Catalytic conversion of CO2 into high value-added hydrocarbons over tandem catalyst
-
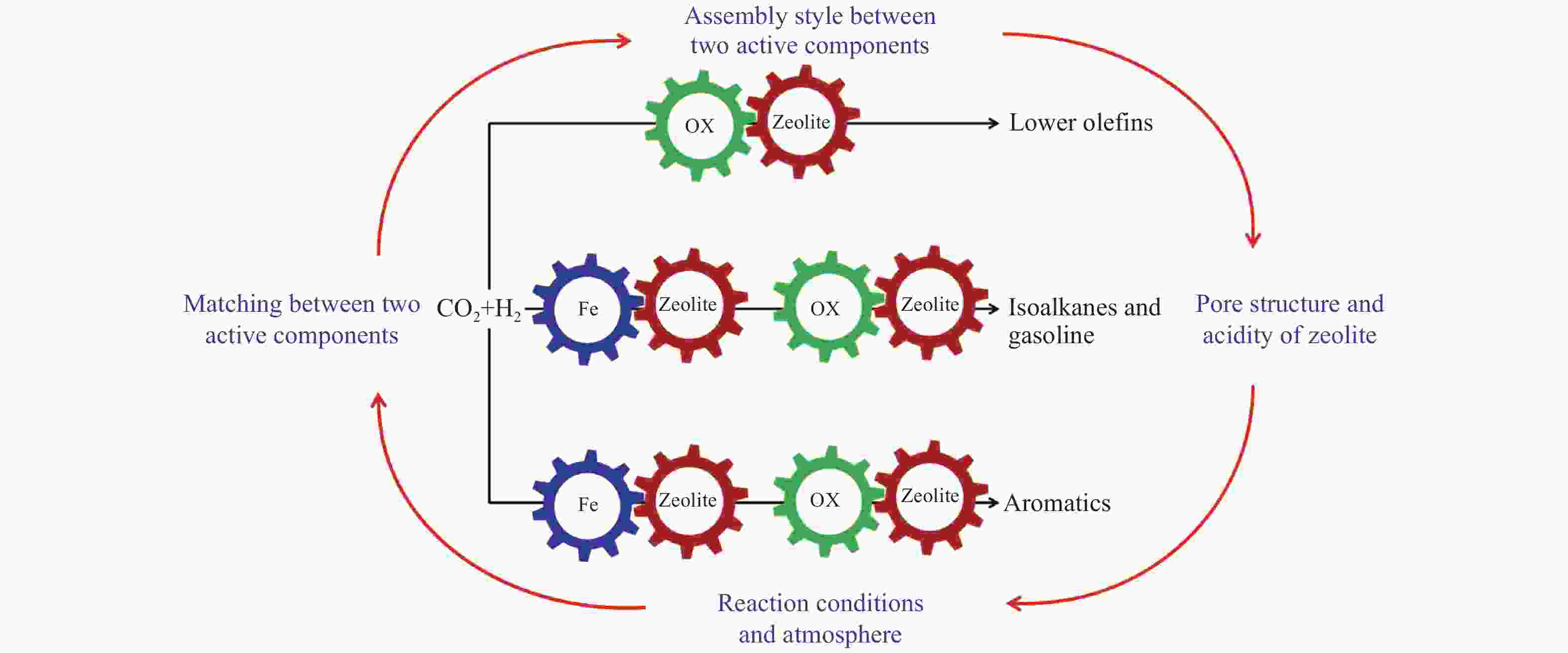
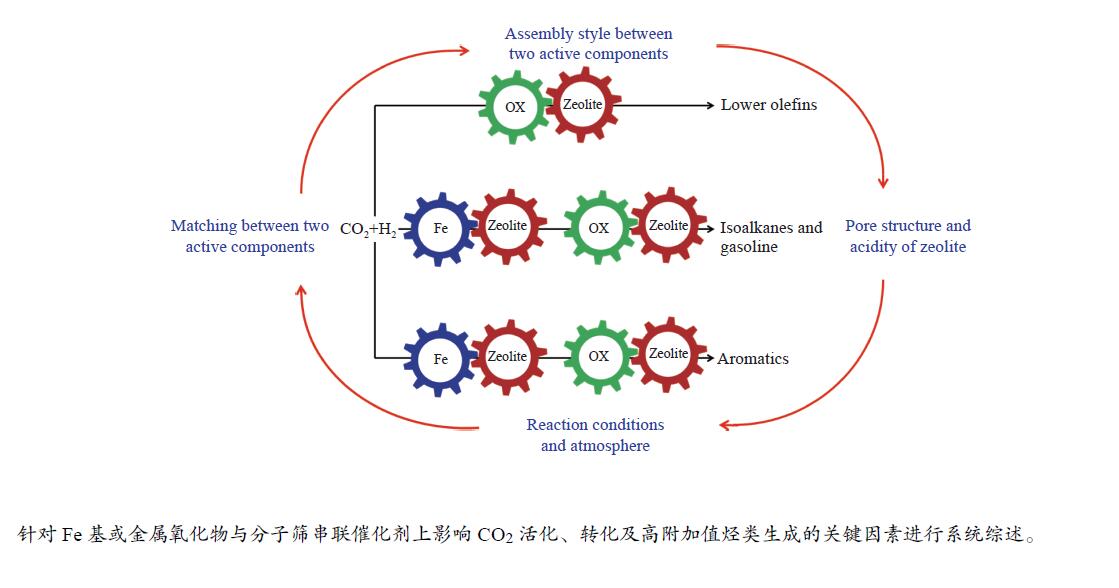
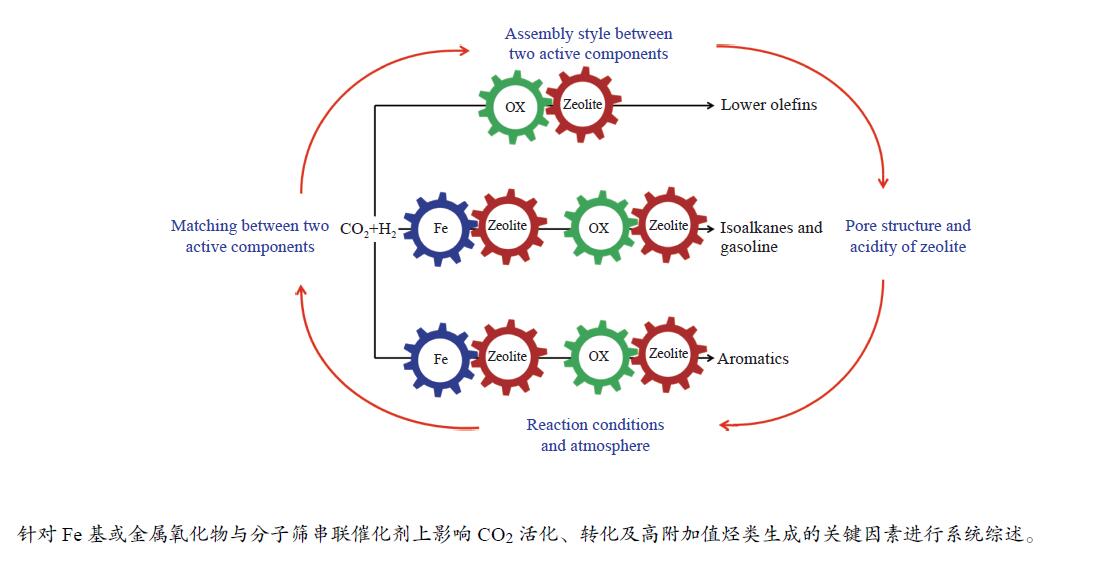
摘要: 将CO2作为可利用的碳资源催化转化为高附加值化学品或液体燃料对于节能减排和碳资源的循环利用具有重要意义。由于CO2分子的化学惰性及高的C–C键耦合能垒,导致CO2的选择性活化及可控转化极具挑战。近年来,随着研究的不断深入及串联催化体系的构建,世界各国研究者在CO2催化加氢制备高附加值烃类方面取得了突破性的研究进展。然而,在串联催化过程中,Fe基催化剂或金属氧化物与分子筛间的协同匹配、活性组分间的组装方式、分子筛的孔道结构及酸性、以及反应条件及气氛均对CO2加氢的产物分布影响显著。有鉴于此,本综述针对CO2加氢制备高附加值烃(低碳烯烃、异构烷烃、汽油及芳烃)的串联催化反应体系,重点介绍串联催化剂上影响CO2活化、转化及目标产物生成的关键因素以及串联催化剂的稳定性,并在此基础上对CO2催化加氢的未来和前景进行总结和展望。Abstract: The conversion of CO2, an abundant carbon resource, into high value-added chemicals or liquid fuels is an attractive way to mitigate carbon emissions, which is also a sustainable approach for the cyclic utilization of carbon resources. However, the selective activation and controllable conversion of CO2 is challenging because of the inertness of CO2 and high C–C coupling barrier. In recent years, some obvious breakthroughs on CO2 hydrogenation to high value-added chemicals or liquid fuels have been made by construction of a tandem catalytic system. For the tandem catalysis, the matching of Fe-based catalyst or metal oxides and zeolites, the assembly between the two active sites, the pore structure and acidity of the zeolites, as well as the reaction conditions and atmosphere all have important effects on the product distribution. Herein, the critical factors affecting the CO2 activation and conversion and the formation of the target products, as well as the stability over the tandem catalysts are summarized. Finally, an outlook is provided.
-
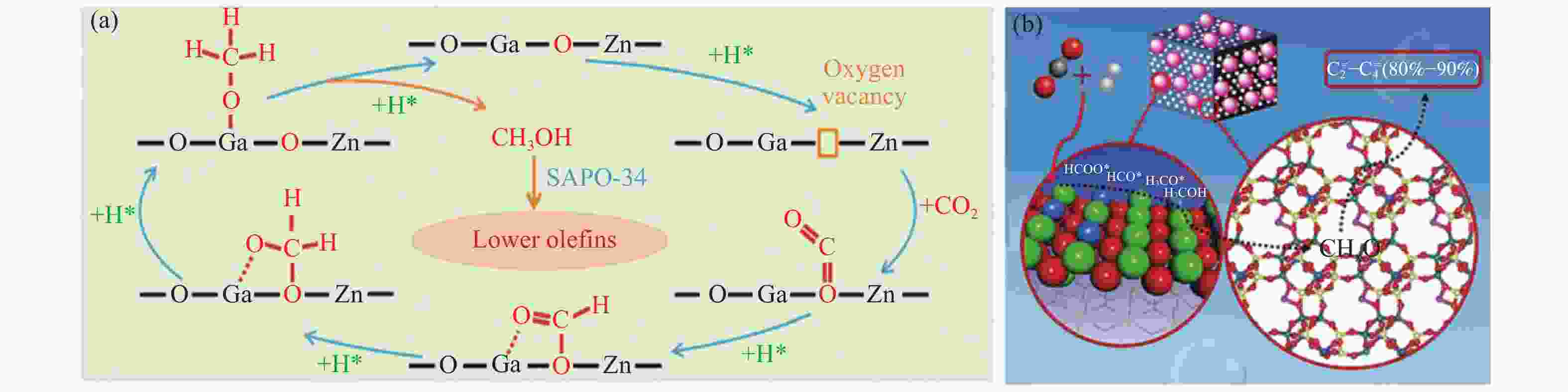
图 2 不同串联催化剂上CO2加氢制低碳烯烃的可能反应机制
Figure 2 Possible reaction mechanisms for CO2 hydrogenation to lower olefins on different tandem catalysts
(a): Zn-Ga-O/SAPO-34[43]; (b): ZnZrO/SAPO[39] reproduced with permission from ref.[43, 39], Copyright (2018) The Royal Society of Chemistry, Copyright (2017) American Chemical Society
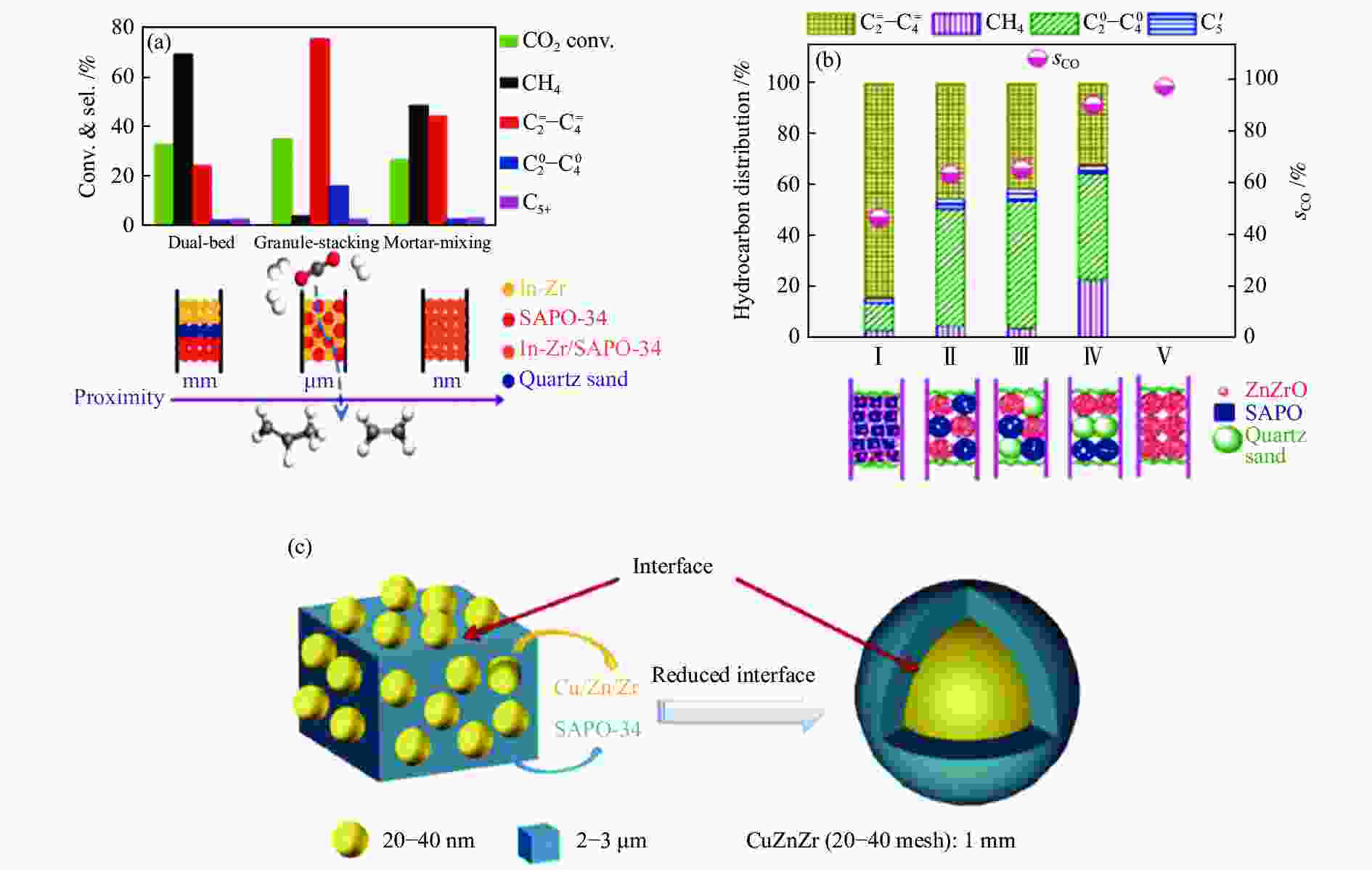
图 3 (a) In-Zr及SAPO-34的组装方式对串联催化剂上CO2加氢制低碳烯烃性能的影响[24];(b) ZnZrO及SAPO的组装方式对串联催化剂上CO2加氢制低碳烯烃性能的影响[39];(c) CuZnZr及SAPO-34两组分间的界面示意图[45]
Figure 3 (a) Effect of the assembly style between In-Zr and SAPO-34 on CO2 hydrogenation to lower olefins[24]; (b) Effect of the assembly style between ZnZrO and SAPO on CO2 hydrogenation to lower olefins[39]; (c) Schematic diagram of the interface between CuZnZr and SAPO-34[45] reproduced with permission from ref.[24, 39, 45], Copyright (2018 & 2017) American Chemical Society, Copyright (2019) Elsevier
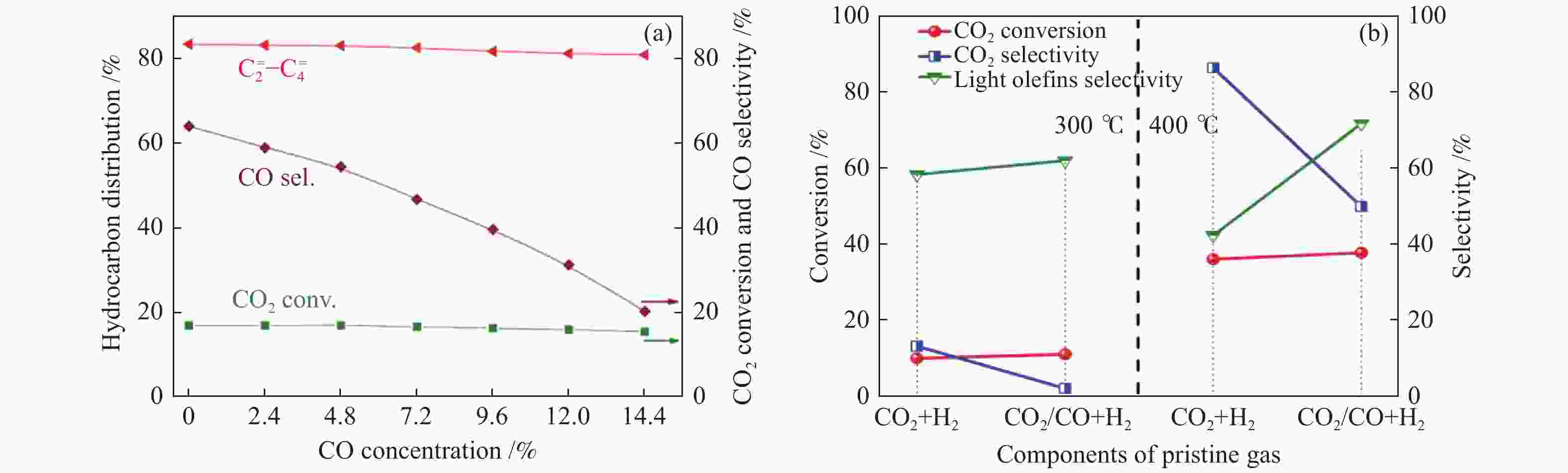
图 5 (a) In-Zr/SAPO-34的催化性能随原料气中CO浓度的变化趋势[24];(b)不同原料气氛下In2O3/ZrO2&SAPO催化剂上CO2转化制备低碳烯烃[68]
Figure 5 (a) Catalytic performance over In-Zr/SAPO-34 as a function of CO concentration[24]; (b) CO2 conversion to lower olefins from different feed gas over In2O3/ZrO2&SAPO catalyst[68] reproduced with permission from ref. [24, 68], Copyright (2018) American Chemical Society, Copyright (2019) Elsevier
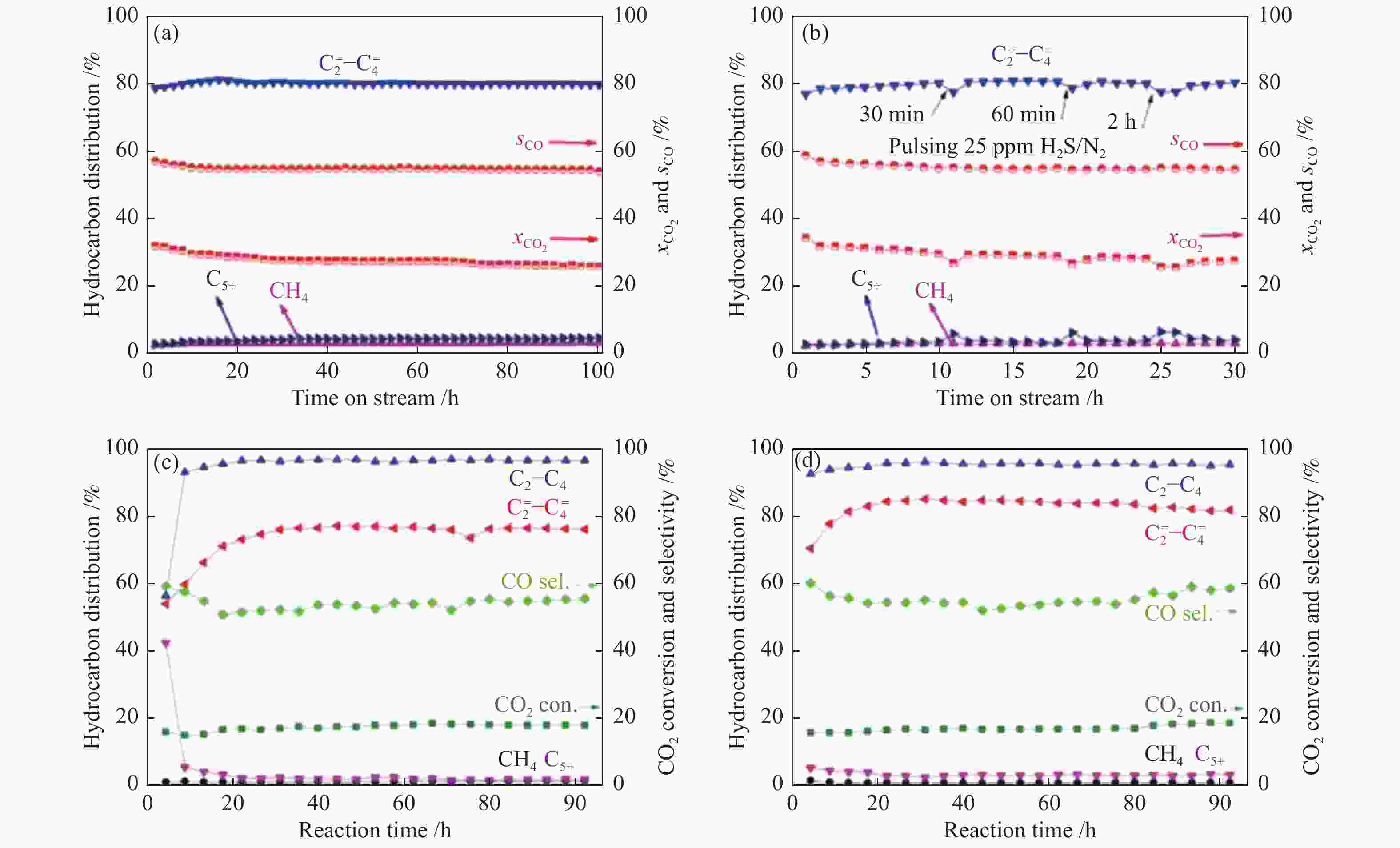
图 6 不同串联催化剂上CO2加氢制低碳烯烃的(a)、(c)、(d)稳定性及(b)抗硫性能测试
Figure 6 (a), (c), (d) Stability and (b) sulfur tolerance test of different tandem catalysts for CO2 hydrogenation to lower olefins
(a), (b): 20%Mn2O3-ZnO/SAPO-34[58]; (c): In2O3-ZnZrOx/SAPO-34-C[41]; (d): In2O3-ZnZrOx/SAPO-34-H-a[41] reproduced with permission from ref. [58, 41], Copyright (2021) Elsevier, Copyright (2019) John Wiley and Sons
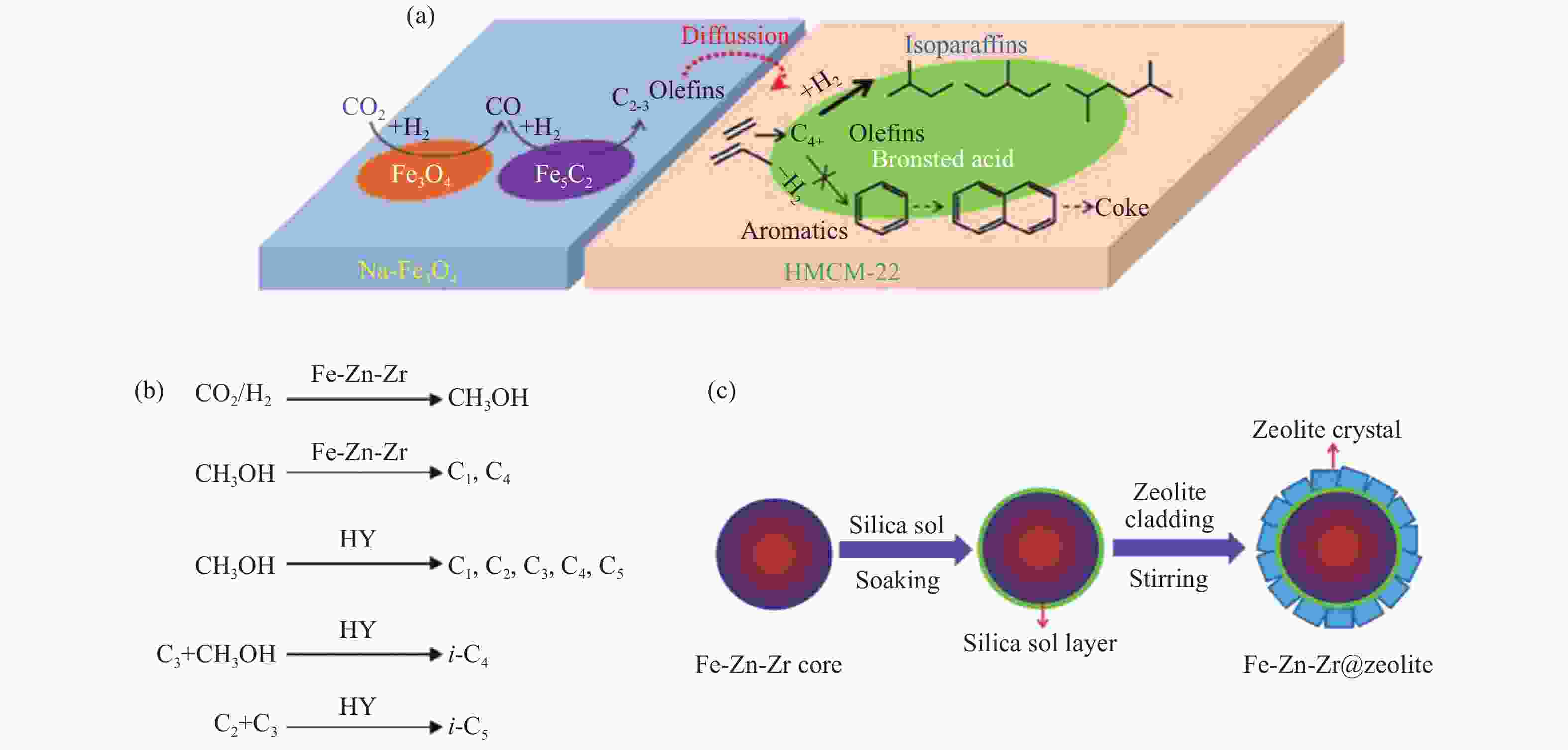
图 7 (a) Na-Fe3O4/HMCM-22串联催化剂上CO2加氢制异构烷烃及积炭形成的反应机制[72];(b) Fe-Zn-Zr/HY串联催化剂上CO2加氢制异构烷烃的反应路径[78];(c) 物理黏接法制备Fe-Zn-Zr@zeolite核壳催化剂的示意图[46]
Figure 7 (a) Reaction scheme of isoalkanes synthesis and coke formation during CO2 hydrogenation over Na-Fe3O4/HMCM-22 catalyst[72]; (b) Proposed reaction path of isoalkanes formation from CO2 hydrogenation over Fe-Zn-Zr/HY composite catalyst[78]; (c) Illustration for the Fe-Zn-Zr@zeolite core-shell catalyst preparation by a cladding method[46] reproduced with permission from ref. [72, 78, 46], Copyright (2018) American Chemical Society, Copyright (2007) Elsevier, Copyright (2016) The Royal Society of Chemistry
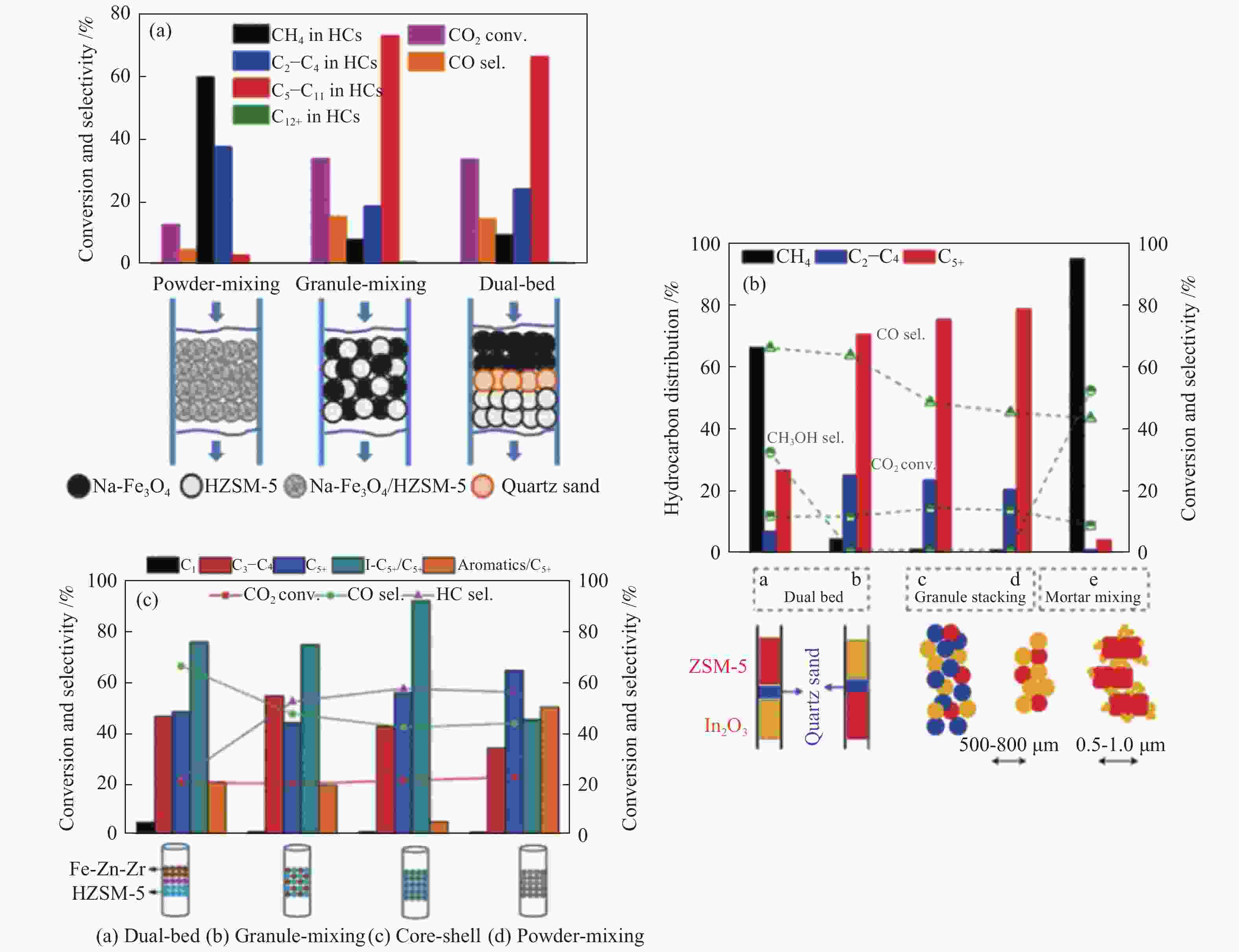
图 8 两类活性组分间的组装方式对不同串联催化剂上CO2加氢制汽油性能的影响
Figure 8 Effect of the assembly style between the two active sites on CO2 hydrogenation to gasoline over the different tandem catalysts
(a): Na-Fe3O4/HZSM-5[79]; (b): In2O3/HZSM-5[80]; (c): Fe-Zn-Zr@HZSM-5[47] reproduced with permission from ref. [79, 80, 47], Copyright (2017) Springer Nature, Copyright (2019) The Royal Society of Chemistry
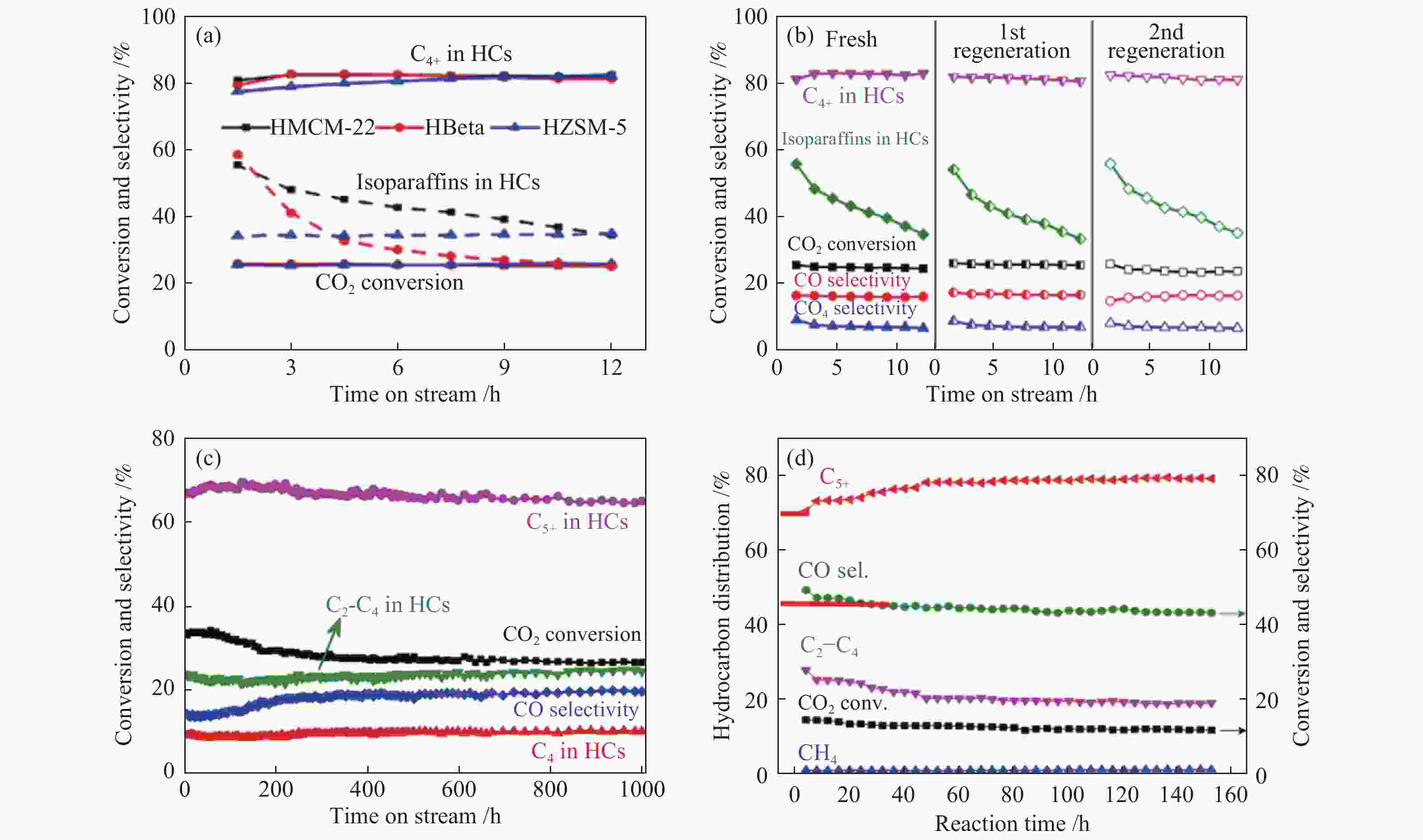
图 9 不同串联催化剂上CO2加氢制(a)、(b)异构烷烃及(c)、(d)汽油的稳定性测试
Figure 9 Stability tests of the different tandem catalysts for CO2 hydrogenation to (a), (b) isoalkanes and (c), (d) gasolines
(a): Fresh Na-Fe3O4/zeolite[72]; (b): Regenerated Na-Fe3O4/HMCM-22[72]; (c): Na-Fe3O4/HZSM-5[79]; (d): In2O3/HZSM-5[80] reproduced with permission from ref. [72, 79, 80], Copyright (2018) American Chemical Society, Copyright (2017) Springer Nature
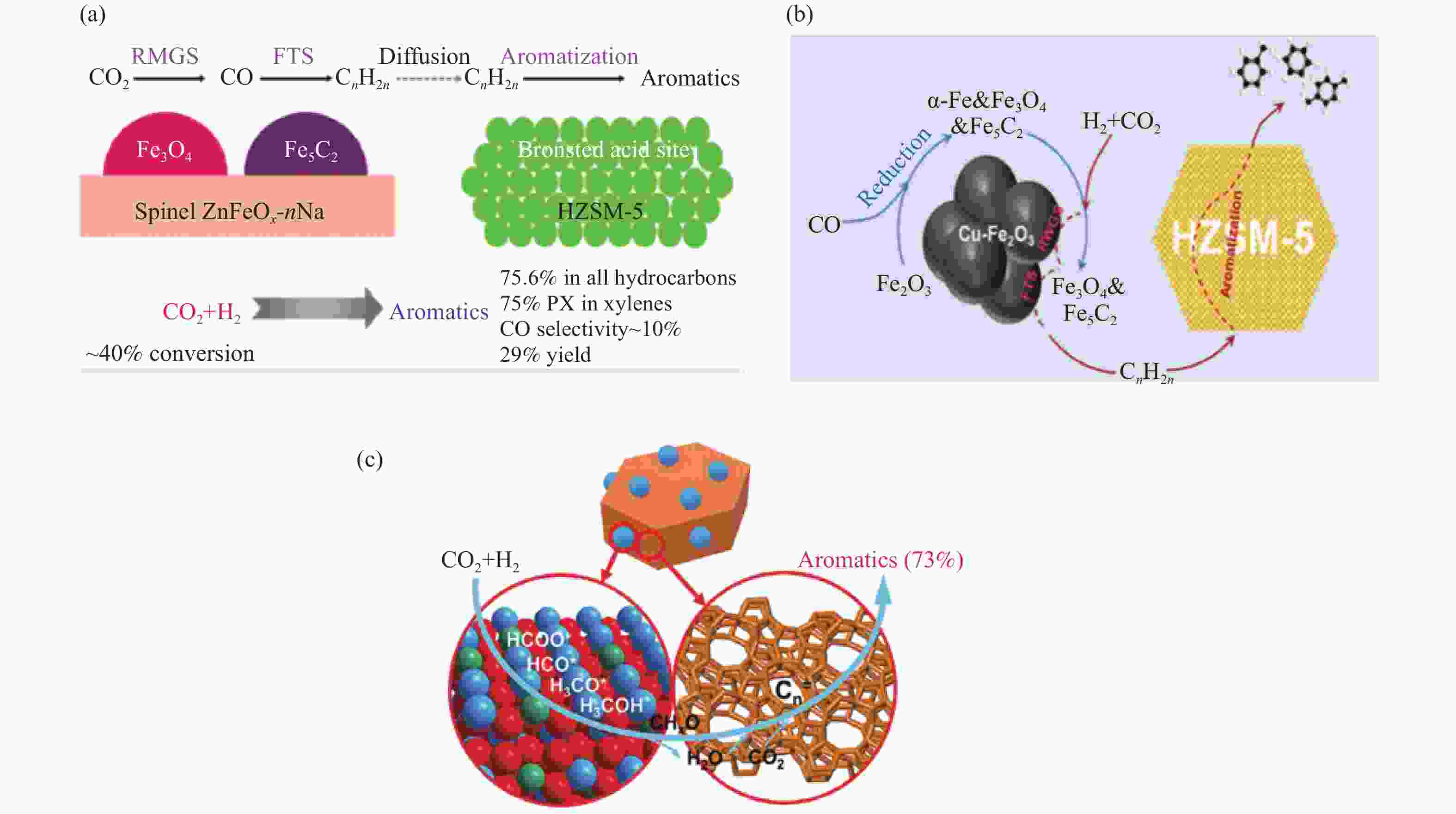
图 10 不同串联催化剂上CO2加氢制芳烃的反应路径及机制
Figure 10 Reaction paths and schematics of CO2 hydrogenation to aromatics on different tandem catalysts
(a): ZnFeOx-4.25Na/S-HZSM-5[81]; (b): 6.25Cu-Fe2O3/HZSM-5[84]; (c); ZnZrO/HZSM-5[40] reproduced with permission from ref. [81, 84, 40], Copyright (2019 & 2020) American Chemical Society, Copyright (2019) Elsevier
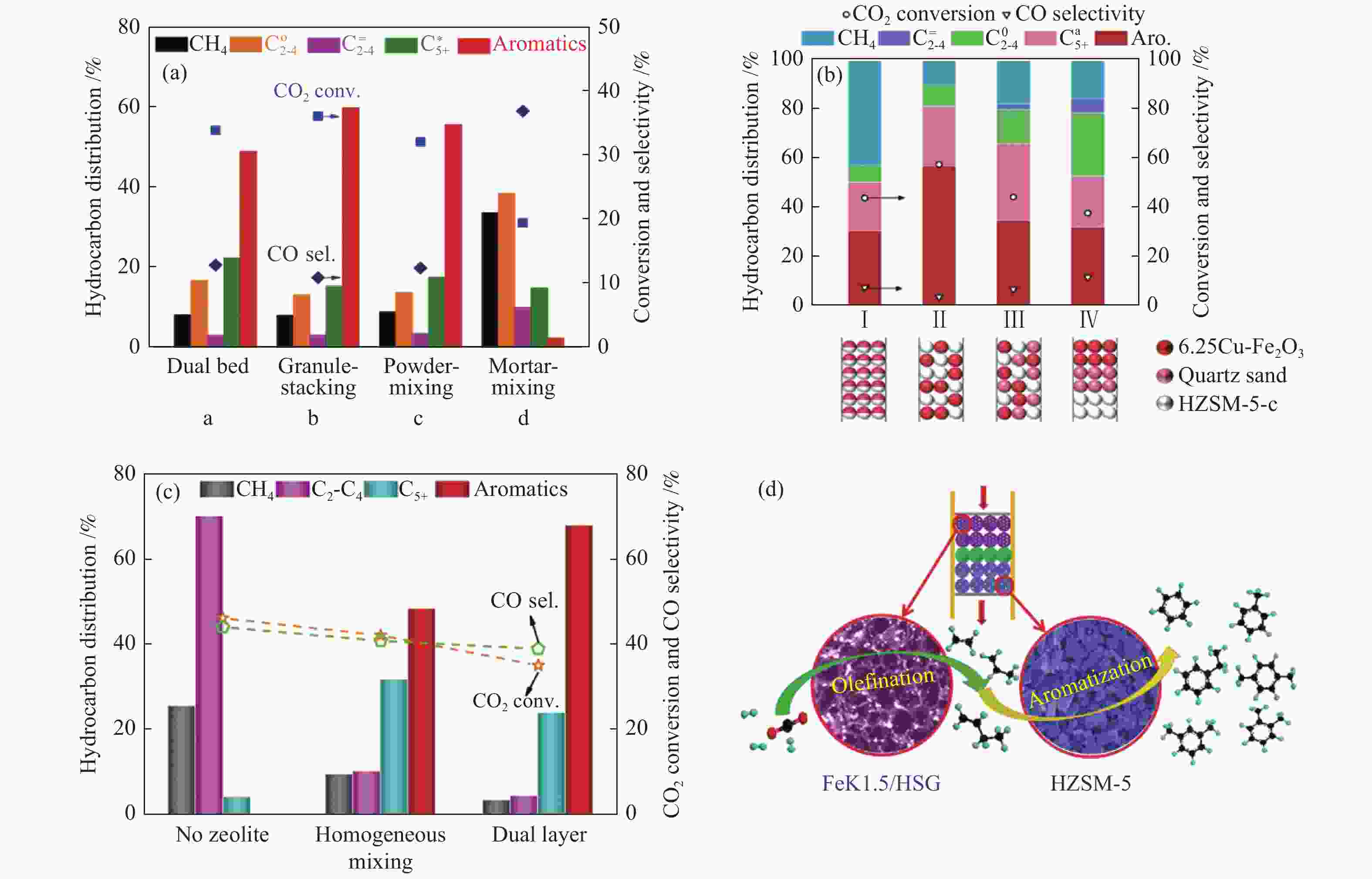
图 11 (a)、(b)、(c) 组装方式对不同串联催化剂上CO2加氢制芳烃性能的影响;(d) FeK1.5/HSG|HZSM-5(50)串联催化剂上CO2转化制芳烃的烯化-芳构化反应路径
Figure 11 (a), (b), (c) Effect of the assembly style between the two active sites on CO2 hydrogenation to aromatics over the different tandem catalysts; (d) Illustration of the olefination-aromatization reaction pathway for converting CO2 to aromatics over the FeK1.5/HSG|HZSM-5(50) tandem catalyst
(a): ZnFeOx-4.25Na/S-HZSM-5[81]; (b): 6.25Cu-Fe2O3/HZSM-5-c[84]; (c), (d): FeK1.5/HSG-HZSM-5(50)[83] reproduced with permission from ref. [81, 84, 83], Copyright (2019 & 2020 & 2019) American Chemical Society
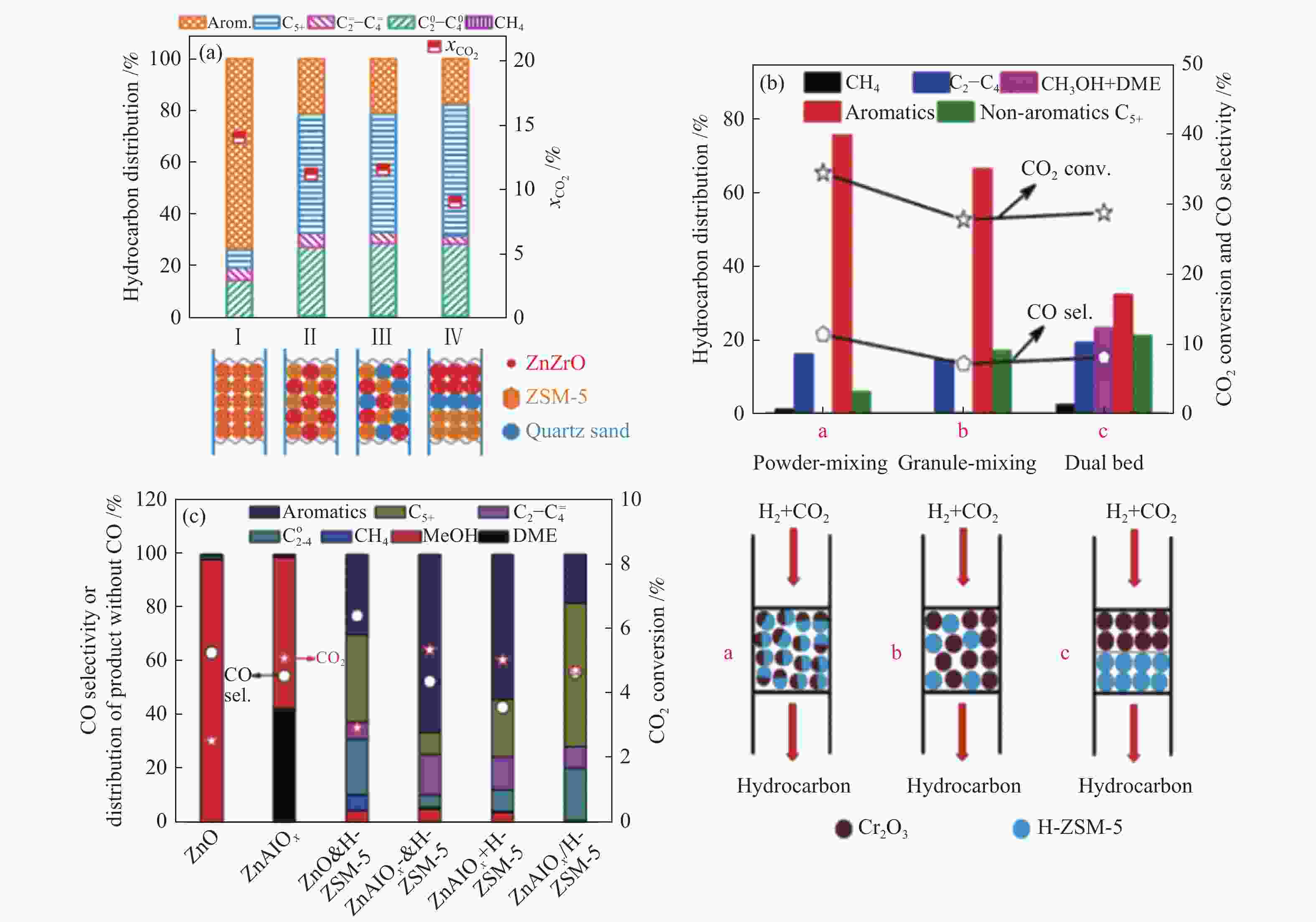
图 12 两类活性组分间的组装方式对不同串联催化剂上CO2加氢制芳烃性能的影响
Figure 12 Effect of the assembly style between two active sites on CO2 hydrogenation to aromatics over different tandem catalysts
(a): ZnZrO/HZSM-5[40]; (b): Cr2O3/HZSM-5[27]; (c): ZnAlOx&HZSM-5[87] reproduced with permission from ref. [40, 27, 87], Copyright (2019) Elsevier, Copyright (2019) American Chemical Society
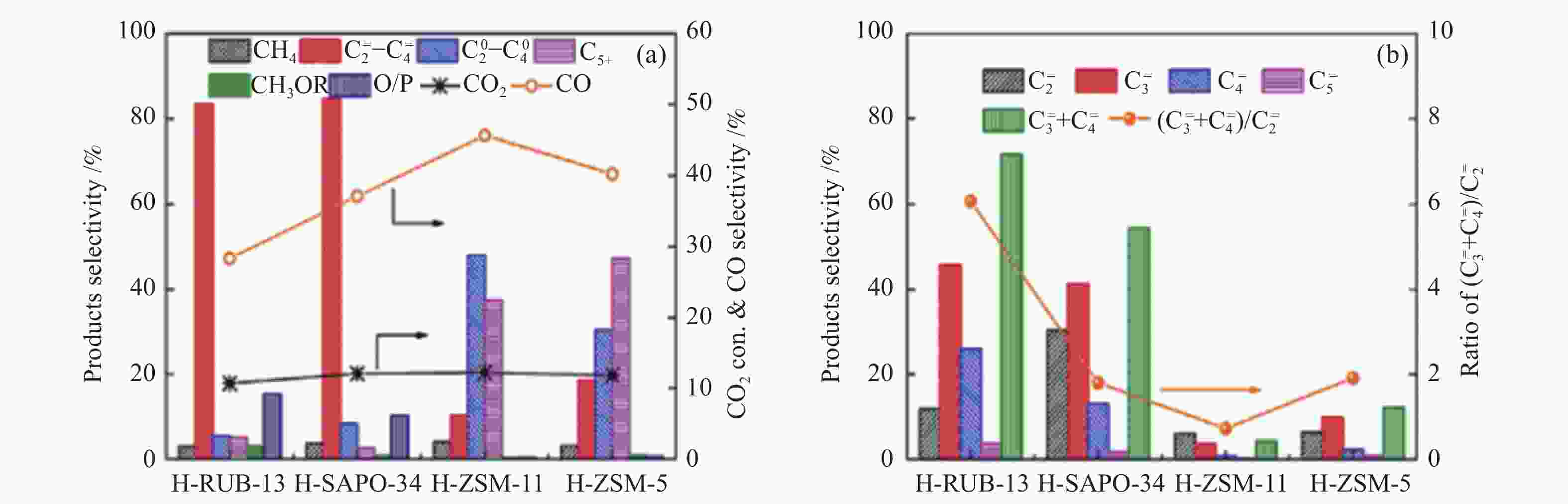
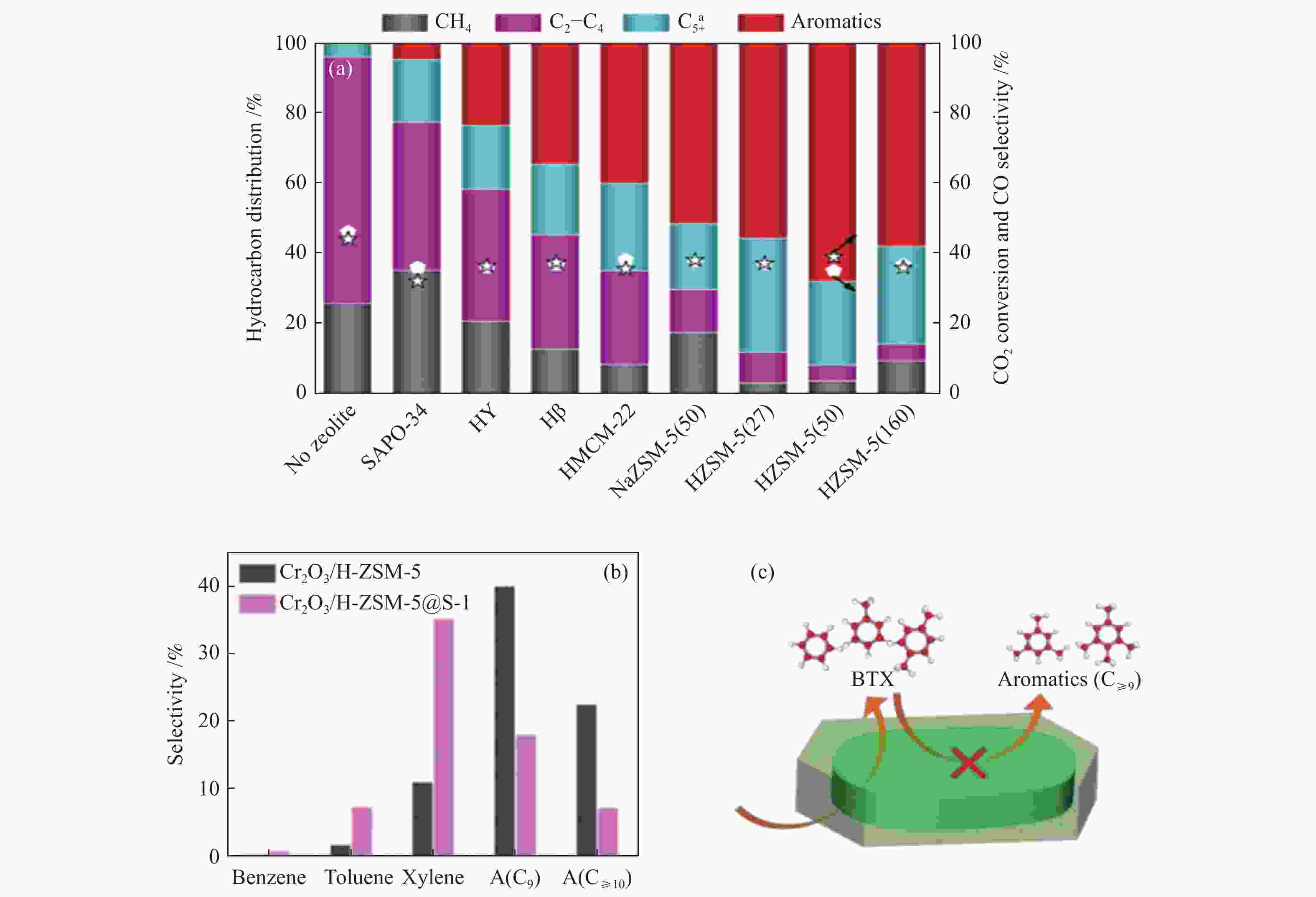
图 13 (a) 分子筛类型对FeK1.5/HSG|zeolite催化剂上CO2转化率及产物选择性的影响[83];(b) 串联催化剂上芳烃分布及 (c)Cr2O3/H-ZSM-5@S-1上高选择性生成BTX的机制[27]
Figure 13 (a) Effect of zeolite types on the CO2 conversion and product selectivity over the FeK1.5/HSG|zeolite catalysts[83]; (b) Fractions of products in aromatics over the tandem catalysts and (C) Scheme of highly selective production of BTX over Cr2O3/H-ZSM-5@S-1[27] reproduced with permission from ref. [83, 27], Copyright (2019) American Chemical Society
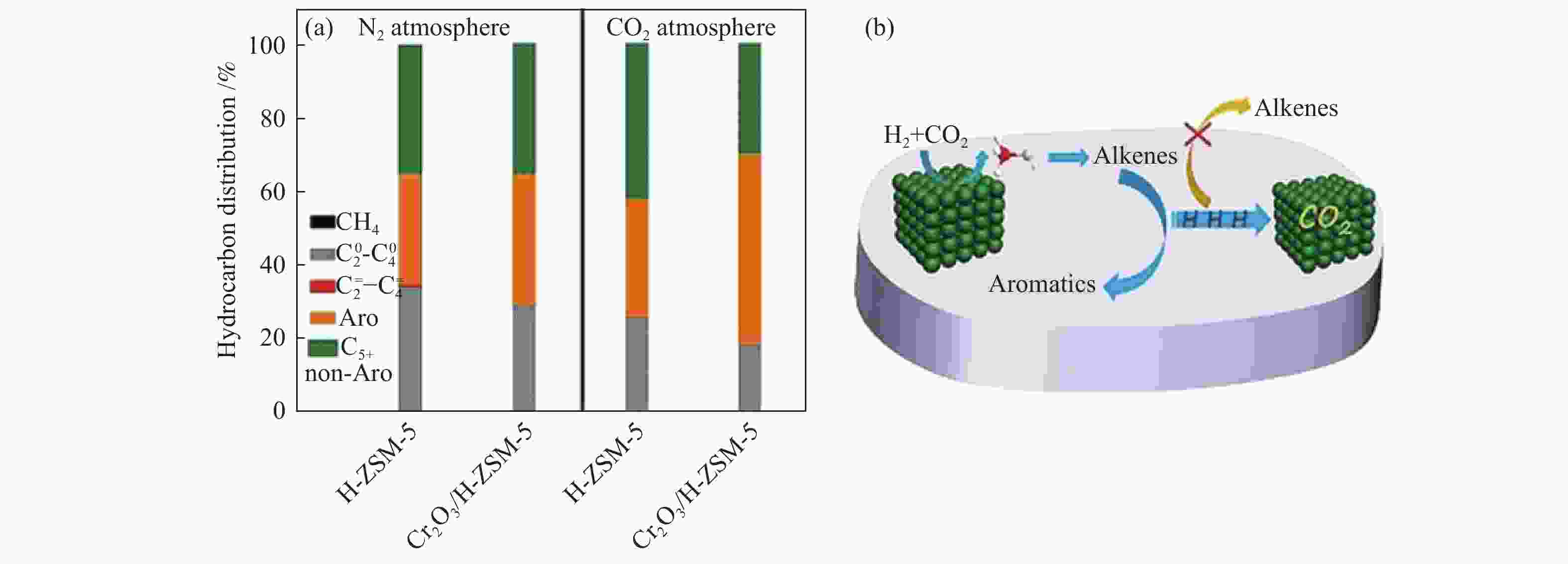
图 14 (a) N2及CO2气氛下HZSM-5及Cr2O3/H-ZSM-5催化剂上甲醇芳构化性能及(b)串联反应中合成芳烃的CO2-辅助作用示意图[89]
Figure 14 (a) Catalytic performances of H-ZSM-5 and Cr2O3/H-ZSM-5 for MTA under N2 and CO2 atmospheres and (b) Schematic representation of the CO2-assisted effect for the synthesis of aromatics in the tandem reaction[89] reproduced with permission from ref. [89], Copyright (2019) John Wiley and Sons
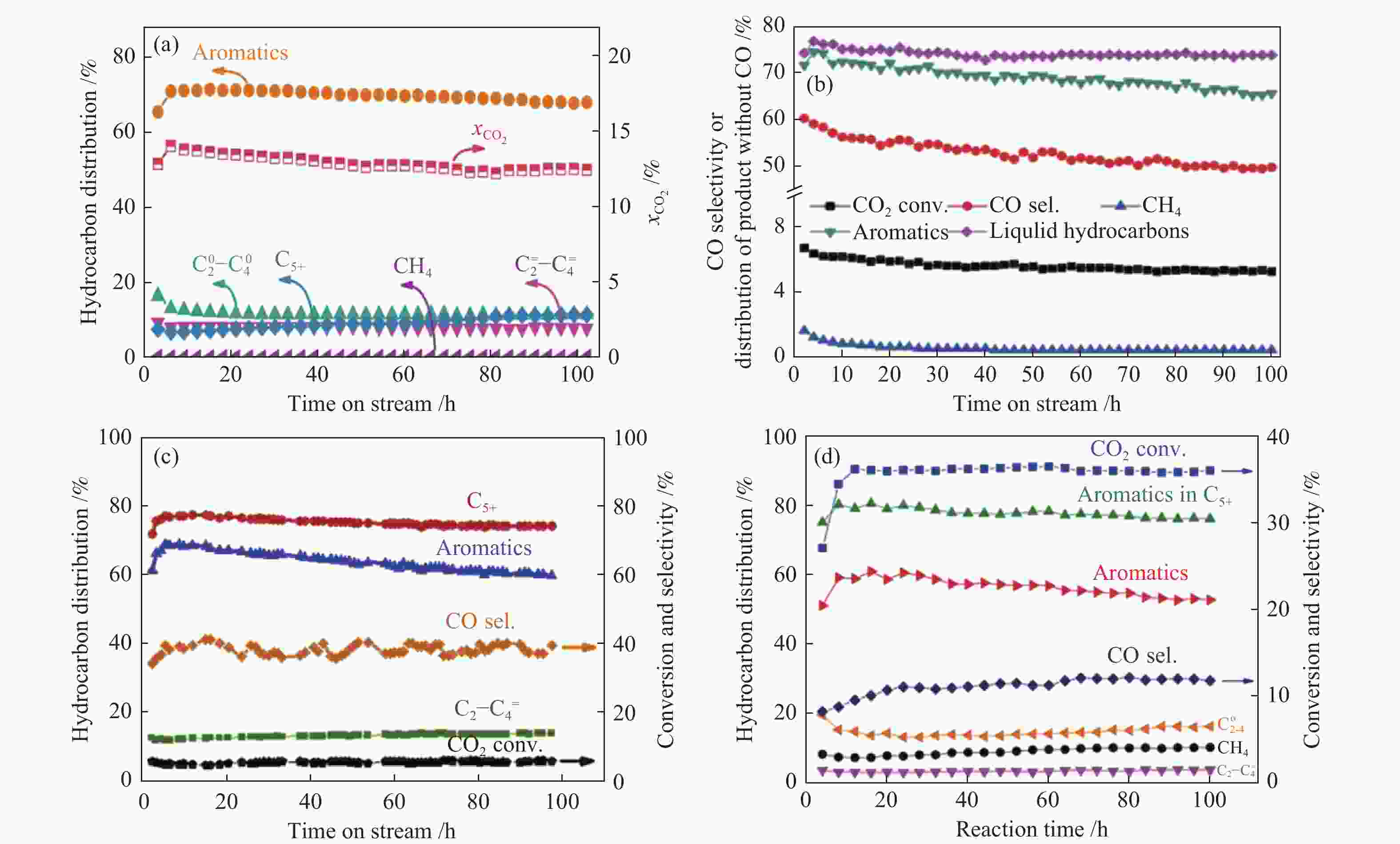
图 15 不同串联催化剂上CO2加氢制芳烃的稳定性测试
Figure 15 Stability tests of the different tandem catalysts for CO2 hydrogenation to aromatics
(a) ZnZrO/ZSM-5[40]; (b) ZnAlOx&H-ZSM-5[87]; (c) ZnO/ZrO2-ZSM5[86]; (d) ZnFeOx-4.25Na/S-HZSM-5[81] reproduced with permission from ref. [40, 87, 86, 81], Copyright (2019 & 2019) Elsevier, Copyright (2019) American Chemical Society
表 1 串联催化剂上CO2加氢制低碳烯烃的催化性能
Table 1 Catalytic performances of CO2 hydrogenation to lower olefins over the tandem catalysts
Catalyst t /℃ p /MPa ${x_{{\rm{C}}{{\rm{O}}_2}}} $ /% ${s_{{\rm{C}}_2^ = - {\rm{C}}_4^ = } }$ /%* In-Zr/SAPO-34 (Granule-mixing)[24] 400 3 35.5 76.4 In2O3-ZnZrOx/SAPO-34 (Granule-mixing)[41] 380 3 17.0 85.0 1In2O3/ZrO2-1SAPO-34 (Powder-mixing)[42] 400 1.5 ~20.0 80.0–90.0 InCrOx(0.13)/SAPO-34 (Powder-mixing)[25] 350 1 17.2 89.1 ZnGa2O4/SAPO-34 (Powder-mixing)[43] 370 3 13.0 86.0 ZnZrO/SAPO-34 (Powder-mixing)[39] 380 2 12.6 80.0 Zr8Cd1/SAPO-18 (Powder-mixing)[44] 370 2.5 17.8 85.6 CuZnZr@Zn–SAPO-34 (Core-shell)[45] 400 2 19.6 60.5 Zn0.5Ce0.2Zr1.8O4/H-RUB-13 (Powder-mixing)[57] 350 1 10.7 83.4 Mn2O3-ZnO/SAPO-34 (Ball milling-mixing)[58] 380 3 29.8 80.2 *: ${\rm{C} }_{2}^{=}$–${\rm{C} }_{4}^{=}$ selectivity in all hydrocarbons 表 2 串联催化剂上CO2加氢制异构烷烃及汽油的催化性能
Table 2 Catalytic performances of CO2 hydrogenation to isoalkanes and gasoline over the tandem catalysts
Catalyst t /℃ p /MPa ${x_{{\rm{C}}{{\rm{O}}_2}}} $ /% sisoalkanes or gasoline /% Fe-Cu-Na/US-Y(10.7) (Physical-mixing)[71] 250 2 11.5 77.3a Na-Fe3O4/HMCM-22(Dual-bed)[72] 320 3 26.0 74.0b 92.6Fe7.4K+HZSM-5 (Granule-mixing)[73] 300 2.5 43.9 69.7a NaFe+SAPO-11+HZSM-5(Triple-bed)[74] 320 3 31.2 38.2c Fe-Zn-Zr/HY (Granule-mixing)[76] 340 5 22.4 55.3a Fe-Zn-Zr@HZSM-5-Hbeta (Core-shell)[46] 340 5 14.9 81.3d Fe-Zn-Zr@HZSM-5 (Core-shell)[47] 340 5 21.5 91.9e Na-Fe3O4/HZSM-5 (Granule-mixing)[79] 320 3 22.0 78.0f In2O3/HZSM-5 (Granule-mixing)[80] 340 3 13.1 78.6f a: C4–C6 isoalkanes selectivity in C4–C6 hydrocarbons;b: C4+ isoalkanes selectivity in C4+ hydrocarbons;c: C5+ isoalkanes selectivity in all hydrocarbons;d: C4+ isoalkanes selectivity in all hydrocarbons;e: C5+ isoalkanes selectivity in C5+ hydrocarbons;f: C5–C11 or C5+ hydrocarbons in all hydrocarbons 表 3 串联催化剂上CO2加氢制芳烃的催化性能
Table 3 Catalytic performances of CO2 hydrogenation to aromatics over the tandem catalysts
Catalyst t /℃ p /MPa ${x_{{\rm{C}}{{\rm{O}}_2}}}$ /% saromatics /% ZnFeOx-4.25Na/S-HZSM-5 (Granule-mixing)[81] 320 3 41.2 75.6 Na/Fe-HZSM-5 (Granule-mixing)[82] 320 1 29.4 54.3 FeK1.5/HSG-HZSM-5 (Dual-bed)[83] 340 2 35.0 68.0 6.25Cu-Fe2O3/HZSM-5 (Granule-mixing)[84] 320 3 57.3 56.6 ZnZrO/ZSM-5 (Powder-mixing)[40] 320 4 14.0 73.0 ae-ZnO-ZrO2/Z5 (Powder-mixing)[85] 340 4 16.0 76.0 ZnO/ZrO2-ZSM-5 (Powder-mixing)[86] 340 3 9.0 70.0 ZnAlOx & HZSM-5 (Powder-mixing)[87] 320 3 9.1 73.9 Cr2O3/HZSM-5 (Powder-mixing)[27] 350 3 34.5 76.0 ZnCrOx-ZnZSM-5 (Powder-mixing)[88] 320 5 19.9 81.1* * Aromatics selectivity in C5+ hydrocarbons, the others are the aromatics selectivity in all hydrocarbons -
[1] SHA F, HAN Z, TANG S, WANG J, LI C. Hydrogenation of carbon dioxide to methanol over non Cu-based heterogeneous catalysts[J]. ChemSusChem,2020,13:6160−6181. [2] 成康, 张庆红, 康金灿, 王野. 二氧化碳直接制备高值化学品中的接力催化方法[J]. 中国科学:化学,2020,50(7):743−755. doi: 10.1360/SSC-2020-0053CHENG Kang, ZHANG Qing-hong, KANG Jin-can, WANG Ye. The tandem catalysis of CO2 conversion to high value–added chemicals[J]. Sci Sin Chim,2020,50(7):743−755. doi: 10.1360/SSC-2020-0053 [3] BANDO K, SOGA K, KUNIMORI K, ICHIKUNI N, OKABE K, KUSAMA H, SAYAMA K, ARAKAWA H. CO2 hydrogenation activity and surface structure of zeolite-supported Rh catalysts[J]. Appl Catal A: Gen,1998,173(1):47−60. [4] JOO O-S, JUNG K-D, MOON I, ROZOVSKII A, LIN G, HAN S, UHM S. Carbon dioxide hydrogenation to form methanol via a reverse-water-gas-shift reaction (the CAMERE Process) [J]. Ind Eng Chem Res, 1999, 38: 1808–1812. [5] 张鲁湘, 张永春, 陈绍云, 助剂TiO2对CO2催化加氢制甲醇催化剂CuO-ZnO-Al2O3性能的影响 [J]. 燃料化学学报, 2011, 39(12): 912–917.ZHANG Lu-xiang, ZHANG Yong-chun, CHEN Shao-yun. Effect of promoter TiO2 on the performance of CuO-ZnO-Al2O3 catalyst for CO2 catalytic hydrogenation to methanol [J]. J Fuel Chem Technol, 2011, 39(12): 912–917. [6] ZHANG L, ZHANG Y, CHEN S. Effect of promoter SiO2, TiO2 or SiO2-TiO2 on the performance of CuO-ZnO-Al2O3 catalyst for methanol synthesis from CO2 hydrogenation [J]. Appl Catal A: Gen, 2012, 415–416: 118–123. [7] KOIZUMI N, JIANG X, KUGAI J, SONG C. Effects of mesoporous silica supports and alkaline promoters on activity of Pd catalysts in CO2 hydrogenation for methanol synthesis [J]. Catal Today, 2012, 194: 16–24. [8] STUDT F, SHARAFUTDINOV I, ABILD-PEDERSEN F, ELKJÆR C, HUMMELSHØJ J, DAHL S, CHORKENDORFF I, NØRSKOV J. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol [J]. Nat Chem, 2014, 6: 320–324. [9] XIAO J, MAO D, GUO X, YU J. Effect of TiO2, ZrO2, and TiO2-ZrO2 on the performance of CuO-ZnO catalyst for CO2 hydrogenation to methanol [J]. Appl Sur Sci, 2015, 338: 146–153. [10] CAI W, DE LA PISCINA P, TOYIR J, HOMS N. CO2 hydrogenation to methanol over CuZnGa catalysts prepared using microwave-assisted methods [J]. Catal Today, 2015, 242: 193–199. [11] KUSAMA H, OKABE K, SAYAMA K, ARAKAWA H. Ethanol synthesis by catalytic hydrogenation of CO2 over Rh-Fe/SiO2 catalysts [J]. Energy, 1997, 22(2/3): 343–348. [12] KUSAMA H, OKABE K, SAYAMA K, ARAKAWA H. CO2 hydrogenation to ethanol over promoted Rh/SiO2 catalysts [J]. Catal Today, 1996, 28: 261–266. [13] INUI T, YAMAMOTO T. Effective synthesis of ethanol from CO2 on polyfunctional composite catalysts [J]. Catal Today, 1998, 45: 209–214. [14] NAIK S, RYU T, BUI V, MILLER J, DRINNAN N, ZMIERCZAK W, Synthesis of DME from CO2/H2 gas mixture [J]. Chem Eng J, 2011, 167: 362–368. [15] BONURA G, CORDARO M, SPADARO L, CANNILLA C, ARENA F, FRUSTERI F. Hybrid Cu-ZnO-ZrO2/H-ZSM5 system for the direct synthesis of DME by CO2 hydrogenation[J]. Appl Catal B: Environ,2013,140–141:16−24. [16] WANG Y, WANG W, CHEN Y, MA J, LI R. Synthesis of dimethyl ether from syngas over core-shell structure catalyst CuO-ZnO-Al2O3@SiO2-Al2O3[J]. Chem Eng J,2014,250:248−256. [17] FRUSTERI F, CORDARO M, CANNILLA C, BONURA G. Multifunctionality of Cu-ZnO-ZrO2/H-ZSM5 catalysts for the one-step CO2-to-DME hydrogenation reaction[J]. Appl Catal B: Environ,2015,162:57−65. [18] SUO Z, KOU Y, NIU J, ZHANG W, WANG H. Characterization of TiO2-, ZrO2- and Al2O3-supported iron catalysts as used for CO2 hydrogenation[J]. Appl Catal A: Gen,1997,148:301−313. [19] LI C, YUAN X, FUJIMOTO K. Direct synthesis of LPG from carbon dioxide over hybrid catalysts comprising modified methanol synthesis catalyst and β-type zeolite[J]. Appl Catal A: Gen,2014,475:155−160. [20] FUJIWARA M, SOUMA Y. Hydrocarbon synthesis from carbon dioxide and hydrogen over Cu-Zn-Cr oxide/zeolite hybrid catalysts[J]. J Chem Soc, Chem Commun,1992,23(42):767−768. [21] LI C, FUJIMOTO K. Efficient conversion of carbon dioxide to non-methane light hydrocarbons-two stage process with intercooler[J]. Fuel Process Technol,2015,136:50−55. [22] AL-DOSSARY M, ISMAIL A, FIERRO J, BOUZID H, AL-SAYARI S. Effect of Mn loading onto MnFeO nanocomposites for the CO2 hydrogenation reaction[J]. Appl Catal B: Environ,2015,165:651−660. [23] FUJIWARA M, SAKURAI H, SHIOKAWA K, LIZUKA Y. Synthesis of C2+ hydrocarbons by CO2 hydrogenation over the composite catalyst of Cu-Zn-Al oxide and Hβ zeolite using two-stage reactor system under low pressure[J]. Catal Today,2015,242:255−260. [24] GAO P, DANG S, LI S, BU X, LIU Z, QIU M, YANG C, WANG H, ZHONG L, HAN Y, LIU Q, WEI W, SUN Y. Direct production of lower olefins from CO2 conversion via bifunctional catalysis[J]. ACS Catal,2018,8:571−578. doi: 10.1021/acscatal.7b02649 [25] WANG S, WANG P, QIN Z, YAN W, DONG M, LI J, WANG J, FAN W. Enhancement of light olefin production in CO2 hydrogenation over In2O3-based oxide and SAPO-34 composite[J]. J Catal,2020,391:459−470. doi: 10.1016/j.jcat.2020.09.010 [26] WANG X, ZENG C, GONG N, ZHANG T, WU Y, ZHANG J, SONG F, YANG G, TAN Y. Effective suppression of CO selectivity for CO2 hydrogenation to high-quality gasoline[J]. ACS Catal,2021,11:1528−1547. doi: 10.1021/acscatal.0c04155 [27] WANG Y, TAN L, TAN M, ZHANG P, FANG Y, YONEYAMA Y, YANG G, TSUBAKI N. Rationally designing bifunctional catalysts as an efficient strategy to boost CO2 hydrogenation producing value-added aromatics[J]. ACS Catal,2019,9:895−901. doi: 10.1021/acscatal.8b01344 [28] ZHANG J, LU S, SU X, FAN S, MA Q, ZHAO T. Selective formation of light olefins from CO2 hydrogenation over Fe-Zn-K catalysts[J]. J CO2 Util,2015,12:95−100. doi: 10.1016/j.jcou.2015.05.004 [29] BURK M, LEE J, MARTINEZ J. A versatile tandem catalysis procedure for the preparation of novel amino acids and peptides[J]. J Am Chem Soc,1994,116:10847−10848. [30] BALEMA V, HEY-HAWKINS E. The CuCl-catalyzed reaction of trimethylsilyl(t-butyl) chlorophosphane with dimethylzirco-nocene: An example for tandem catalysis[J]. Z Anorg Allg Chem,1996,622:2053−2056. doi: 10.1002/zaac.19966221209 [31] WILSON E. Heterogeneous tandem catalysis[J]. Chem Eng News,2011,89(16):9. [32] LOHR T, MARKS T. Orthogonal tandem catalysis[J]. Nat Chem,2015,7:477−482. doi: 10.1038/nchem.2262 [33] CHENG K, GU B, LIU X, KANG J, ZHANG Q, WANG Y. Direct and highly selective conversion of synthesis gas to lower olefins: Design of a bifunctional catalyst combining methanol synthesis and carbon-carbon coupling[J]. Angew Chem Int Ed,2016,55:4725−4728. doi: 10.1002/anie.201601208 [34] LIU X, ZHOU W, YANG Y, CHENG K, KANG J, ZHANG L, ZHANG G, MIN X, ZHANG Q, WANG Y. Design of efficient bifunctional catalysts for direct conversion of syngas into lower olefins via methanol/dimethyl ether intermediates[J]. Chem Sci,2018,9:4708−4718. doi: 10.1039/C8SC01597J [35] LI N, JIAO F, PAN X, CHEN Y, FENG J, LI G, BAO X. High-quality gasoline directly from syngas by dual metal oxide-zeolite (OX-ZEO) catalysis[J]. Angew Chem Int Ed,2019,58:1−6. doi: 10.1002/anie.201813481 [36] CHENG K, ZHOU W, KANG J, HE S, SHI S, ZHANG Q, PAN Y, WEN W, WANG Y. Bifunctional catalysts for one-step conversion of syngas into aromatics with excellent selectivity and stability[J]. Chem,2017,3:334−347. doi: 10.1016/j.chempr.2017.05.007 [37] ZHANG P, TAN L, YANG G, TSUBAKI N. One-pass selective conversion of syngas to para–xylene[J]. Chem Sci,2017,8:7941−7946. doi: 10.1039/C7SC03427J [38] XU Y, LIU J, WANG J, MA G, LIN J, YANG Y, LI Y, ZHANG C, DING M. Selective conversion of syngas to aromatics over Fe3O4@MnO2 and hollow HZSM-5 bifunctional catalysts[J]. ACS Catal,2019,9:5147−5156. doi: 10.1021/acscatal.9b01045 [39] LI Z, WANG J, QU Y, LIU H, TANG C, MIAO S, FENG Z, AN H, LI C. Highly selective conversion of carbon dioxide to lower olefins[J]. ACS Catal,2017,7:8544−8548. doi: 10.1021/acscatal.7b03251 [40] LI Z, QU Y, WANG J, LIU H, LI M, MIAO S, LI C. Highly selective conversion of carbon dioxide to aromatics over tandem catalysts[J]. Joule,2019,3:570−583. doi: 10.1016/j.joule.2018.10.027 [41] DANG S, LI S, YANG C, CHEN X, LI X, ZHONG L, GAO P, SUN Y. Selective transformation of CO2 and H2 into lower olefins over In2O3-ZnZrOx/SAPO-34 bifunctional catalysts[J]. ChemSusChem,2019,12:3582−3591. doi: 10.1002/cssc.201900958 [42] GAO J, JIA C, LIU B. Direct and selective hydrogenation of CO2 to ethylene and propene by bifunctional catalysts[J]. Catal Sci Technol,2017,7:5602−5607. doi: 10.1039/C7CY01549F [43] LIU X, WANG M, ZHOU C, ZHOU W, CHENG K, KANG J, ZHANG Q, DENG W, WANG Y. Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34[J]. Chem Commun,2018,54:140−143. doi: 10.1039/C7CC08642C [44] 杨浪浪, 孟凡会, 张 鹏, 梁晓彤, 李 忠. ZrCdOx/SAPO-18双功能催化剂催化CO2加氢合成低碳烯烃性能 [J]. 无机化学学报, 2021, 37(3): 448–456.YANG Lang-lang, MENG Fan-hui, ZHANG Peng, LIANG Xiao-tong, LI Zhong, Catalytic performance for CO2 hydrogenation to light olefins Over ZrCdOx/SAPO-18 bifunctional catalyst [J]. Chin J Inorg Chem, 2021, 37(3): 448–456. [45] CHEN J, WANG X, WU D, ZHANG J, MA Q, GAO X, LAI X, XIA H, FAN S, ZHAO T-S. Hydrogenation of CO2 to light olefins on CuZnZr@(Zn-)SAPO-34 catalysts: Strategy for product distribution[J]. Fuel,2019,239:44−52. doi: 10.1016/j.fuel.2018.10.148 [46] WANG X, YANG G, ZHANG J, CHEN S, WU Y, ZHANG Q, WANG J, HAN Y, TAN Y. Synthesis of isoalkanes over a core (Fe-Zn-Zr)-shell (zeolite) catalyst by CO2 hydrogenation[J]. Chem Commun,2016,52:7352−7355. doi: 10.1039/C6CC01965J [47] WANG X, YANG G, ZHANG J, SONG F, WU Y, ZHANG T, ZHANG Q, TSUBAKI N, TAN Y. Macroscopic assembly style of catalysts significantly determining their efficiency for converting CO2 to gasoline[J]. Catal Sci Technol,2019,9:5401−5412. doi: 10.1039/C9CY01470E [48] WEI J, SUN J, WEN Z, FANG C, GE Q, XU H. New insights into the effect of sodium on Fe3O4-based nanocatalysts for CO2 hydrogenation to light olefins[J]. Catal Sci Technol,2016,6:4786−4793. doi: 10.1039/C6CY00160B [49] WEI C, TU W, JIA L, LIU Y, LIAN H, WANG P, ZHANG Z. The evolutions of carbon and iron species modified by Na and their tuning effect on the hydrogenation of CO2 to olefins[J]. Appl Surf Sci,2020,525:146622. [50] ZHANG Z, WEI C, JIA L, LIU Y, SUN C, WANG P, TU W. Insights into the regulation of FeNa catalysts modified by Mn promoter and their tuning effect on the hydrogenation of CO2 to light olefins[J]. J Catal,2020,390:12−22. [51] ZHANG Y, CAO C, ZHANG C, ZHANG Z, LIU X, YANG Z, ZHU M, MENG B, XU J, HAN Y–F. The study of structure–performance relationship of iron catalyst during a full life cycle for CO2 hydrogenation[J]. J Catal,2019,378:51−62. [52] ZHANG Y, FU D, LIU X, ZHANG Z, ZHANG C, SHI B, XU J, HAN Y–F. Operando spectroscopic study of dynamic structure of iron oxide catalysts during CO2 hydrogenation[J]. ChemCatChem,2018,10:1272−1276. [53] HUANG J, JIANG S, WANG M, WANG X, GAO J, SONG C. Dynamic evolution of Fe and carbon species over different ZrO2 supports during CO prereduction and their effects on CO2 hydrogenation to light olefins[J]. ACS Sustainable Chem Eng,2021,9:7891−7903. [54] YUAN F, ZHANG G, ZHU J, DING F, ZHANG A, SONG C, GUO X. Boosting light olefin selectivity in CO2 hydrogenation by adding Co to Fe catalysts within close proximity[J]. Catal Today,2021,371:142−149. [55] LIU J, ZHANG G, JIANG X, WANG J, SONG C, GUO X. Insight into the role of Fe5C2 in CO2 catalytic hydrogenation to hydrocarbons[J]. Catal Today,2021,371:162−170. [56] LIU J, LI K, SONG Y, SONG C, GUO X. Selective hydrogenation of CO2 to hydrocarbons: effects of Fe3O4 particle size on reduction, carburization, and catalytic performance[J]. Energy Fuels,2021,35:10703−10709. [57] PHONGAMWONG T, CHANTAPRASERTPORN U, WITOON T, NUMPILAI T, POO-ARPORN Y, LIMPHIRAT W, DONPHAI W, DITTANET P, CHAREONPANICH M, LIMTRAKUL J. CO2 hydrogenation to methanol over CuO-ZnO-ZrO2-SiO2 catalysts: Effects of SiO2 contents[J]. Chem Eng J,2017,316:692−703. doi: 10.1016/j.cej.2017.02.010 [58] MOU J, FAN X, LIU F, WANG X, ZHAO T, CHEN P, LI Z, YANG C, CAO J. CO2 hydrogenation to lower olefins over Mn2O3-ZnO/SAPO-34 tandem catalysts[J]. Chem Eng J,2021,421:129978. [59] YE J, LIU C, MEI D, GE Q. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): A DFT study[J]. ACS Catal,2013,3:1296−1306. [60] SUN K, FAN Z, YE J, YAN J, GE Q, LI Y, HE W, YANG W, LIU C. Hydrogenation of CO2 to methanol over In2O3 catalyst[J]. J CO2 Utili,2015,12:1−6. [61] WANG J, SUN K, JIA X, LIU C. CO2 hydrogenation to methanol over Rh/In2O3 catalyst[J]. Catal Today,2021,365:341−347. [62] WANG W, CHEN Y, ZHANG M. Facet effect of In2O3 for methanol synthesis by CO2 hydrogenation: A mechanistic and kinetic study[J]. Surf Interfaces,2021,25:101244. [63] DONG X, LI F, ZHAO N, XIAO F, WANG J, TAN Y. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts prepared by precipitation-reduction method[J]. Appl Catal B: Environ,2016,191:8−17. doi: 10.1016/j.apcatb.2016.03.014 [64] CHEN S, ZHANG J, SONG F, ZHANG Q, YANG G, ZHANG M, WANG X, XIE H, TAN Y. Induced high selectivity methanol formation during CO2 hydrogenation over a CuBr2-modified CuZnZr catalyst[J]. J Catal,2020,389:47−59. doi: 10.1016/j.jcat.2020.05.023 [65] FUJIWARA M, SOUMA Y. Hydrocarbon synthesis from carbon dioxide and hydrogen over Cu-Zn-Cr oxide/zeolite hybrid catalysts[J]. J Chem Soc, Chem Commun,1992,10:767−768. [66] PARK YK, PARK KC, IHM SK. Hydrocarbon synthesis through CO2 hydrogenation over CuZnOZrO2/zeolite hybrid catalysts[J]. Catal Today,1998,44:165−73. doi: 10.1016/S0920-5861(98)00187-4 [67] WANG S, ZHANG L, ZHANG W, WANG P, QIN Z, YAN W, DONG M, LI J, WANG J, HE L, OLSBYE U, FAN W. Selective conversion of CO2 into propene and butene[J]. Chem,2020,6:3344−3363. doi: 10.1016/j.chempr.2020.09.025 [68] TAN L, ZHANG P, CUI Y, SUZUKI Y, LI H, GUO L, YANG G, TSUBAKI N. Direct CO2 hydrogenation to light olefins by suppressing CO by-product formation[J]. Fuel Process Technol,2019,196:106174. doi: 10.1016/j.fuproc.2019.106174 [69] MARTIN O, MARTIN A J, MONDELLI C, MITCHELL S, SEGAWA T F, HAUERT R, DROUILLY C, FERRE D C, RAMÍREZ J P. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angew Chem Int Ed,2016,55:6261−6265. doi: 10.1002/anie.201600943 [70] XU Q, HE D, FUJIWARA M, SOUMA Y. Improved activity of Fe-Cu catalysts by physical mixing with zeolites for the hydrogenation of carbon dioxide[J]. J Mol Catal A: Chem,1997,120:L23−L26. doi: 10.1016/S1381-1169(97)00014-9 [71] Xu Q, HE D, FUJIWARA M, TANAKA M, SOUMA Y, YAMANAKA H. Hydrogenation of carbon dioxide over Fe-Cu-Na/zeolite composite catalysts: Na migration via solid–solid reaction and its effects on the catalytic activity[J]. J Mol Catal A: Chem,1998,136:161−168. doi: 10.1016/S1381-1169(98)00047-8 [72] WEI J, YAO R, GE Q, WEN Z, JI X, FANG C, ZHANG J, XU H, SUN J. Catalytic hydrogenation of CO2 to isoparaffins over Fe-based multifunctional catalysts[J]. ACS Catal,2018,8:9958−9967. doi: 10.1021/acscatal.8b02267 [73] GENG S, JIANG F, XU Y, LIU X. Iron-based Fischer-Tropsch synthesis for the efficient conversion of carbon dioxide into isoparaffins[J]. ChemCatChem,2016,8:1303−1307. doi: 10.1002/cctc.201600058 [74] NOREEN A, LI M, FU Y, AMOO C, WANG J, MATURURA E, DU C, YANG R, XING C, SUN J. One-pass hydrogenation of CO2 to multibranched isoparaffins over bifunctional zeolite-based catalysts[J]. ACS Catal,2020,10:14186−14194. doi: 10.1021/acscatal.0c03292 [75] TAN Y, FUJIWARA M, ANDO H, XU Q, SOUMA Y. Syntheses of isobutane and branched higher hydrocarbons from carbon dioxide and hydrogen over composite catalysts[J]. Ind Eng Chem Res,1999,38:3225−3229. doi: 10.1021/ie980672m [76] BAI R, TAN Y, HAN Y. Study on the carbon dioxide hydrogenation to iso−alkanes over Fe-Zn-M/zeolite composite catalysts[J]. Fuel Process Technol,2004,86:293−301. doi: 10.1016/j.fuproc.2004.05.001 [77] 白荣献, 谭猗生, 韩怡卓. Fe-Zn-Zr/分子筛复合催化剂上CO2加氢合成异构烷烃Ⅰ不同分子筛对催化剂性能的影响[J]. 催化学报,2004,25(3):223−226. doi: 10.3321/j.issn:0253-9837.2004.03.013BAI Rong-xian, TAN Yi-sheng, HAN Yi-zhuo. Hydrogenation of carbon dioxide to isoalkanes over Fe-Zn-Zr/zeolite composite catalysts Ⅰ Effects of zeolites on catalytic performance of the catalysts[J]. Chin J Catal,2004,25(3):223−226. doi: 10.3321/j.issn:0253-9837.2004.03.013 [78] NI X, TAN Y, HAN Y, TSUBAKI N. Synthesis of isoalkanes over Fe-Zn-Zr/HY composite catalyst through carbon dioxide hydrogenation[J]. Catal Commun,2007,8:1711−1714. doi: 10.1016/j.catcom.2007.01.023 [79] WEI J, GE Q, YAO R, WEN Z, FANG C, GUO L, XU H, SUN J. Directly converting CO2 into a gasoline fuel[J]. Nat Commun,2017,8:15174−15181. doi: 10.1038/ncomms15174 [80] GAO P, LI S, BU X, DANG S, LIU Z, WANG H, ZHONG L, QIU M, YANG C, CAI J, WEI W, SUN Y. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst[J]. Nat Chem,2017,9:1019−1024. doi: 10.1038/nchem.2794 [81] CUI X, GAO P, LI S, YANG C, LIU Z, WANG H, ZHONG L, SUN Y. Selective production of aromatics directly from carbon dioxide hydrogenation[J]. ACS Catal,2019,9:3866−3876. doi: 10.1021/acscatal.9b00640 [82] XU Y, SHI C, LIU B, WANG T, ZHENG J, LI W, LIU D, LIU X. Selective production of aromatics from CO2[J]. Catal Sci Technol,2019,9:593−610. doi: 10.1039/C8CY02024H [83] WANG S, WU T, LIN J, TIAN J, JIY, PEI Y, YAN S, QIAO M, XU H, ZONG B. FeK on 3D graphene-zeolite tandem catalyst with high efficiency and versatility in direct CO2 conversion to aromatics[J]. ACS Sustainable Chem Eng,2019,7:17825−17833. doi: 10.1021/acssuschemeng.9b04328 [84] SONG G, LI M, YAN P, ASIF NAWAZ M, LIU D. High conversion to aromatics via CO2-FT over a CO-reduced Cu-Fe2O3 catalyst integrated with HZSM-5[J]. ACS Catal,2020,10:11268−11279. doi: 10.1021/acscatal.0c02722 [85] ZHOU C, SHI J, ZHOU W, CHENG K, ZHANG Q, KANG J, WANG Y. Highly active ZnO-ZrO2 aerogels integrated with H-ZSM-5 for aromatics synthesis from carbon dioxide[J]. ACS Catal,2020,10:302−310. doi: 10.1021/acscatal.9b04309 [86] ZHANG X, ZHANG A, JIANG X, ZHU J, LIU J, LI J, ZHANG G, SONG C, GUO X. Utilization of CO2 for aromatics production over ZnO/ZrO2-ZSM-5 tandem catalyst[J]. J CO2 Util,2019,29:140−145. doi: 10.1016/j.jcou.2018.12.002 [87] NI Y, CHEN Z, FU Y, LIU Y, ZHU W, LIU Z. Selective conversion of CO2 and H2 into aromatics[J]. Nat Commun,2018,9:3457−3463. doi: 10.1038/s41467-018-05880-4 [88] ZHANG J, ZHANG M, CHEN S, WANG X, ZHOU Z, WU Y, ZHANG T, YANG G, HAN Y, TAN Y. Hydrogenation of CO2 into aromatics over a ZnCrOx-zeolite composite catalyst[J]. Chem Commun,2019,55:973−976. doi: 10.1039/C8CC09019J [89] WANG Y, GAO W, KAZUMI S, LI H, YANG G, TSUBAKI N. Direct and oriented conversion of CO2 into value-added aromatics[J]. Chem Eur J,2019,25:5149−5153. doi: 10.1002/chem.201806165 -




 下载:
下载: