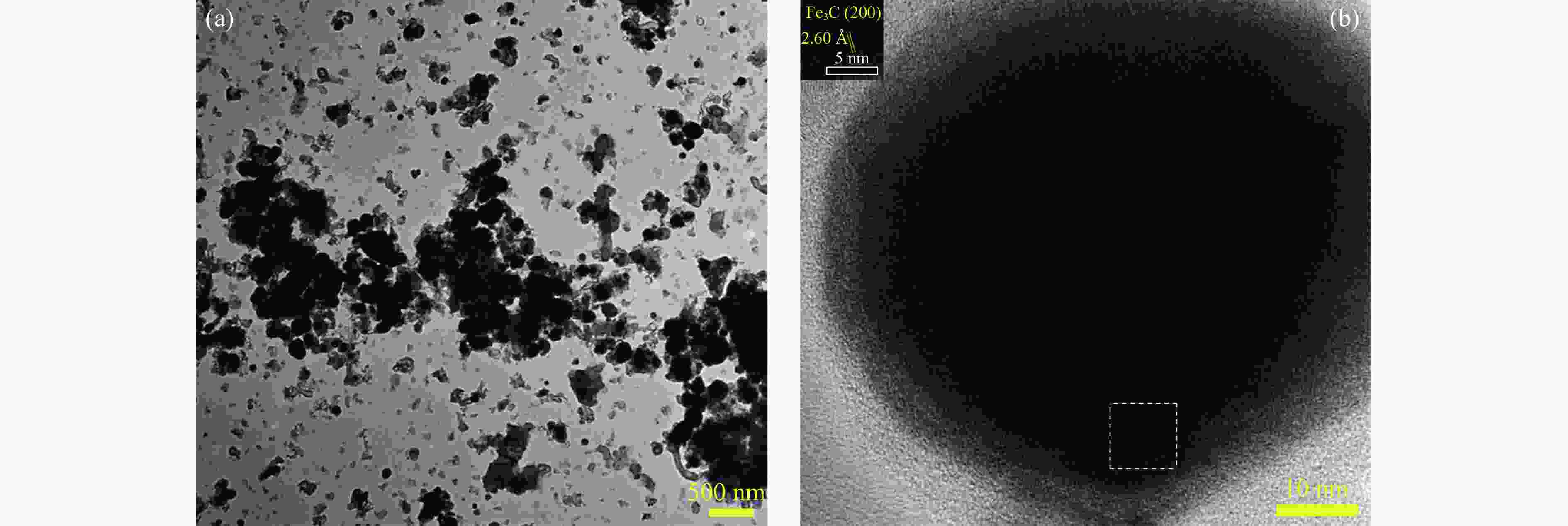
In situ reduction and carbonation of organogel containing Fe and Mn and their catalytic performance in Fischer-Tropsch synthesis
-
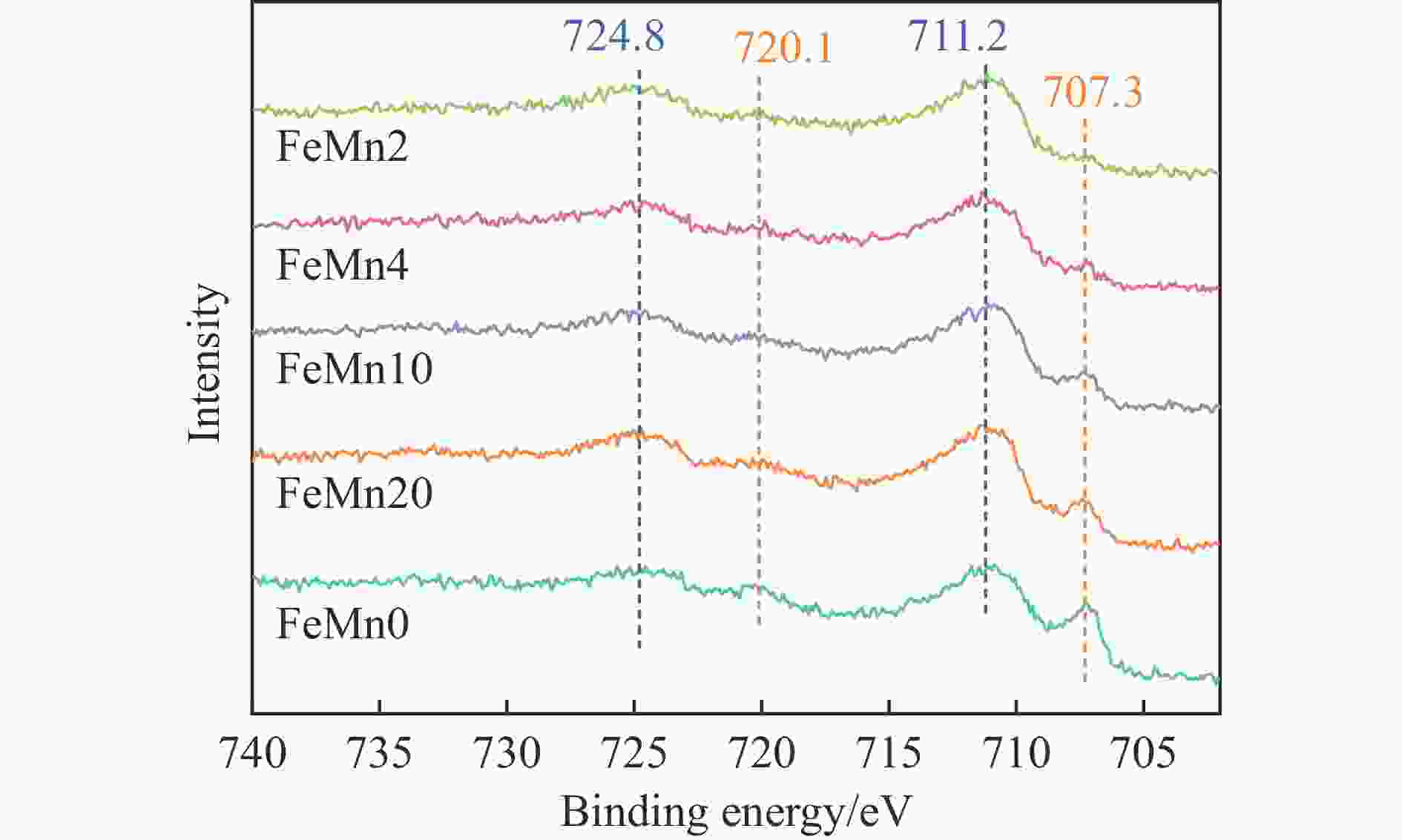
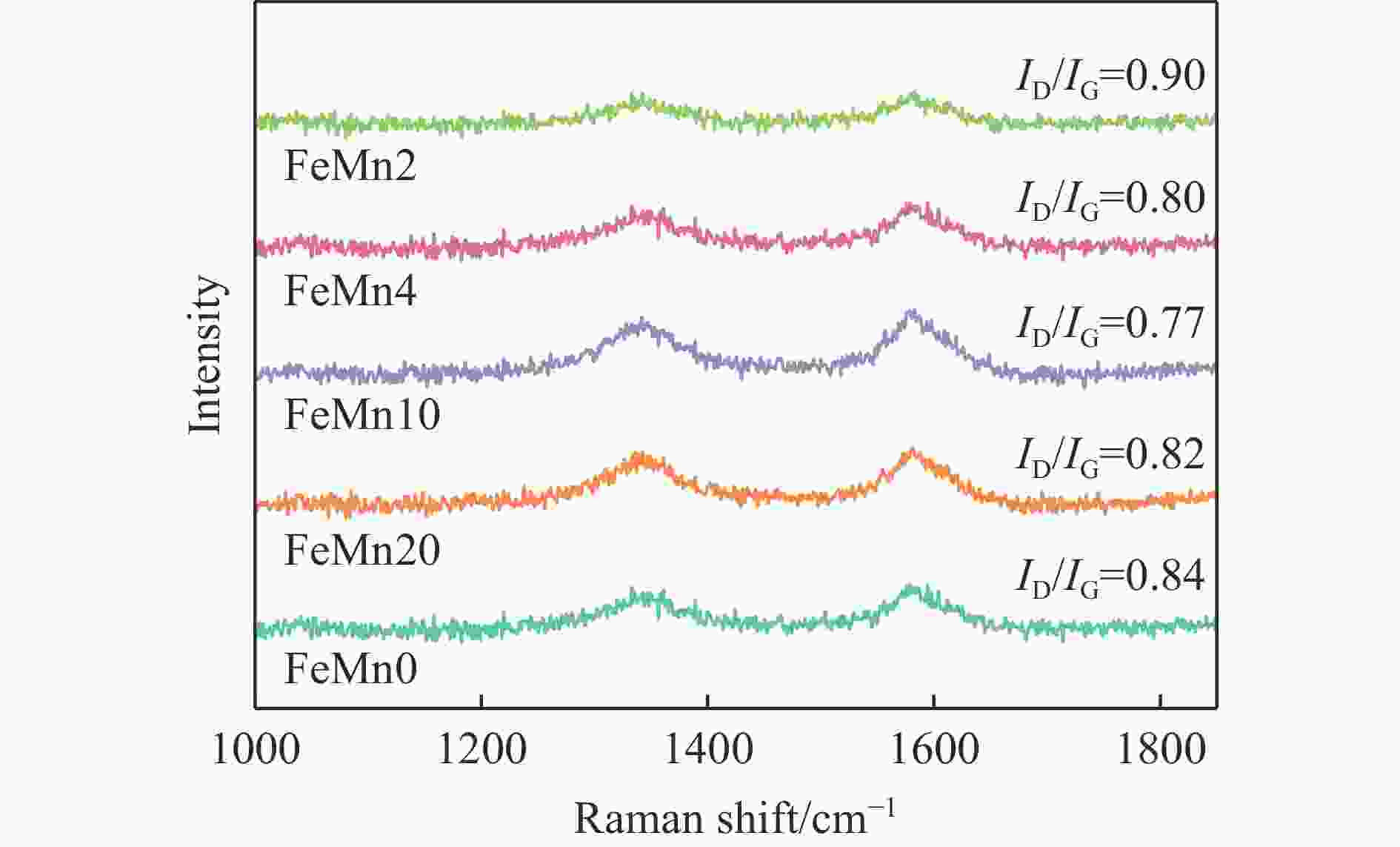
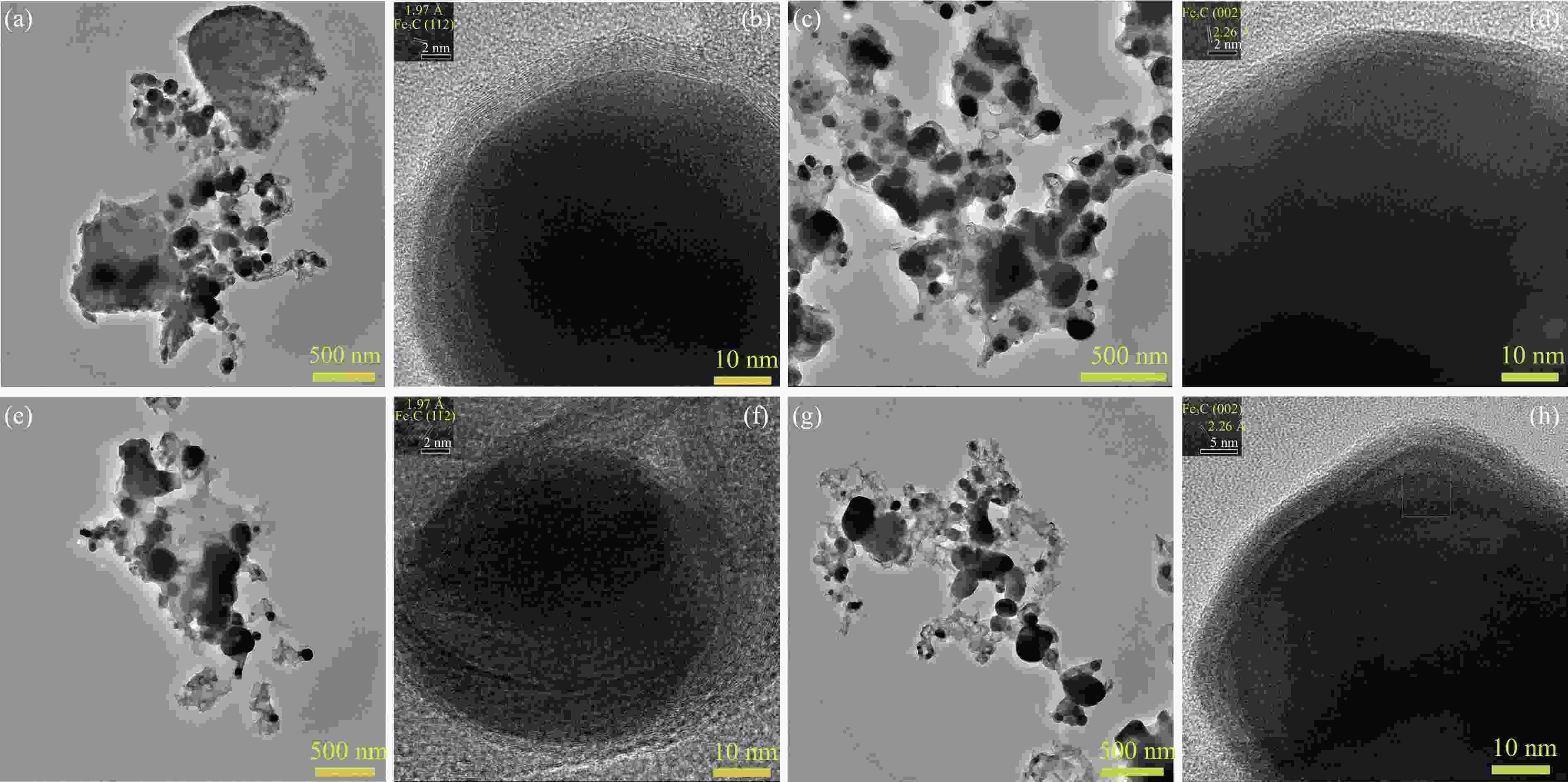
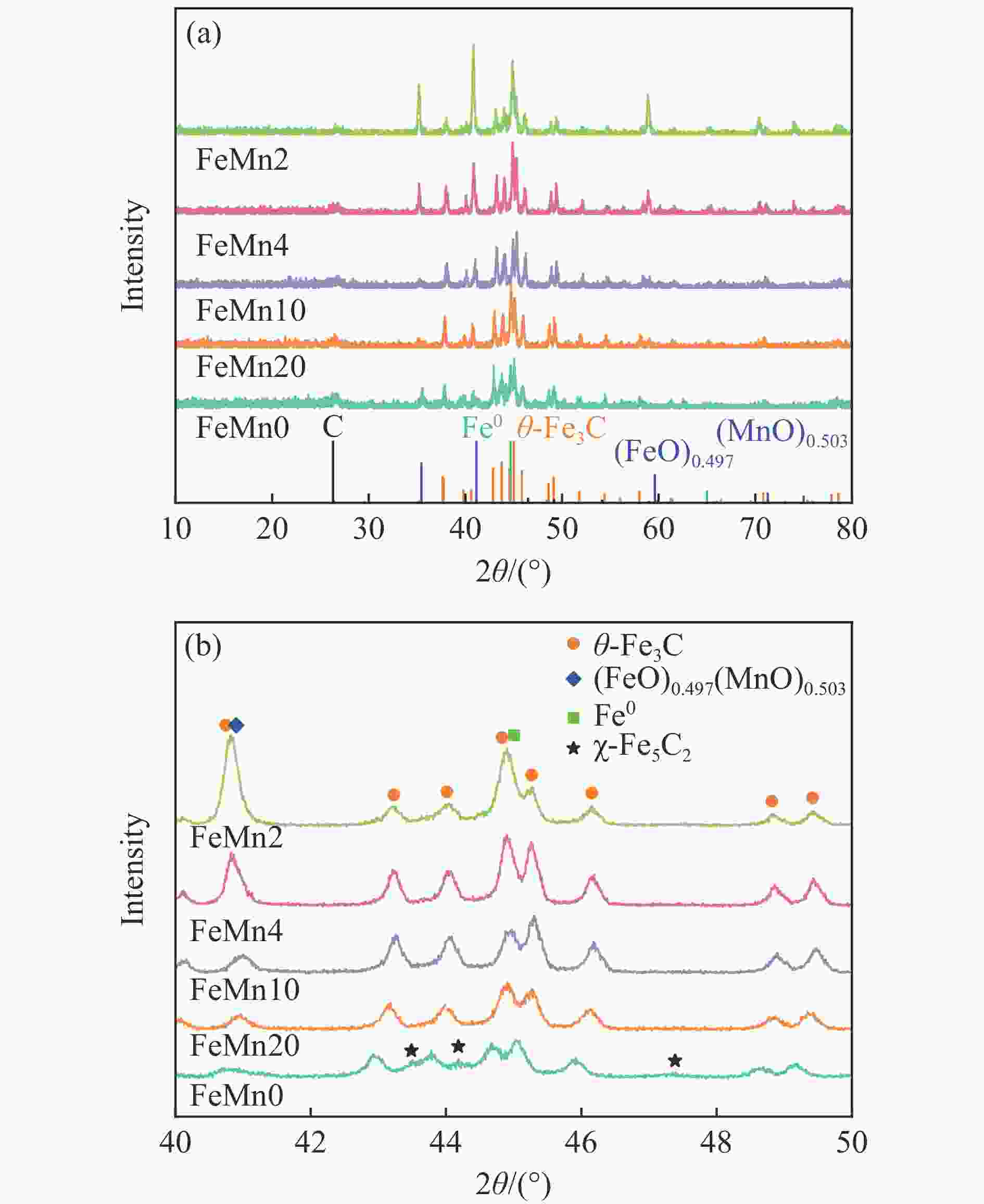
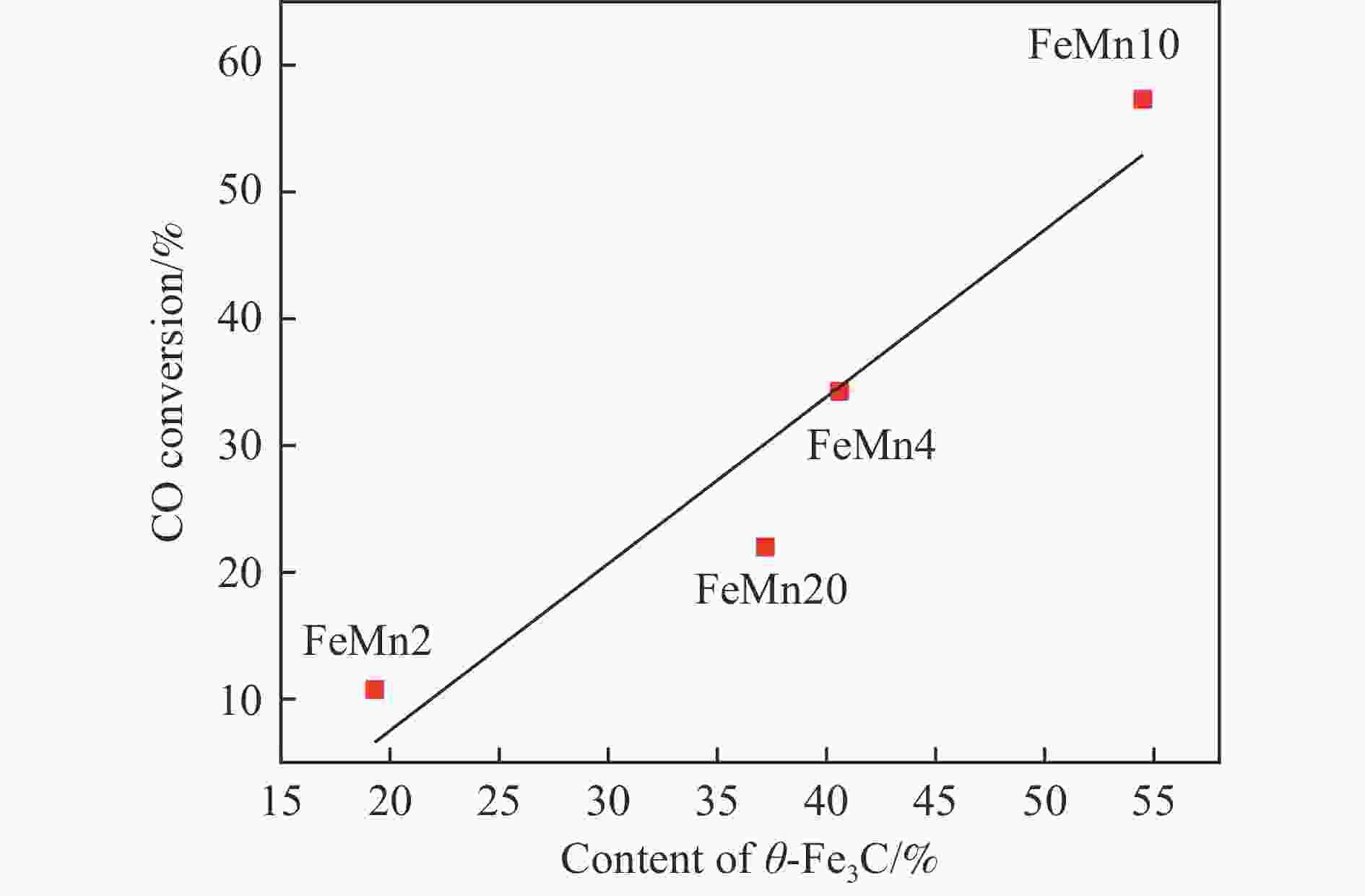
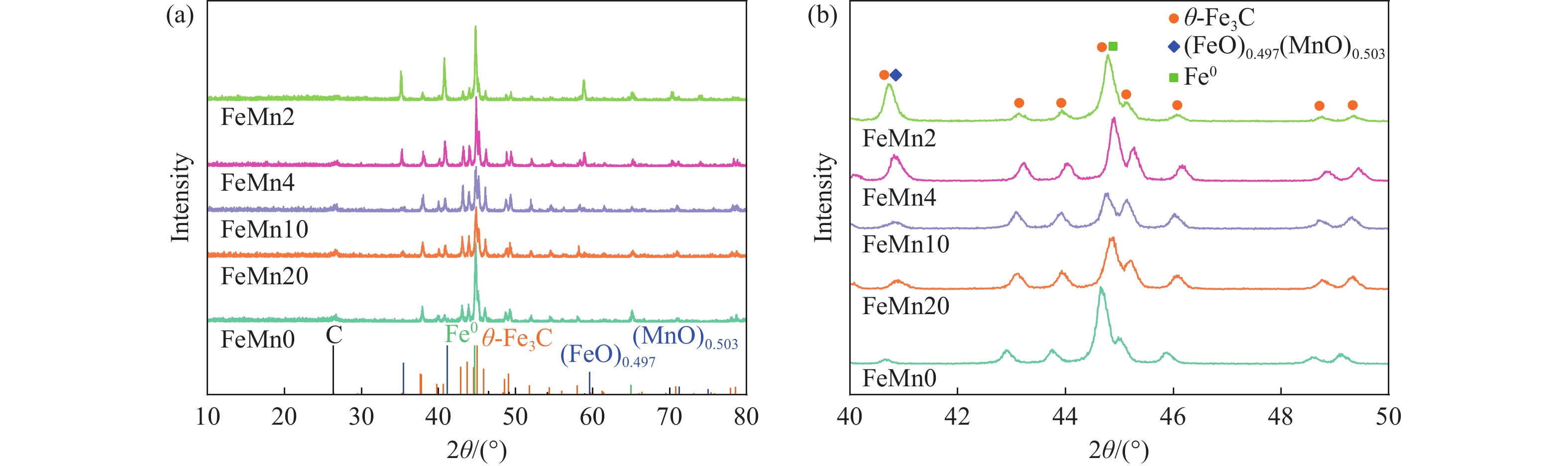
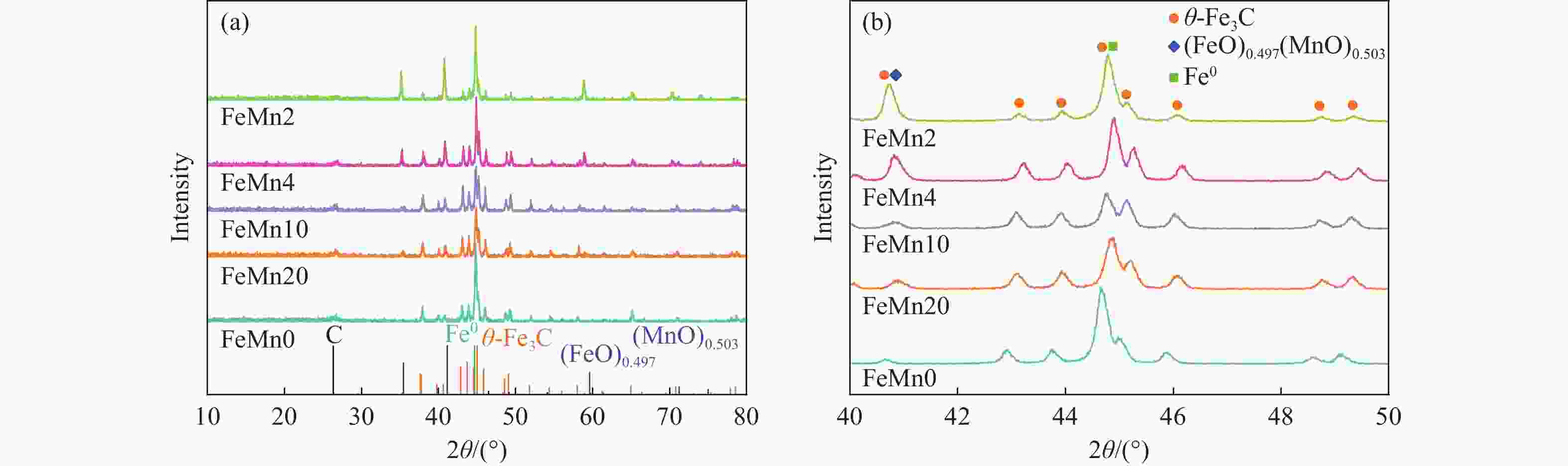
摘要: 论文制备了系列含Fe、Mn的有机凝胶前驱体,在氩气氛围下通过高温热处理,凝胶中铁物种被有机物原位分解进行还原和碳化,制备出了θ-Fe3C含量不同的费托合成催化剂。采用XRD、N2吸附、Raman、CO-TPD、CO2-TPD、XPS和TEM等手段对催化剂的结构组成、表面性质以及活性物种的电子价态进行了系统的表征和分析。实验结果表明,热处理后获得的催化剂含石墨碳、θ-Fe3C、Fe0和(FeO)0.497(MnO)0.503物相,费托反应后催化剂的结构保持稳定,物相种类不发生变化。系统地考察了反应条件对催化性能的影响,FeMn10催化剂具有较优的催化性能,CO转化率为57.3%,低碳烯烃(C2-C4)选择性为37.1%,其中θ-Fe3C物相作为催化活性位点,催化剂的活性和低碳烯烃的选择性与θ-Fe3C的含量具有正相关性。Abstract: Light olefins constitute crucial chemical commodities primarily obtained from petroleum through naphtha cracking processes. Given China's energy landscape, characterized by a scarcity of oil, limited natural gas resources, and substantial coal reserves, leveraging coal for synthesizing light olefins emerges as a strategic pathway. This approach not only reduces reliance on petroleum resources but also enhances the value proposition of coal reservoirs. Coal-to-olefin conversion pathways encompass both direct (FTO) and indirect (MTO) methodologies. Notably, the FTO route stands out as a more efficiently and economically viable strategy for coal resource utilization. Fischer-Tropsch synthesis relies on iron carbides as active sites, posing a challenge in elucidating the distinct roles of single-phase iron carbide species within catalysts derived from CO or syngas. To address this challenge, we synthesized a range of organogel precursors incorporating Fe and Mn species. Subsequent in-situ reduction and carbonization of Fe species within the gel matrix under high-temperature conditions in an argon environment yielded Fischer-Tropsch catalysts featuring varying contents of θ-Fe3C species. The structural composition, surface properties and electronic valence states of the active species of the catalysts were systematically characterised and analysed by XRD, N2 adsorption, Raman spectroscopy, CO-TPD, CO2-TPD, XPS, and TEM measurements. The resulting catalysts exhibited a composite composition comprising graphitic carbon, θ-Fe3C, Fe0, and (FeO)0.497(MnO)0.503 phases. Catalysts lacking Mn promoter demonstrated superior catalytic activity (91.4%) but lower selectivity towards light olefins (16.0%), with the emergence of the χ-Fe5C2 phase post-reaction. This was attributed to the χ-Fe5C2 species had higher intrinsic catalytic activity than θ-Fe3C species. For the catalysts with Mn promoter, the structure of the catalysts and the species of the physical phase remained stable after the Fischer-Tropsch reaction. We believed that Mn promoter played the role of structural promoter and displayed a stabilizing role in the phase structure of the catalysts. Fine-tuning the content of θ-Fe3C within the catalysts by varying Mn promoter addition enabled a deeper exploration of the correlation between catalytic performance and content of θ-Fe3C. Fine-tuning the content of θ-Fe3C within the catalysts by varying Mn promoter addition enabled a deeper exploration of the correlation between catalytic performance and content of θ-Fe3C. Quantification of θ-Fe3C content via XRD revealed that content of θ-Fe3C of the FeMn10 catalysts exhibited approximately 54.5%, resulting in a CO conversion rate of 57.3% and light olefins selectivity of 37.1%. In contrast, content of θ-Fe3C of the FeMn2 catalysts displayed roughly 19.3%, yielding a CO conversion rate of 10.7% and light olefins selectivity of 24.1%. These findings underscored the pivotal role of θ-Fe3C as the catalytic core in Fischer-Tropsch reactions, positively correlating with both CO conversion and light olefins selectivity. In addition, the FeMn catalysts exhibited low CO2 selectivity attributed to the hydrophobic nature of carbon material generated from organic gel pyrolysis. This phenomenon curbed iron carbide oxidation by water, thereby reducing the formation of Fe3O4 species and exerting a suppressive effect on the water-gas shift (WGS) reaction. θ-Fe3C catalysts exhibited excellent light olefins selectivity and low CO2 selectivity in Fischer-Tropsch synthesis, and had potential for industrial applications.
-
Key words:
- Fischer-Tropsch synthesis /
- light olefins /
- θ-Fe3C /
- Mn
-
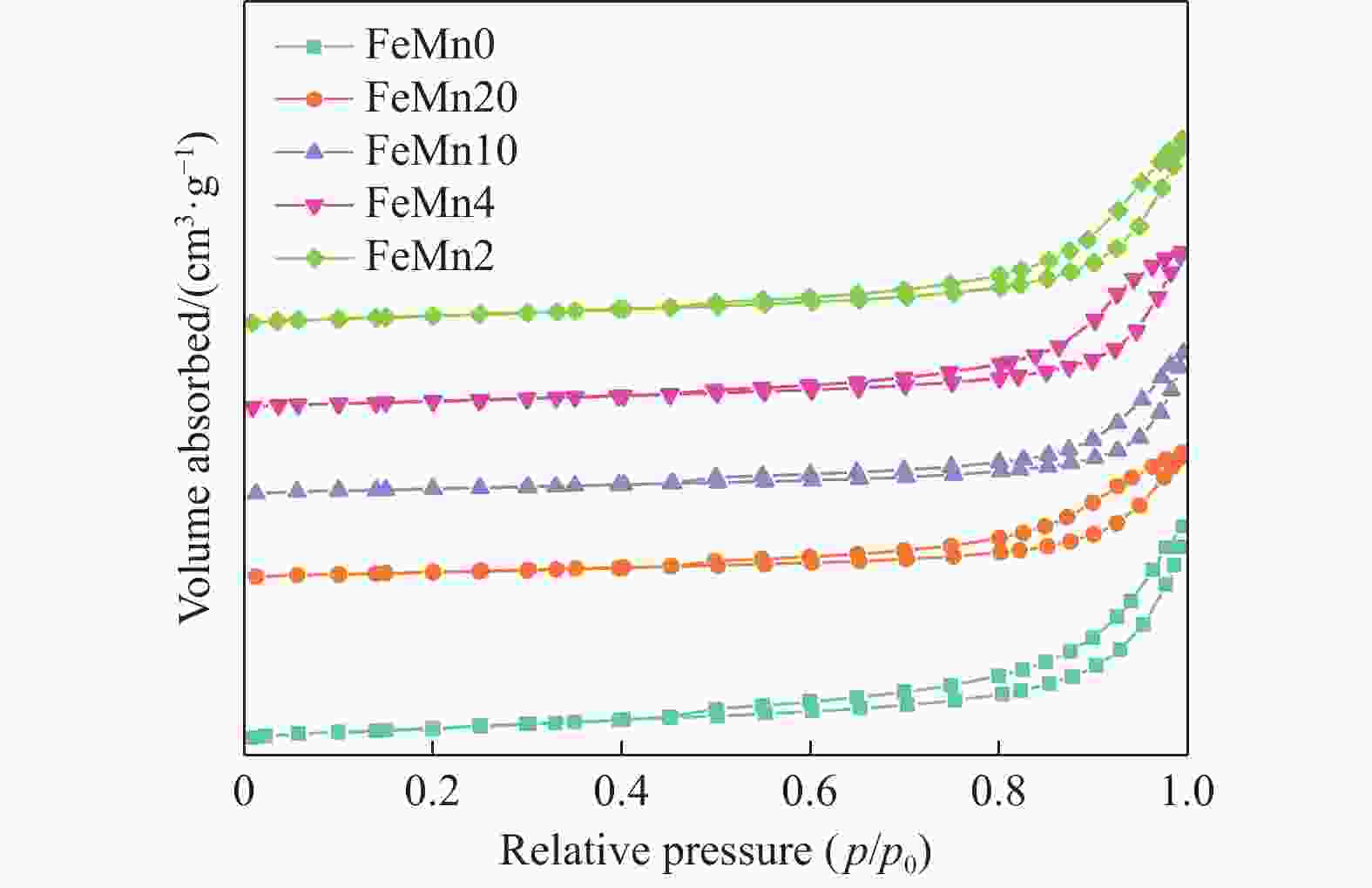
表 1 FeMn催化剂的物理化学性质
Table 1 Physico-chemical properties of the FeMn catalysts
Samples SBET/(m2·g−1) Pore volume/(cm3·g−1) Pore size/nm n(Fe)∶n(Mn)* FeMn0 46.8 0.17 14.7 − FeMn20 26.4 0.10 15.2 15.0 FeMn10 25.6 0.10 17.1 7.8 FeMn4 32.1 0.13 16.1 3.4 FeMn2 35.3 0.15 15.8 1.9 *: Results were obtained by ICP-OES. 表 2 FeMn催化剂的CO2脱附量
Table 2 CO2 desorption amount of the FeMn catalysts
Samples CO2 desorption amount (μmol gcat−1) T<200 ℃ T>200 ℃ FeMn0 49.0 20.0 FeMn20 62.0 31.4 FeMn10 71.4 45.1 FeMn4 88.0 32.0 FeMn2 71.8 17.5 表 3 不同Mn含量的催化剂的催化性能
Table 3 3 Catalytic performance of catalysts with different Mn contents
Samples CO conv.
(%)CO2 sel.
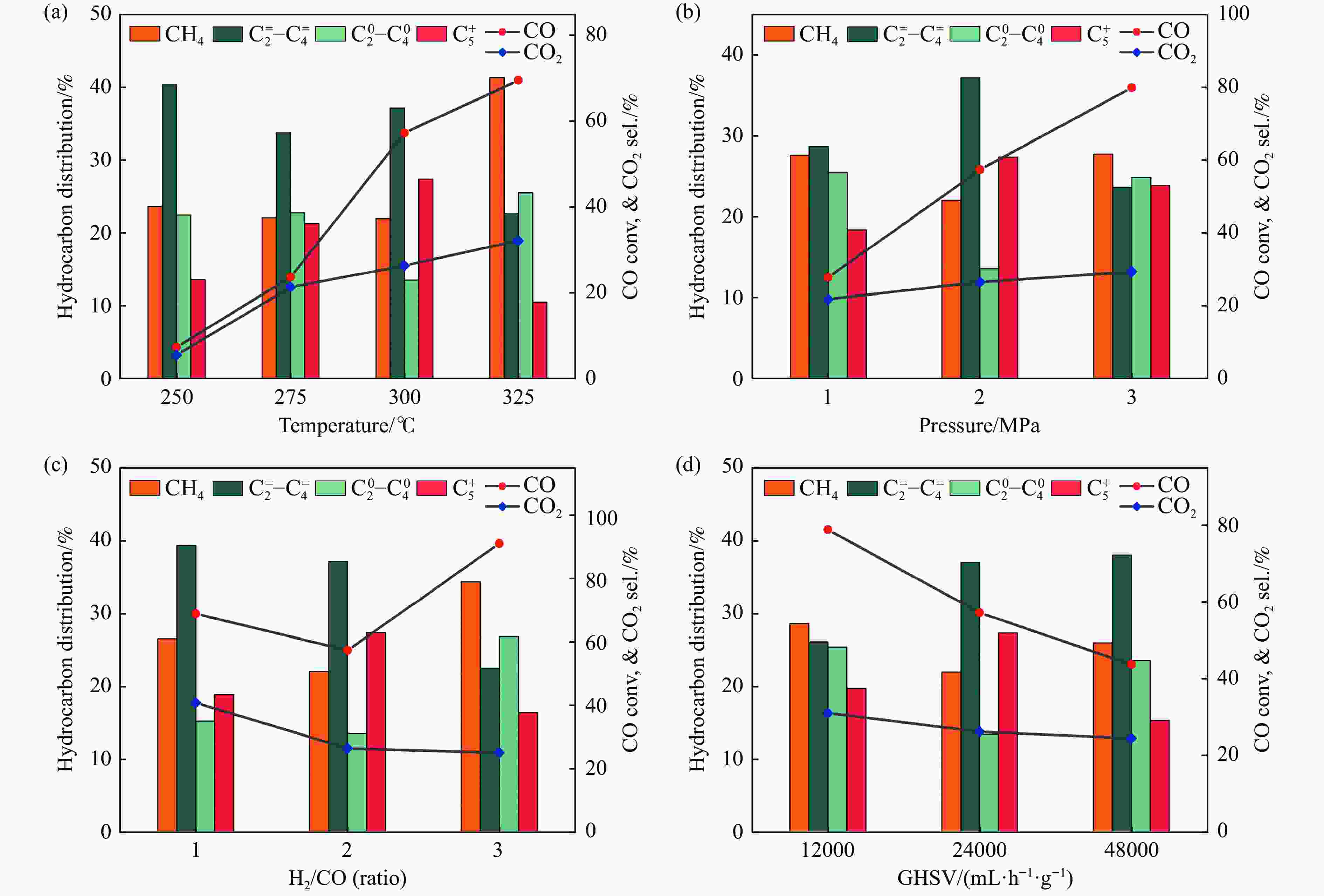
(%)Selectivity (%) O/P CH4 C2=-C4= C20-C40 C5 + FeMn0 91.4 28.6 33.0 16.0 33.2 17.8 0.48 FeMn20 22.0 12.2 29.9 27.5 27.1 15.5 1.02 FeMn10 57.3 26.4 22.0 37.1 13.5 27.4 2.75 FeMn4 34.3 19.6 29.5 30.2 27.9 12.4 1.08 FeMn2 10.7 11.1 33.1 24.1 32.9 9.9 0.73 Reaction conditions:H2/CO = 2,GHSV = 24000 mL·h−1·g−1,t = 300 ℃,p = 2 MPa,TOS = 20 h;O/P = C2=-C4=/C20-C40。 -
[1] TORRES GALVIS H M, DE JONG K P. Catalysts for production of lower olefins from synthesis gas: A review[J]. ACS Catal,2013,3(9):2130−2149. doi: 10.1021/cs4003436 [2] 王佳, 高秀娟, 宋法恩, 等. 低钼锡比催化剂中钼的价态对甲醇氧化制甲缩醛反应性能的影响[J]. 燃料化学学报(中英文),2024,52(1):38−46.WANG Jia, GAO Xiujuan, SONG Faen, et al. Effect of molybdenum valence in low Mo/Sn ratio catalysts for the oxidation of methanol to dimethoxymethane[J]. J Fuel Chem Technol,2024,52(1):38−46. [3] GAO X, ZHANG J, CHEN N, et al. Effects of zinc on Fe-based catalysts during the synthesis of light olefins from the Fischer-Tropsch process[J]. Chin J Catal,2016,37(4):510−516. doi: 10.1016/S1872-2067(15)61051-8 [4] 刘赛赛, 姚金刚, 陈冠益, 等. 合成气一步法制备低碳烯烃和液体燃料催化剂研究进展[J]. 燃料化学学报(中英文),2023,51(1):34−51.LIU Saisai, YAO Jingang, CHEN Guanyi, et al. One-step catalyst for the preparation of light olefins and liquid fuels from syngas[J]. J Fuel Chem Technol,2023,51(1):34−51. [5] CHEN X, DENG D, PAN X, et al. Iron catalyst encapsulated in carbon nanotubes for CO hydrogenation to light olefins[J]. Chin J Catal,2015,36(9):1631−1637. doi: 10.1016/S1872-2067(15)60882-8 [6] LI Z, ZHONG L, YU F, et al. Effects of sodium on the catalytic performance of CoMn catalysts for Fischer-Tropsch to olefin reactions[J]. ACS Catal,2017,7(5):3622−3631. doi: 10.1021/acscatal.6b03478 [7] MENG G, SUN J, TAO L, et al. Ru1Con Single-Atom Alloy for Enhancing Fischer-Tropsch Synthesis[J]. ACS Catal,2021,11(3):1886−1896. doi: 10.1021/acscatal.0c04162 [8] 陈治平, 张智, 周文武, 等. 碳化铁的制备及其在费托合成中的应用研究进展[J]. 燃料化学学报(中英文),2022,50(11):1381−1392.CHEN Zhiping, ZHANG Zhi, ZHOU Wenwu, et al. Preparation of iron carbide and its application in Fischer-Tropsch synthesis[J]. J Fuel Chem Technol,2022,50(11):1381−1392. [9] DE S E, BEALE A M, NIKITENKO S, et al. Local and long range order in promoted iron-based Fischer-Tropsch catalysts: a combined in situ X-ray absorption spectroscopy/wide angle X-ray scattering study[J]. J Catal,2009,262(2):244−256. doi: 10.1016/j.jcat.2008.12.021 [10] DING M, YANG Y, WU B, et al. Study on reduction and carburization behaviors of iron phases for iron-based Fischer-Tropsch synthesis catalyst[J]. Appl Energy,2015,160:982−989. doi: 10.1016/j.apenergy.2014.12.042 [11] MA C, ZHANG W, CHANG Q, et al. θ-Fe3C dominated Fe@C core–shell catalysts for Fischer-Tropsch synthesis: Roles of θ-Fe3C and carbon shell[J]. J Catal,2021,393:238−246. doi: 10.1016/j.jcat.2020.11.033 [12] ZHANG W, MA C, LIU X, et al. Single-phase θ-Fe3C derived from prussian blue and its catalytic application in fischer-tropsch synthesis[J]. Catalysts,2022,12(10):1140. doi: 10.3390/catal12101140 [13] LIU Y, CHEN J, BAO J, et al. Manganese-modified Fe3O4 microsphere catalyst with effective active phase of forming light olefins from syngas[J]. ACS Catal,2015,5(6):3905−3909. doi: 10.1021/acscatal.5b00492 [14] YANG Z, ZHANG Z, LIU Y, et al. Tuning direct CO hydrogenation reaction over Fe-Mn bimetallic catalysts toward light olefins: Effects of Mn promotion[J]. Appl Catal B:Environ,2021,285:119815. doi: 10.1016/j.apcatb.2020.119815 [15] 张建利, 王旭, 马丽萍, 等. MgFeMn-HTLcs的制备、改性及其CO加氢性能[J]. 化工学报,2018,69(5):2073−2080.ZHANG Jianli, WANG Xu, MA Liping, et al. Preparation of modified MgFeMn-HTLcs and catalytic performance in CO hydrogenation[J]. CIESC J,2018,69(5):2073−2080. [16] SHI B, ZHANG Z, LIU Y, et al. Promotional effect of Mn-doping on the structure and performance of spinel ferrite microspheres for CO hydrogenation[J]. J Catal,2020,381:150−162. doi: 10.1016/j.jcat.2019.10.034 [17] 张庆玲, 郭荷芹, 侯博, 等. Mn和Zr助剂对介孔碳负载钴基催化剂费托合成反应性能的影响[J]. 燃料化学学报(中英文),2017,45(6):682−688.ZHANG Qingling, GUO Heqin, HOU Bo, et al. Effects of Mn and Zr promoters on the performance of ordered mesoporous carbon supported Co catalyst in Fischer-Tropsch synthesis[J]. J Fuel Chem Technol,2017,45(6):682−688. [18] 马丽萍, 张建利, 马清祥, 等. K/MgFeZn-HTLcs催化CO加氢制低碳烯烃性能研究[J]. 燃料化学学报(中英文),2016,44(4):449−456.MA Liping, ZHANG Jianli, MA Qingxiang, et al. Direct synthesis of light olefins from CO hydrogenation over K/MgFeZn-HTLcs catalysts[J]. J Fuel Chem Technol,2016,44(4):449−456. [19] 张建利, 王旭, 马丽萍, 等. 层状K/Mg-Fe-Al催化剂的制备及其CO加氢性能研究[J]. 燃料化学学报(中英文),2017,45(12):1489−1498. doi: 10.1016/S1872-5813(17)30066-XZHANG Jianli, WANG Xu, MA Liping, et al. Preparation of layered K/Mg-Fe-Al catalysts and its catalytic performances in CO hydrogenation[J]. J Fuel Chem Technol,2017,45(12):1489−1498. doi: 10.1016/S1872-5813(17)30066-X [20] 杨向平, 郭晓雪, 张成华, 等. 金属有机骨架材料Fe-MIL-100诱导的铁基费托催化剂的合成及催化性能研究[J]. 化学学报,2017,75(4):360−366. doi: 10.6023/A16100549YANG Xiangping, GUO Xiaoxue, ZHANG Chenghua, et al. Synthesis and Catalytic Properties of Iron Based Fischer-Tropsch Catalyst Mediated by MOFs Fe-MIL-100[J]. Acta Chim Sinica,2017,75(4):360−366. doi: 10.6023/A16100549 [21] 李宁, 马彩萍, 张成华, 等. MOFs衍生炭负载的钴基催化剂的廉价制备及其CO加氢催化性能[J]. 燃料化学学报(中英文),2019,47(4):428−437. doi: 10.1016/S1872-5813(19)30020-9LI Ning, MA Cai-ping, ZHANG Cheng-hua, et al. Low-cost preparation of carbon-supported cobalt catalysts from MOFs and their performance in CO hydrogenation[J]. J Fuel Chem Technol,2019,47(4):428−437. doi: 10.1016/S1872-5813(19)30020-9 [22] SUN J, ZHENG S, CHEN J. Influence of pretreatment conditions on the structure and catalytic performance of supported cobalt catalysts derived from metal-organic frameworks[J]. J Fuel Chem Technol,2023,51(9):1291−1297. doi: 10.1016/S1872-5813(23)60352-4 [23] 赵云鹏, 赵薇, 司兴刚, 等. Co@C催化木质素衍生酚类化合物的加氢转化[J]. 燃料化学学报(中英文),2021,49(1):55−62.ZHAO Yunpeng, ZHAO Wei, SI Xinggang, et al. Hydrogenation of lignin-derived phenolic compounds over Co@C catalysts[J]. J Fuel Chem Technol,2021,49(1):55−62. [24] WANG A, LUO M, LV B, et al. MOF-derived porous carbon-supported bimetallic Fischer-Tropsch synthesis catalysts[J]. Ind Eng Chem Res,2022,61(11):3941−3951. doi: 10.1021/acs.iecr.1c03810 [25] AN B, CHENG K, WANG C, et al. Pyrolysis of metal–organic frameworks to Fe3O4@Fe5C2 core-shell nanoparticles for Fischer-Tropsch synthesis[J]. ACS Catal,2016,6(6):3610−3618. doi: 10.1021/acscatal.6b00464 [26] WEZENDONK T A, SANTOS V P, NASALEVICH M A, et al. Elucidating the nature of Fe species during pyrolysis of the Fe-BTC MOF into highly active and stable Fischer-Tropsch catalysts[J]. ACS Catal,2016,6(6):3236−3247. [27] KODAMA T, OOKUBO M, MIURA S, et al. Synthesis and characterization of ultrafine Mn(II)-bearing ferrite of type MnxFe3-xO4 by coprecipitation[J]. Mater Res Bull,1996,31(12):1501−1512. doi: 10.1016/S0025-5408(96)00146-8 [28] MUNIR S, AMIN M, IQBAL N, et al. Effect of Pyrolysis on iron-metal organic frameworks (MOFs) to Fe3C@ Fe5C2 for diesel production in Fischer-Tropsch Synthesis[J]. Front Chem,2023,11:1150565. doi: 10.3389/fchem.2023.1150565 [29] AL-DOSSARY M, FIERRO J, SPIVEY J. Cu-promoted Fe2O3/MgO-based Fischer-Tropsch catalysts of biomass-derived syngas[J]. Ind Eng Chem Res,2015,54(3):911−921. doi: 10.1021/ie504473a [30] WU X, MA H, ZHANG H, et al. High-temperature Fischer-Tropsch synthesis of light olefins over nano-Fe3O4@MnO2 core-shell catalysts[J]. Ind Eng Chem Res,2019,58(47):21350−21362. doi: 10.1021/acs.iecr.9b04221 [31] WAN H, WU B, LI T, et al. Effects of SiO2 and Al2O3 on performances of iron-basedcatalysts for slurry Fischer-Tropsch synthesis[J]. J Fuel Chem Technol,2007,35(5):589−594. doi: 10.1016/S1872-5813(07)60036-X [32] HAN Z, QIAN W, ZHANG H, et al. Effect of rare-earth promoters on precipitated iron-based catalysts for Fischer-Tropsch synthesis[J]. Ind. Eng. Chem. Res,2020,59(33):14598−14605. doi: 10.1021/acs.iecr.9b06760 [33] LYU S, LIU C, WANG G, et al. Structural evolution of carbon in an Fe@C catalyst during the Fischer-Tropsch synthesis reaction[J]. Catal Sci Technol,2019,9(4):1013−1020. doi: 10.1039/C8CY02420K [34] SALAZAR-CONTRERAS H G, MARTINEZ-HERNANDEZ A, BOIX AA, et al. Effect of Mn on Co/HMS-Mn and Co/SiO2-Mn catalysts for the Fischer-Tropsch reaction[J]. Appl Catal B:Environ,2019,244:414−426. doi: 10.1016/j.apcatb.2018.11.067 [35] LI T, WANG H, YANG Y, et al. Effect of manganese on the catalytic performance of an iron-manganese bimetallic catalyst for light olefin synthesis[J]. J Energy Chem,2013,22(4):624−632. doi: 10.1016/S2095-4956(13)60082-0 [36] ZHAO Q, HUANG S, HAN X, et al. Highly active and controllable MOF-derived carbon nanosheets supported iron catalysts for Fischer-Tropsch synthesis[J]. Carbon,2021,173:364−375. doi: 10.1016/j.carbon.2020.11.019 [37] MALHI H S, SUN C, ZHANG Z, et al. Catalytic consequences of the decoration of sodium and zinc atoms during CO2 hydrogenation to olefins over iron-based catalyst[J]. Catal Today,2022,387:28−37. doi: 10.1016/j.cattod.2021.03.009 [38] WEI C, TU W, JIA L, et al. The evolutions of carbon and iron species modified by Na and their tuning effect on the hydrogenation of CO2 to olefins[J]. Appl Surf Sci,2020,525:146622. doi: 10.1016/j.apsusc.2020.146622 [39] ZHANG J, ABBAS M, ZHAO W, et al. Enhanced stability of a fused iron catalyst under realistic Fischer-Tropsch synthesis conditions: insights into the role of iron phases (χ-Fe5C2, θ-Fe3C and α-Fe)[J]. Catal Sci Technol,2022,12(13):4217−4227. doi: 10.1039/D2CY00703G [40] HERRANZ T, ROJAS S, PEREZ-ALONSO F J, et al. Genesis of iron carbides and their role in the synthesis of hydrocarbons from synthesis gas[J]. J Catal,2006,243(1):199−211. doi: 10.1016/j.jcat.2006.07.012 -




 下载:
下载: