 不同升温速率下玉米秸秆热解反应动力学参数
Table 2 Kinetic parameters of CS pyrolysis under different heating rates
β/(mL·min−1) CS MCh CR E/(kJ·mol−1) A/min−1 E/(kJ·mol−1) A/min−1 E/(kJ·mol−1) A/min−1 10 74.96 1498.02 74.88 1474.39 73.04 1237.76 20 73.21 1253.56 73.50 1290.48 72.27 1150.18 30 72.81 1210.30 74.60 1430.49 72.44 1169.72 40 72.61 1186.40 73.75 1316.83 71.77 1099.88 β/(mL·min−1) WSh WSt PR E/(kJ·mol−1) A/min−1 E/(kJ·mol−1) A/min−1 E/(kJ·mol−1) A/min−1 10 71.33 1059.17 72.48 1178.61 73.89 1341.81 20 71.51 1073.74 72.20 1143.25 72.22 1144.56 30 71.69 1093.54 72.18 1143.54 71.69 1091.38 40 71.95 1119.70 72.48 1179.23 71.63 1084.02 表 3 生物质热解的反应动力学参数
Table 3 Reaction kinetic parameters of biomass pyrolysis
Sample E/(kJ·mol−1) A/min−1 Sample E/(kJ·mol−1) A/min−1 CS 73.39 1237.27 WSh 71.62 1061.48 MCh 74.18 1379.27 WSt 72.34 1142.08 CR 72.38 1147.11 PR 72.36 1144.39 表 4 反应后催化剂的孔道特性
Table 4 Pore characteristics of reacted catalysts
Reacted catalyst ASA HZSM-5 Ni/HZSM-5 Ni-Fe/HZSM-5 Ni-Fe/ASA@HZSM-5 BET surface area/(m2·g−1) 48.95 219.99 235.49 214.4 136.12 t-plot micropore area/(m2·g−1) 33.09 157.67 167.17 172.84 104.48 t-plot external surface area/(m2·g−1) 15.86 62.32 68.32 41.56 31.64 Total pore volume/(cm3·g−1) 0.09 0.15 0.18 0.15 0.1 Pore size/nm 7.13 2.75 3.03 2.72 2.94 Average nanoparticle size/nm 122.58 27.27 25.48 27.99 44.08 -
[1] SALAMA E S, HWANG J H, ELDALATONY M M, et al. Enhancement of microalgal growth and biocomponent-based transformations for improved biofuel recovery: A review[J]. Bioresour Technol,2018,258:365−375. doi: 10.1016/j.biortech.2018.02.006 [2] SURIAPPARAO D V, TEJASVI R. A review on role of process parameters on pyrolysis of biomass and plastics: Present scope and future opportunities in conventional and microwave-assisted pyrolysis technologies[J]. Process Saf Environ,2022,162:435−462. doi: 10.1016/j.psep.2022.04.024 [3] HU X, GHOLIZADEH M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage[J]. J Energy Chem,2019,39(12):109−143. [4] HUO X, XIAO J, SONG M, et al. Comparison between in-situ and ex-situ catalytic pyrolysis of sawdust for gas production[J]. J Anal Appl Pyrolysis,2018,135:189−198. doi: 10.1016/j.jaap.2018.09.003 [5] GUPTA S, MONDAL P. Catalytic pyrolysis of pine needles with nickel doped gamma-alumina: Reaction kinetics, mechanism, thermodynamics and products analysis[J]. J Clean Prod,2021,286(1):124930.1−124930.12. [6] HERNANDO H, MORENO I, FERMOSO J, et al. Biomass catalytic fast pyrolysis over hierarchical ZSM-5 and Beta zeolites modified with Mg and Zn oxides[J]. Biomass Convers Bior,2017,7(3):289−304. doi: 10.1007/s13399-017-0266-6 [7] LI Y, NI S, YELLEZUOME D, et al. Deactivation mechanism and regeneration effect of bi-metallic Fe-Ni/ZSM-5 catalyst during biomass catalytic pyrolysis[J]. Fuel,2022,312(15):122924.1−122924.10. [8] LI X, LIU P, WU S, et al. Study on the mechanism of syngas production from catalytic pyrolysis of biomass tar by Ni–Fe catalyst in CO2 atmosphere[J]. Fuel,2022,11(17):126705. [9] LI X, LIU P, LEI T, et al. Pyrolysis of biomass Tar model compound with various Ni-based catalysts: Influence of promoters characteristics on hydrogen-rich gas formation[J]. Energy,2022,244(PB):123137. [10] LI X, LIU P, CHEN W, et al. Catalytic pyrolysis of toluene as biomass tar model component using Ni/HZSM-5 modified by CeO2 and MgO promoters[J]. J Anal Appl Pyrolysis,2022,162:105436.1−105436.11. [11] LI X, Chen Z, LIU P, et al. Feasibility assessment of recycling waste aluminum dross as a basic catalyst for biomass pyrolysis to produce hydrogen-rich gas[J]. Int J Hydrog Energy,2023,48(93):36361−36376. doi: 10.1016/j.ijhydene.2023.06.049 [12] SINGH S, CHAKRABORTY J P, MONDAL M K. Intrinsic kinetics, thermodynamic parameters and reaction mechanism of non-isothermal degradation of torrefied Acacia nilotica using isoconversional methods[J]. Fuel,2020,259(1):116263.1−116263.15. [13] ZHAO B, YANG H, ZHANG H, et al. Study on hydrogen-rich gas production by biomass catalytic pyrolysis assisted with magnetic field[J]. J Anal Appl Pyrolysis,2021,157(Aug):105227.1−105227.10. [14] SHARMA P, PANDEY O P, DIWAN P K. Non-isothermal kinetics of pseudo-components of waste biomass[J]. Fuel,2019,253:1149−1161. doi: 10.1016/j.fuel.2019.05.093 [15] 许敏. 生物质热解气化特性分析与试验研究[D]. 天津: 天津大学, 2008.XU MIN. Mechanism and experimental study on biomass gasification and pyrolysis[D]. Tianjin: Tianjin University, 2021.) [16] QIN Z, YOU Z, BOZHILOV K N, et al. Dissolution behavior and varied mesoporosity of zeolites by NH4 F etching[J]. Chem Eur J,2022,28(16):e202104339. doi: 10.1002/chem.202104339 [17] HU Z, HAN J, WEI Y, et al. Dynamic evolution of zeolite framework and metal-zeolite interface[J]. ACS Catal,2022,12(9):5060−5076. doi: 10.1021/acscatal.2c01233 [18] KOSTYNIUK A, BAJEC D, LIKOZAR B. Catalytic hydrocracking reactions of tetralin as aromatic biomass tar model compound to benzene/toluene/xylenes (BTX) over zeolites under ambient pressure conditions[J]. J Ind Eng Chem,2021,96(1):130−143. [19] LI X, LIU P, HUANG S, et al. Study on the mechanism of syngas production from catalytic pyrolysis of biomass tar by Ni–Fe catalyst in CO2 atmosphere[J]. Fuel,2023,335(1):1−12. [20] FATHI S, SOHRABI M, FALAMAKI C. Improvement of HZSM-5 performance by alkaline treatments: Comparative catalytic study in the MTG reactions[J]. Fuel,2014,116:529−537. doi: 10.1016/j.fuel.2013.08.036 [21] TANG W, CAO J, YANG F, et al. Highly active and stable HF acid modified HZSM-5 supported Ni catalysts for steam reforming of toluene and biomass pyrolysis tar[J]. Energy Convers Manag,2020,212:112799.1−112799.12. [22] MAHINROOSTA M, ALLAHVERDI A. Hazardous aluminum dross characterization and recycling strategies: A critical review[J]. J Environ Manage,2018,223:452−468. doi: 10.1016/j.jenvman.2018.06.068 [23] 孙堂磊. 木质纤维素类生物质定向热解产物分布规律及实验研究[D]. 河南: 河南农业大学, 2021.SUN Tanglei. Product distribution and experimental study of lignocellulosic biomass directional pyrolysis[D]. Henan: Henan Agricultural University, 2021.) -





 下载:
下载: