Effect of Al source on the physicochemical properties of Cu-Al spinel catalysts and the catalytic performance for reverse water gas shift
-
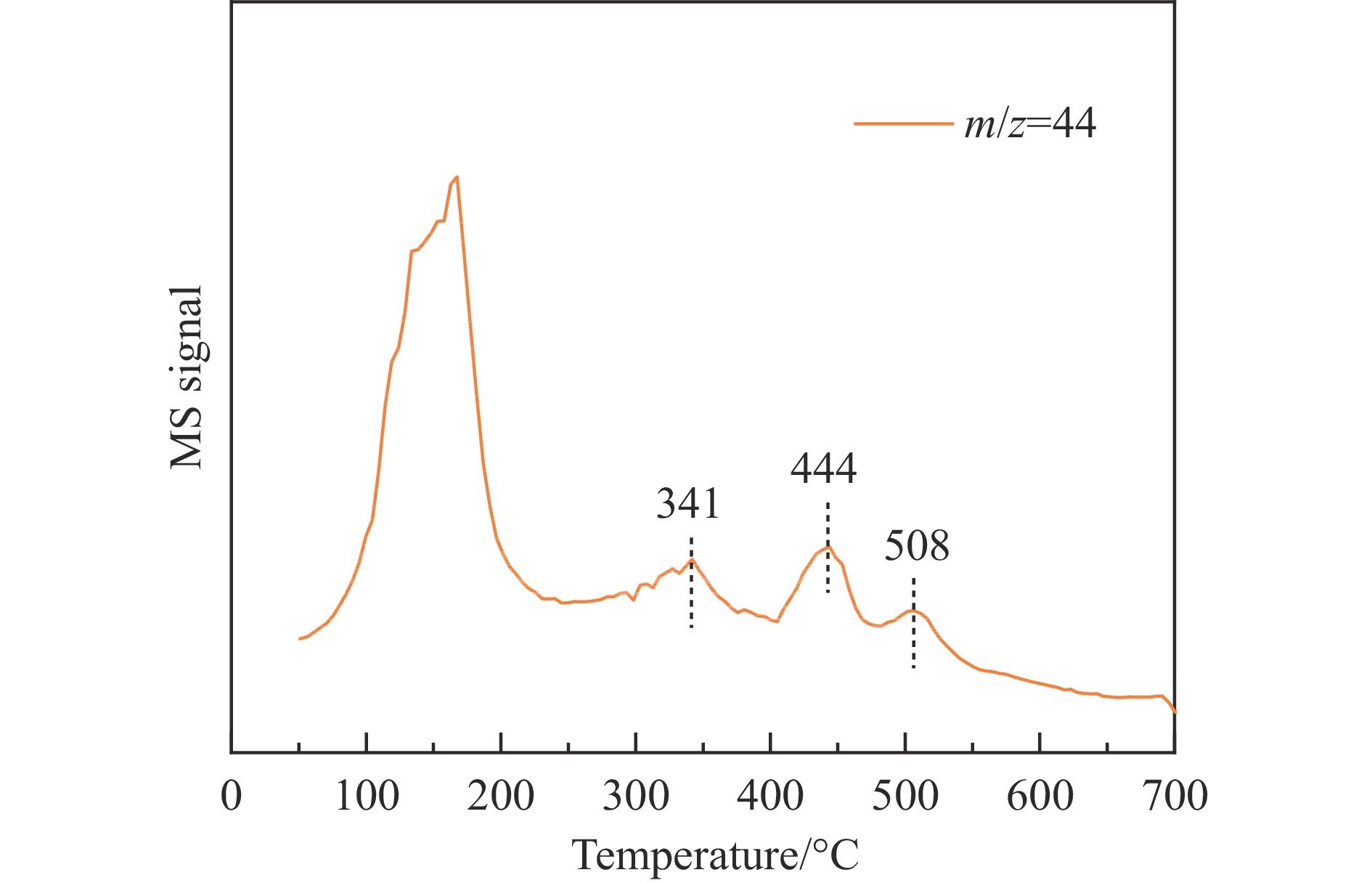
摘要: 基于高能球磨和固相焙烧的方法,采用杂质元素(Na、Fe、Si和S)含量不等的四种拟薄水铝石和氢氧化铜制备了Cu-Al尖晶石固溶体催化剂,通过ICP-AES、TG、XRD、H2-TPR和BET表征了催化剂的物化性质,并考察了对逆水煤气变换反应的催化性能。结果表明,拟薄水铝石中的杂质元素对于Cu-Al尖晶石催化剂的物相性质、还原性能、织构性质和催化性能有显著的影响,Si有助于合成高比表面积的催化剂,但不利于Cu-Al尖晶石生成,导致催化活性低;含有少量Na和Fe的尖晶石的催化活性较低;S物种经高温焙烧后分解,对催化活性没有影响;基于杂质元素含量最低的拟薄水铝石合成的催化剂中难还原尖晶石含量最高,表现出最高的逆水煤气变换催化活性。此外,基于活性最优样品的CO2-TPD-MS和In-situ DRIFTS分析表明,Al上形成的双齿甲酸盐是Cu-Al尖晶石固溶体催化CO2加氢生成CO的主要中间产物,其含量与催化活性随反应时间的变化规律一致。Abstract: The excessive of fossil fuels has caused a swift rise in global carbon dioxide levels, resulting in severe climate change and environmental pollution. The research on the conversion of CO2 into high value-added chemicals is of great significance for CO2 reduction. Due to the high chemical activity of CO, a first conversion of CO2 to CO is meaningful, which makes the subsequent conversions become easier. Therefore, the reverse water gas shift reaction is considered to be an important intermediate step of CO2 hydrogenation to methanol, ethanol and other carbon-containing high value-added industrial products. For the reverse water gas shift reaction, several catalyst systems were researched, including supported catalysts, mixed metal oxide catalysts and transition metal carbide catalysts. Among these catalysts, Cu-based catalysts were widely reported owing to the high activity and CO selectivity. Recently, we found that Cu-Al spinel catalysts can be used as the efficient sustained release catalysts for reverse water gas shift reaction. High surface area pseudo-boehmite acts as an appropriate Al source for the synthesis of Cu-Al spinel catalysts by the mechanochemical method. However, the impurity elements in pseudo-boehmites showed significant influence on the formation and properties of Cu-Al spinel, and the catalytic performance for reverse water gas shift reaction. To unravel this point, four pseudo-boehmites with unequal contents of impurity elements (Na, Fe, Si, and S) and copper hydroxide were used for the synthesis of Cu-Al spinel solid solution catalysts by both high-energy ball milling and solid-phase calcination procedures. The physicochemical properties of the catalysts were characterized by ICP-AES, TG, XRD, H2-TPR, and BET methods, and the catalytic performances were investigated in reverse water gas shift reaction. The results showed that impurity elements in pseudo-boehmite samples had significant effects on the crystal property, reducibility, texture property and catalytic performance of the Cu-Al spinel catalysts. Specifically, Si facilitated the synthesize of high specific surface area catalysts but was detrimental to the formation of Cu-Al spinel, thus leading to a low catalytic activity. Cu-Al spinel catalysts with a small amount of Na and Fe also showed low catalytic activities. S species would be decomposed and removed during the precursor calcination step at high temperature of 950 ℃, thus giving little effect on the catalytic activity. Importantly, the catalyst synthesized based on the pseudo-boehmite with the lowest content of impurity elements had the highest content of hardly-reducible spinel, and exhibited the highest catalytic activity for CO2 hydrogenation to CO. In addition, the Cu-Al spinel catalyst with the highest catalytic activity was selected for the in-situ DRIFTS and CO2-TPD-MS characterizations. The results showed that the formate species, including monodentate formate on Al, bidentate formate on Al, and bidentate formate on Cu, were intermediate species of CO2 hydrogenation to CO over Cu-Al spinel catalysts. Notably, low peak intensities were detected with monodentate formate on Al and bidentate formate on Cu, but the bidentate formate on Al showed higher peak intensity. Especially, the content of bidentate formate on Al was in line with the catalytic activity at different reaction time, implying that the bidentate formate on Al was the main intermediate. This work provides guidance to catalyst synthesis using pseudo-boehmite as raw material.
-
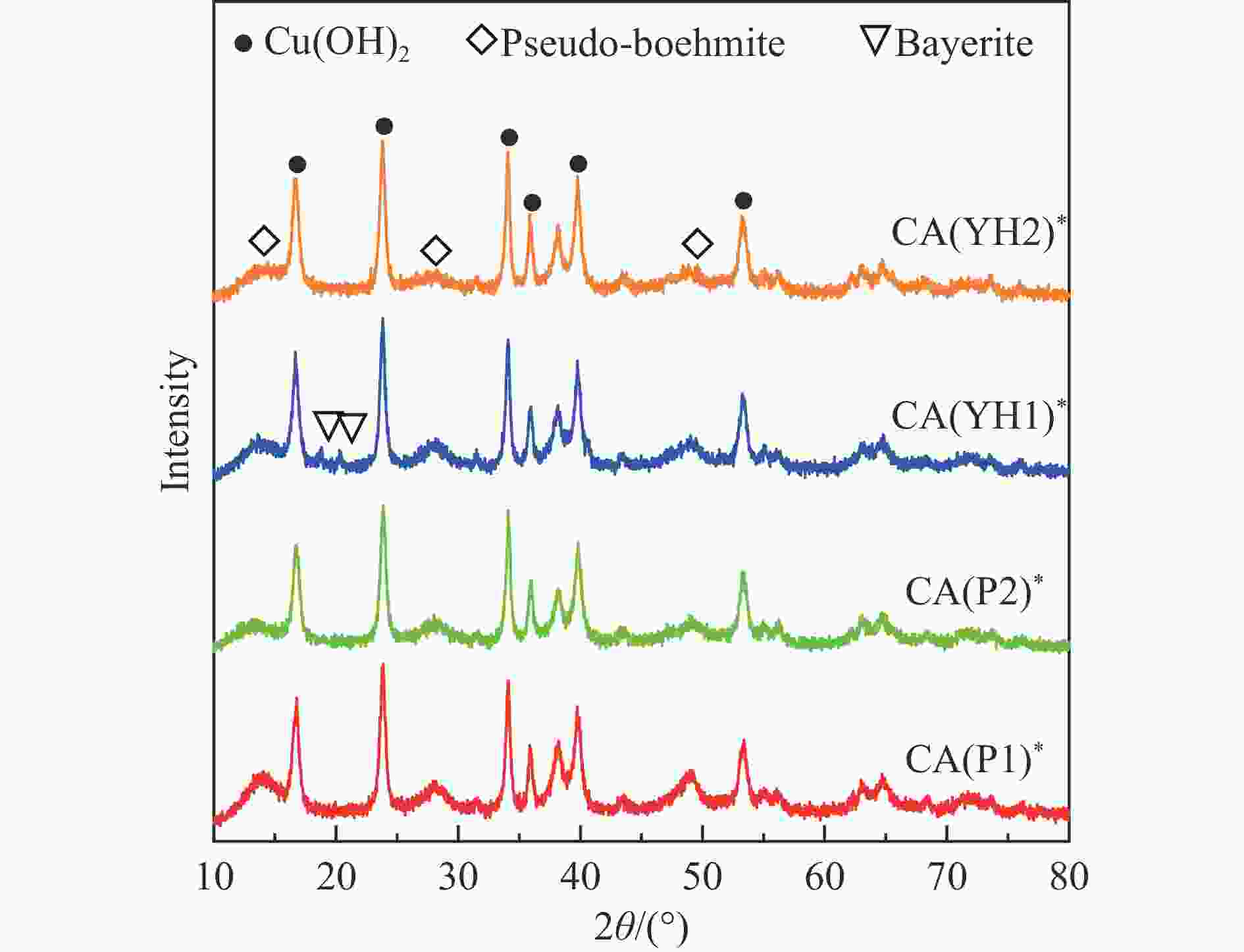
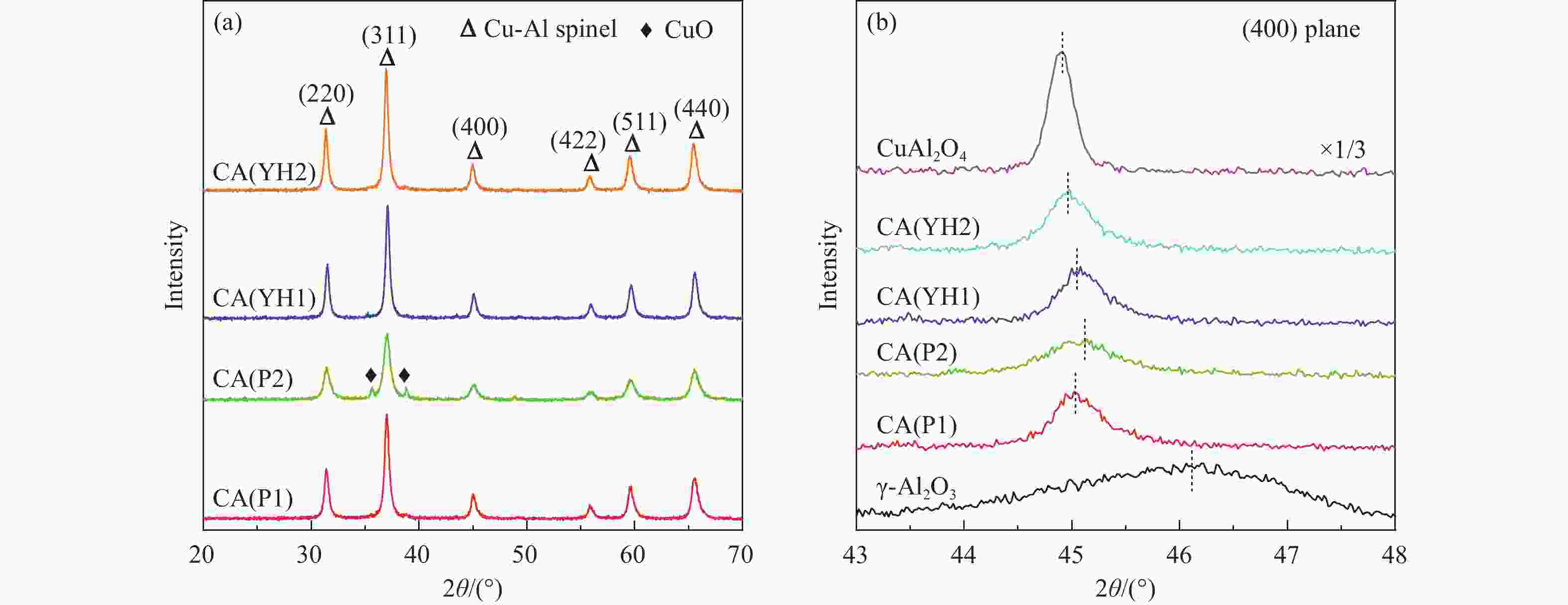
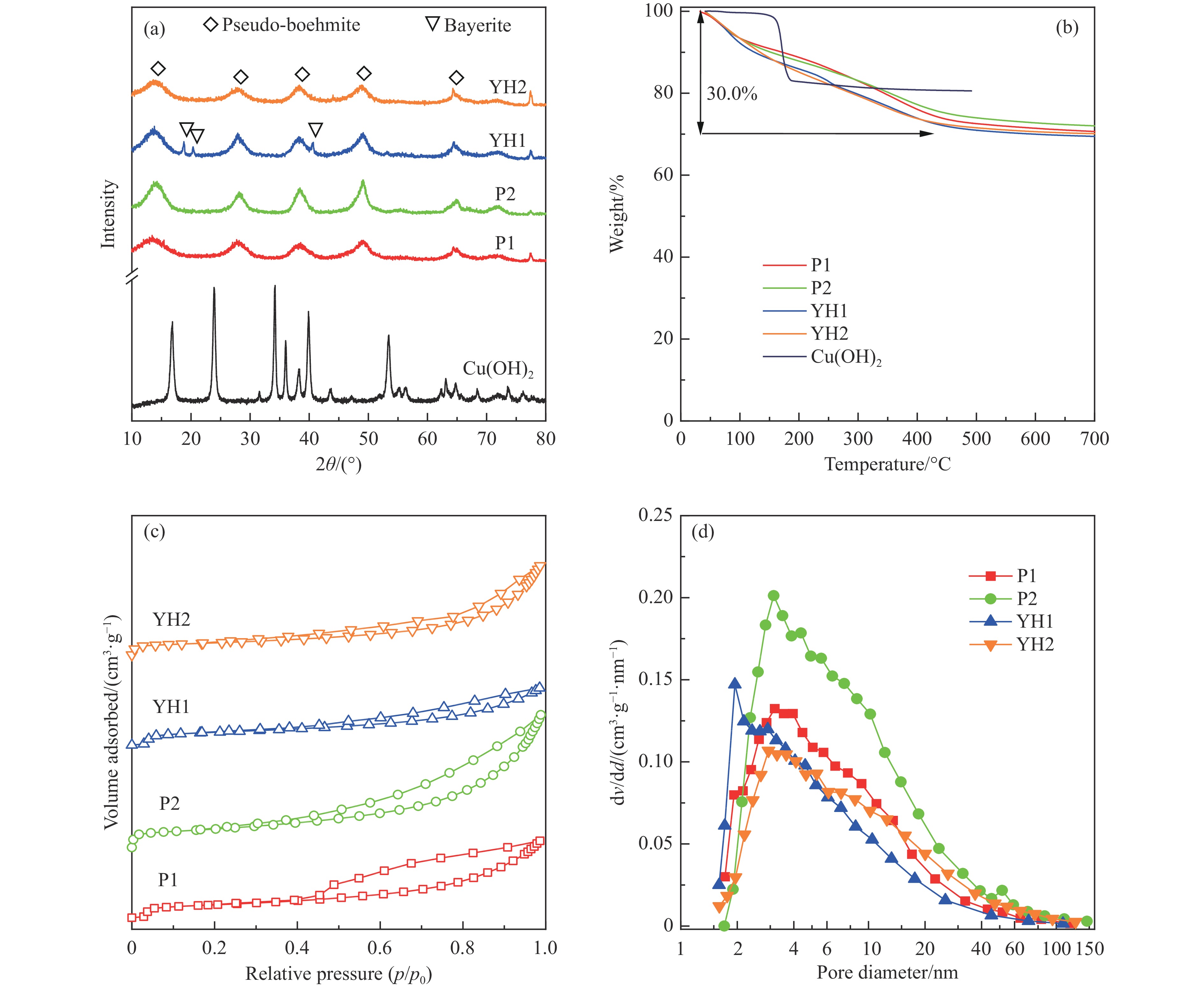
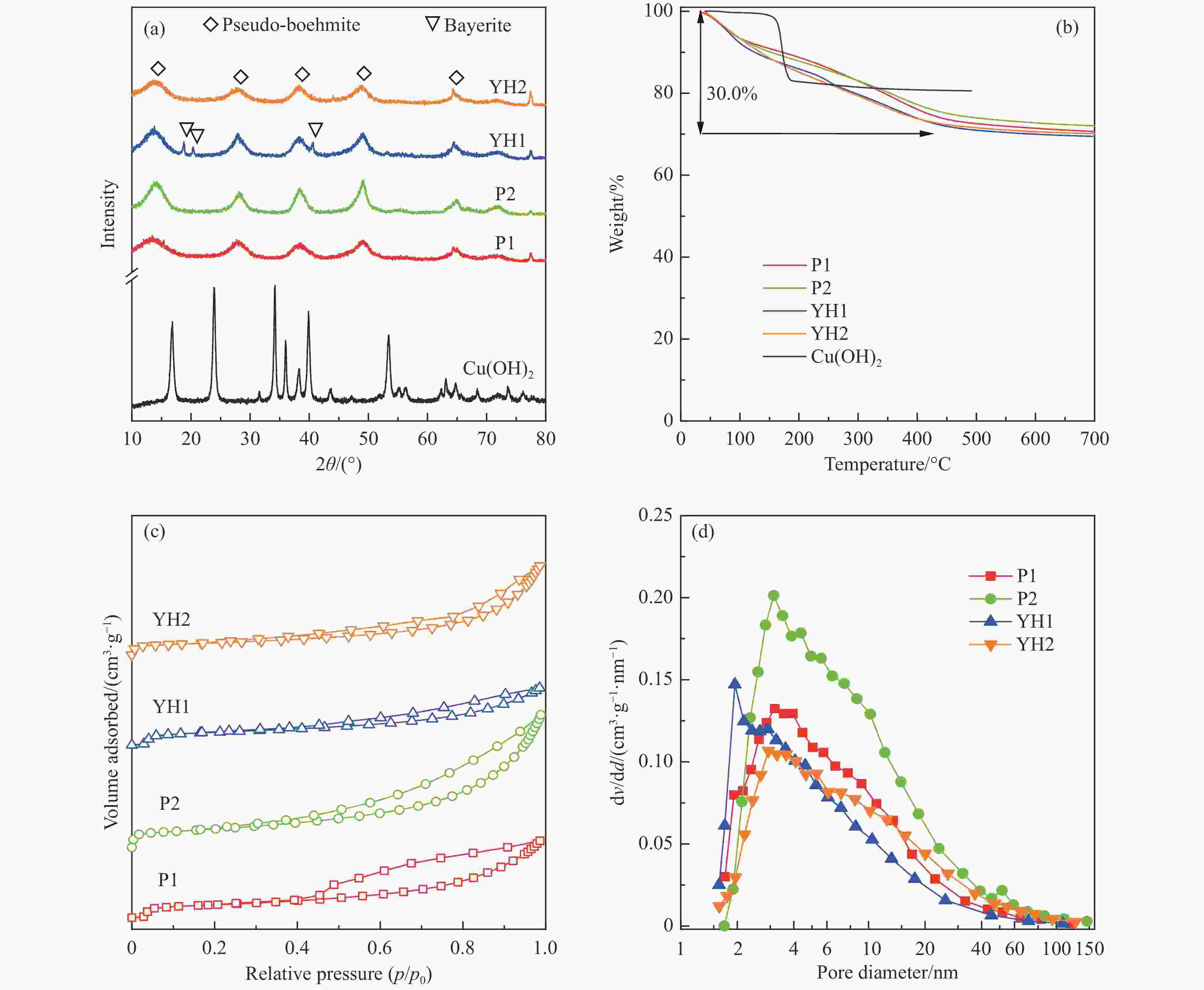
表 1 拟薄水铝石样品中杂质元素的(Na、Fe、Si和S)含量
Table 1 The content of impurity elements (Na, Fe, Si, and S) in the pseudo-boehmite samples
PB Method Content/% Na2O Fe2O3 SiO2 ${\mathrm{SO}}_4^{2-} $ P1 NaAlO2-Al(SO4)3 0.03 0.01 0.01 − P2 NaAlO2-Al(SO4)3 0.07 0.01 2.08 − YH1 Carbonization 0.10 0.05 − − YH2 NaAlO2-Al(SO4)3 0.10 0.05 − 1.95 表 2 Cu-Al尖晶石催化剂的物化性质
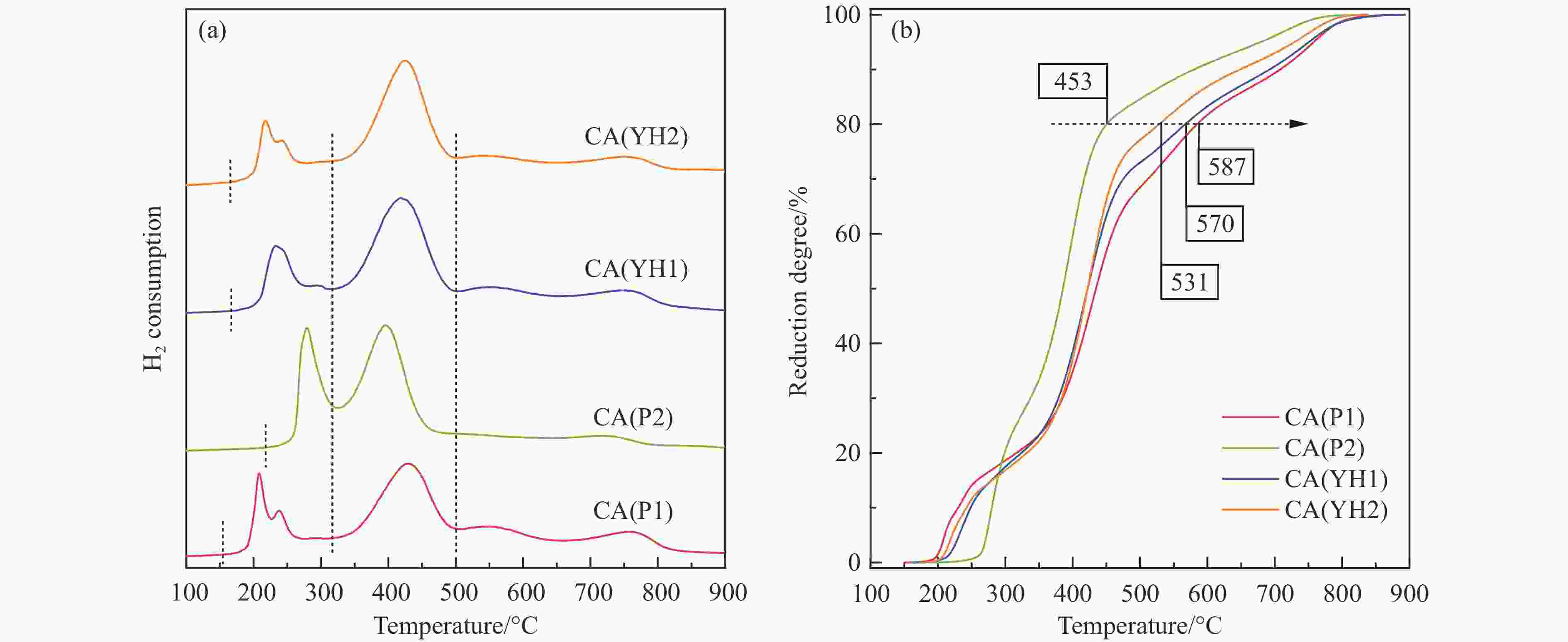
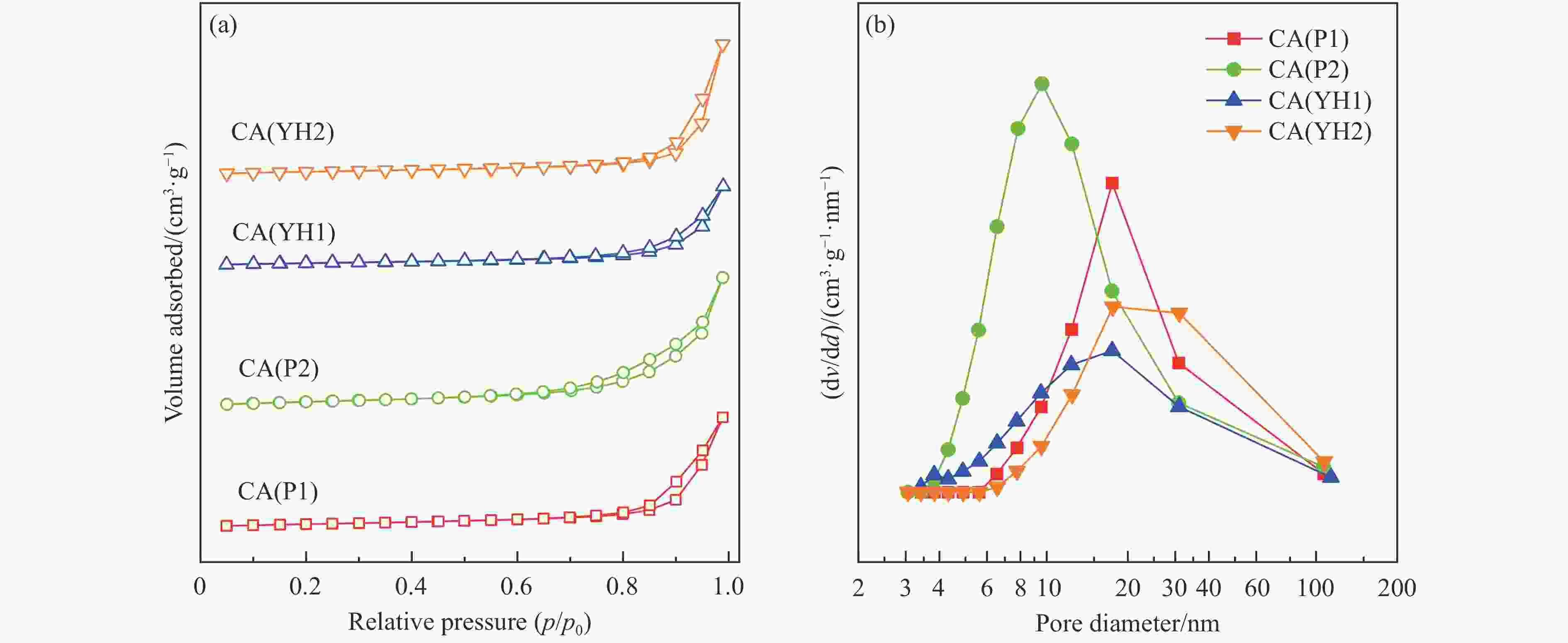
Table 2 Physicochemical properties of Cu-Al spinel catalysts
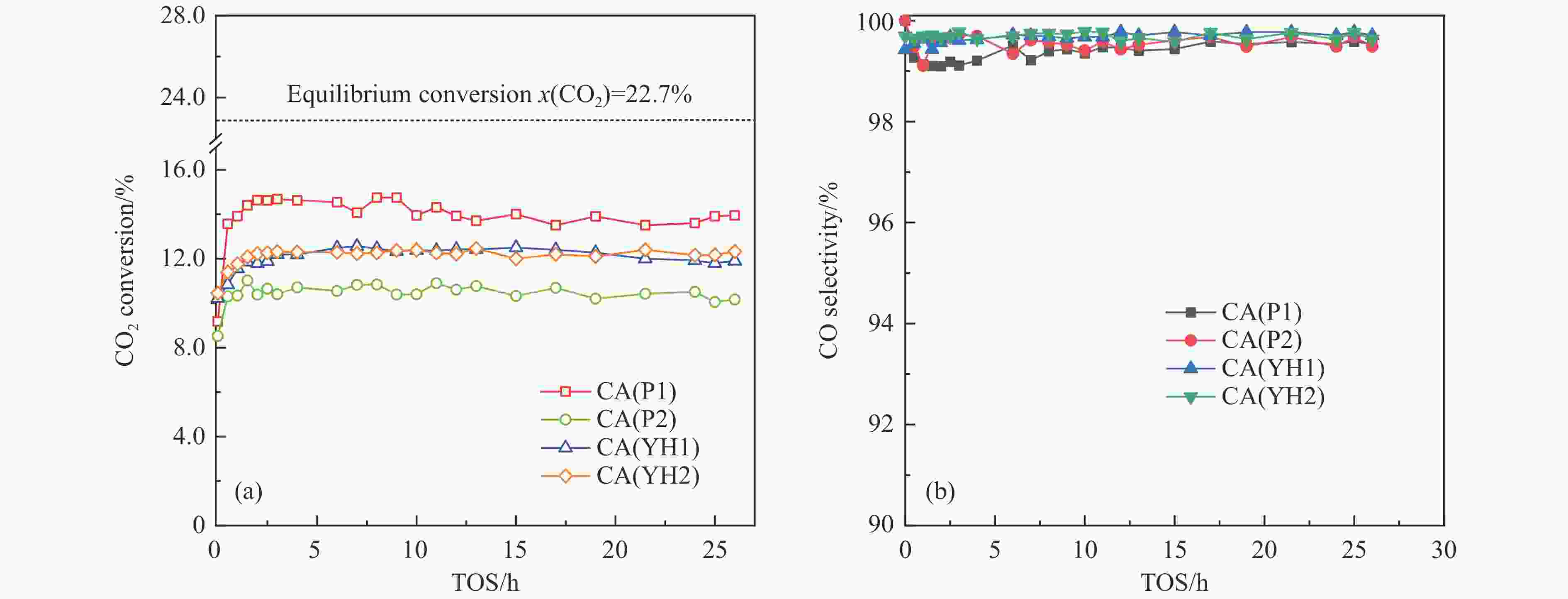
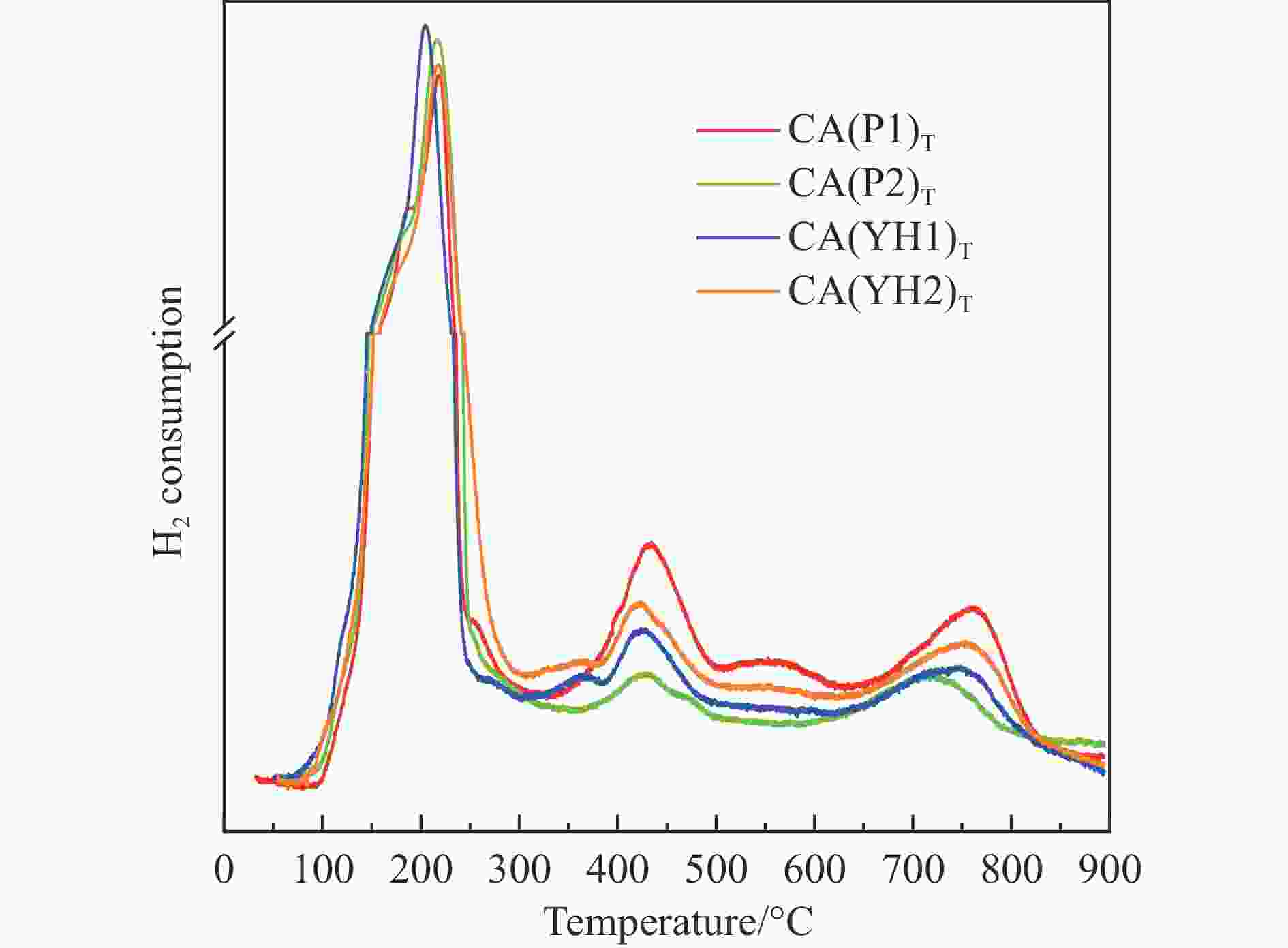
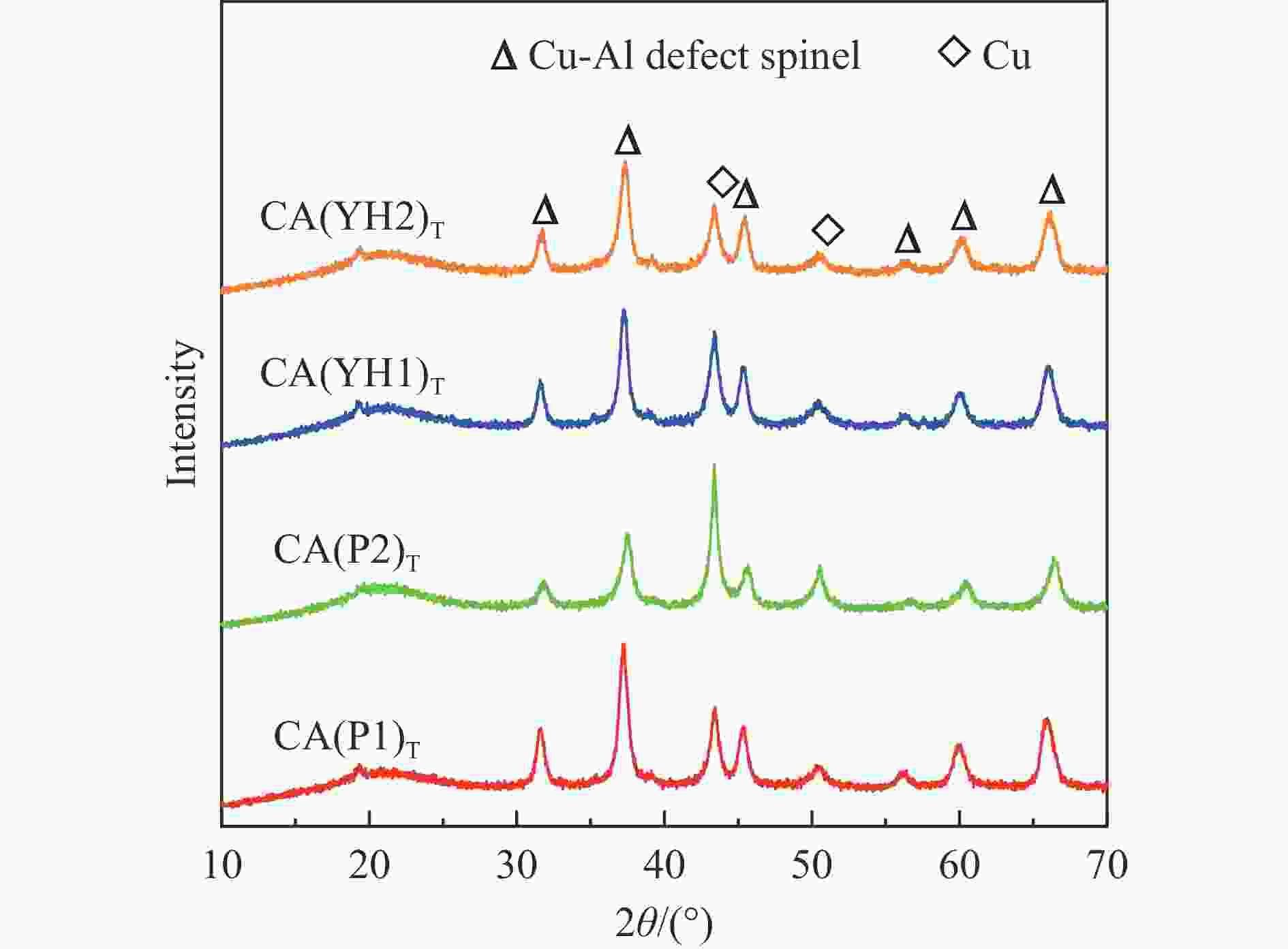
Sample CA(P1) CA(P2) CA(YH1) CA(YH2) a/nm[a] 0.8038 0.8034 0.8032 0.8050 dspinel/nm[b] 14.8 10.8 16.0 16.4 X(spinel Cu2+)/%[c] 83.2 72.8 80.9 85.1 X(non-spinel Cu2+)/%[c] 16.8 27.2 19.1 14.9 X(hardly-reducible spinel Cu2+)/%[c] 30.5 14.8 26.7 22.5 S/(m2·g−1)[d] 38.3 54.6 28.8 34.4 v/(cm3·g−1)[d] 0.283 0.335 0.204 0.334 Tested catalysts RD/%[e] 73.6 88.4 80.6 77.3 Y(hardly-reducible spinel Cu2+)/%[f] 14.7 6.3 10.7 12.4 dCu/nm[g] 10.3 9.6 10.8 10.9 aT/nm[h] 0.7996 0.7961 0.7995 0.7994 [a]: Spinel cell parameter; [b]: Spinel crystallite size; [c]: The conent of spinel Cu2+, non-spinel Cu2+, and hardly-reducible spinel Cu2+ species in the fresh catalysts; [d]: Surface area and pore volume; [e]: Cu releaing degree; [f]: The conent of hardly-reducible spinel Cu2+ species in the tested catalysts; [g]: Copper average size; [h]: The cell parameter of defect spinel in the tested catalysts. -
[1] ARESTA M, DIBENEDETTO A, QUARANTA E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: The distinctive contribution of chemical catalysis and biotechnology[J]. J Catal,2016,343:2−45. doi: 10.1016/j.jcat.2016.04.003 [2] STEINHAUSER G. Cleaner production in the solvay process: general strategies and recent developments[J]. J Clean Prod,2008,16:833−841. doi: 10.1016/j.jclepro.2007.04.005 [3] ARESTA M, DIBENEDETTO A, ANGELINI A. The changing paradigm in CO2 utilization[J]. J CO2 Util, 2013, 3/4 , 65−73. [4] MARTENS J A, BOGAERTS A, DE KIMPE N, et al. The chemical route to a carbon dioxide neutral world[J]. ChemSusChem,2017,10:1039−1055. doi: 10.1002/cssc.201601051 [5] BAHMANPOUR A M, SIGNORILE M, KRÖCHER O. Recent progress in syngas production via catalytic CO2 hydrogenation reaction[J]. Appl Catal B: Environ,2021,295:120319. [6] CHEN X D, CHEN Y, SONG C Y, et al. Recent advances in supported metal catalysts and oxide catalysts for the reverse water-gas shift reaction[J]. Front Chem,2020,8:709. [7] SU X, YANG X, ZHAO B, et al. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: Recent advances and the future directions[J]. J Energy Chem,2017,26:854−867. doi: 10.1016/j.jechem.2017.07.006 [8] XI H J, HOU X N, LIU Y J, et al. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming[J]. Angew Chem Int Ed,2014,53:11886−11889. doi: 10.1002/anie.201405213 [9] LIU Y J, QING S J, HOU X N, et al. Temperature dependence of Cu-Al spinel formation and its catalytic performance in methanol steam reforming[J]. Catal Sci Technol,2017,7:5069−5078. doi: 10.1039/C7CY01236E [10] LIU Y J, QING S J, HOU X N, et al. Cu-Ni-Al spinel oxide as an efficient durable catalyst for methanol steam reforming[J]. ChemCatChem,2018,10:5698−5706. doi: 10.1002/cctc.201801472 [11] HOU X N, QING S J, LIU Y J, et al. Cu1- x Mg x Al3 spinel solid solution as a sustained release catalyst: One-pot green synthesis and catalytic performance in methanol steam reforming[J]. Fuel,2021,284:119041. doi: 10.1016/j.fuel.2020.119041 [12] LIU Y J, KANG H F, HOU X N, et al. Sustained release catalysis: Dynamic copper releasing from stoichiometric spinel CuAl2O4 during methanol steam reforming[J]. Appl Catal B: Environ,2023,323:122043. doi: 10.1016/j.apcatb.2022.122043 [13] 轩晓蝶, 刘锰钰, 郭静静, 等. 拟薄水铝石合成及其性能研究进展[J]. 工业催化,2022,30:21−30.XUAN Xiaodie, LIU Mengyu, GUO Jingjing, et al. Progresson synthesis and properties of pseudo-boehmite[J]. Ind Catal,2022,30:21−30. [14] 朱尔一, 程兆年, 陈念贻. 用PLS方法研究种子分解中拜耳石形成条件[J]. 科学通报,1991,2:157−158.ZHU Eryi, CHENG Zhaonian, CHEN Nianyi. Study on the formation conditions of Bayerite in seed decomposition using PLS method[J]. Chin Sci Bull,1991,2:157−158. [15] YANG Y, XU Y, HAN B, et al. Effects of synthetic conditions on the textural structure of pseudo-boehmite[J]. J Colloid Interf Sci,2016,469:1−7. doi: 10.1016/j.jcis.2016.01.053 [16] 唐国旗, 张春富, 孙长山, 等. 碳化法制备拟薄水铝石技术的研究进展[J]. 工业催化,2011,19:21−24.TAND Guoqi, ZHANG Chunfu, SUN Changshan, et al. Research advance on preparation of pseudo-boehmite with carbonization process[J]. Ind Catal,2011,19:21−24. [17] MICHELENE E M, MISTURE S T. Idealizing γ-Al2O3: In situ determination of nonstoichiometric spinel defect structure[J]. J Phys Chem C,2010,114:13039−13046. doi: 10.1021/jp102759y [18] 刘雅杰, 庆绍军, 侯晓宁, 等. Cu-Al 尖晶石的合成及非等温生成动力学分析[J]. 燃料化学学报,2020,48:338−348.LIU Yajie, Kang Hefei, HOU Xiaoning, et al. Synthesis of Cu-Al spinels and its non-isothermal formation kinetics analysis[J]. J Fuel Chem Technol,2020,48:338−348. [19] LAM E, CORRAL-PEREZ J J, LARMIER K, et al. CO2 Hydrogenation on Cu/Al2O3: Role of the metal/support interface in driving activity and selectivity of a bifunctional catalyst[J]. Angew Chem Int Ed,2019,58:13989−13996. doi: 10.1002/anie.201908060 [20] HU J, LI Y, ZHEN Y, et al. In situ FTIR and ex situ XPS/HS-LEIS study of supported Cu/Al2O3 and Cu/ZnO catalysts for CO2 hydrogenation[J]. Chin J Catal,2021,42:367−375. doi: 10.1016/S1872-2067(20)63672-5 [21] WU C, LIN L, LIU J, et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation[J]. Nat Commun,2020,11:5767. doi: 10.1038/s41467-020-19634-8 [22] YANG Y, MIMS C A, DISSELKAMP R S, et al. Isotope effects in methanol synthesis and the reactivity of copper formates on a Cu/SiO2 catalyst[J]. Catal Lett,2008,125:201−208. doi: 10.1007/s10562-008-9592-4 [23] FISHER I A, BELL A T. In-situ infrared study of methanol synthesis from H2[J]. J Catal,1998,178:153−173. doi: 10.1006/jcat.1998.2134 [24] ATSBHA T A, YOON T, SEONGHO P, et al. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons[J]. J CO2 Util,2021,44:101413. doi: 10.1016/j.jcou.2020.101413 [25] PAHIJA E, PANARITIS C, GUSAROV S, et al. Experimental and computational synergistic design of Cu and Fe catalysts for the reverse water-gas shift: A review[J]. ACS Catal,2022,12:6887−6905. doi: 10.1021/acscatal.2c01099 -




 下载:
下载: