Surface reaction and lattice oxygen transfer in chemical looping oxidative coupling of methane: Molecular dynamics simulations
-
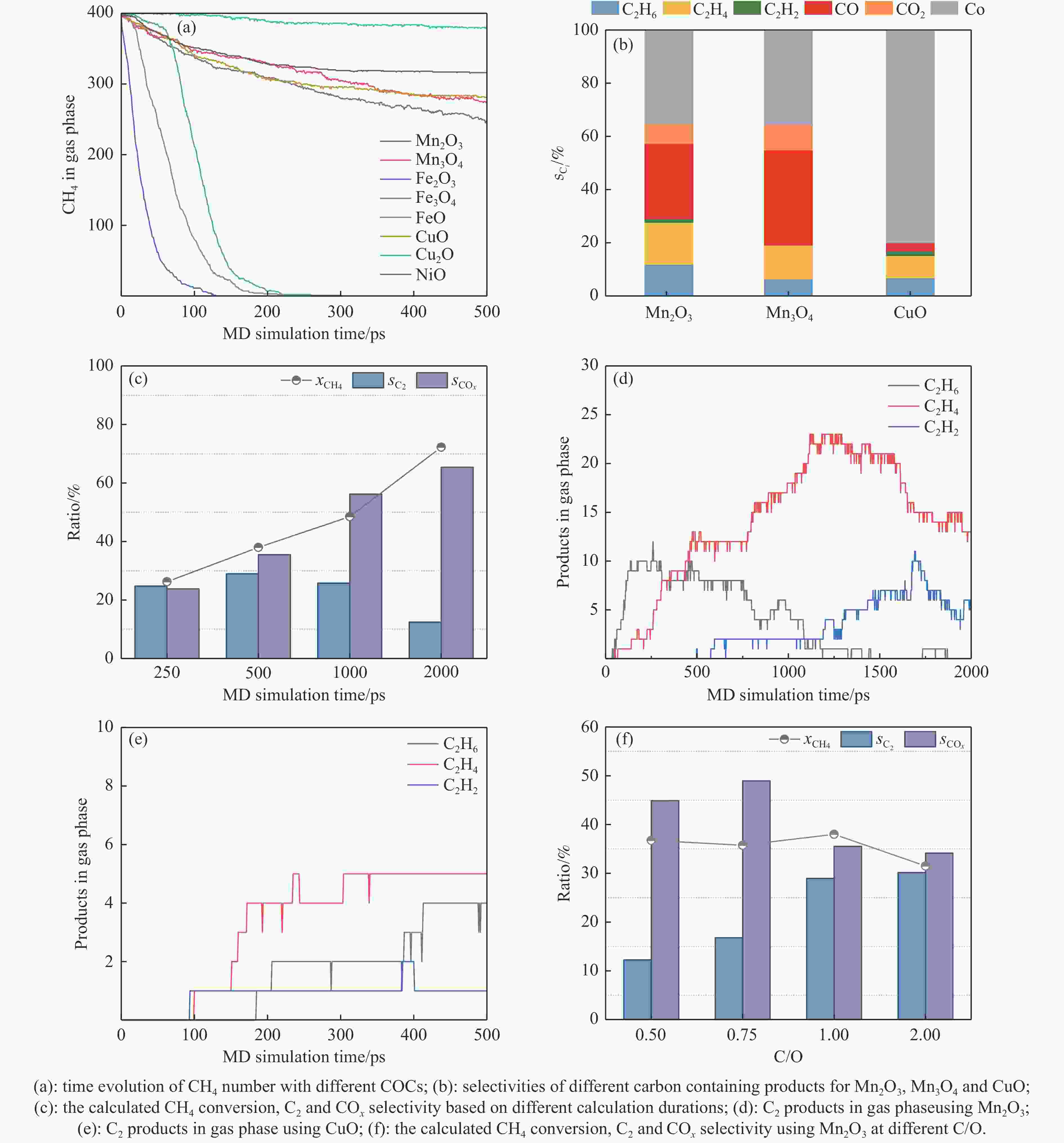
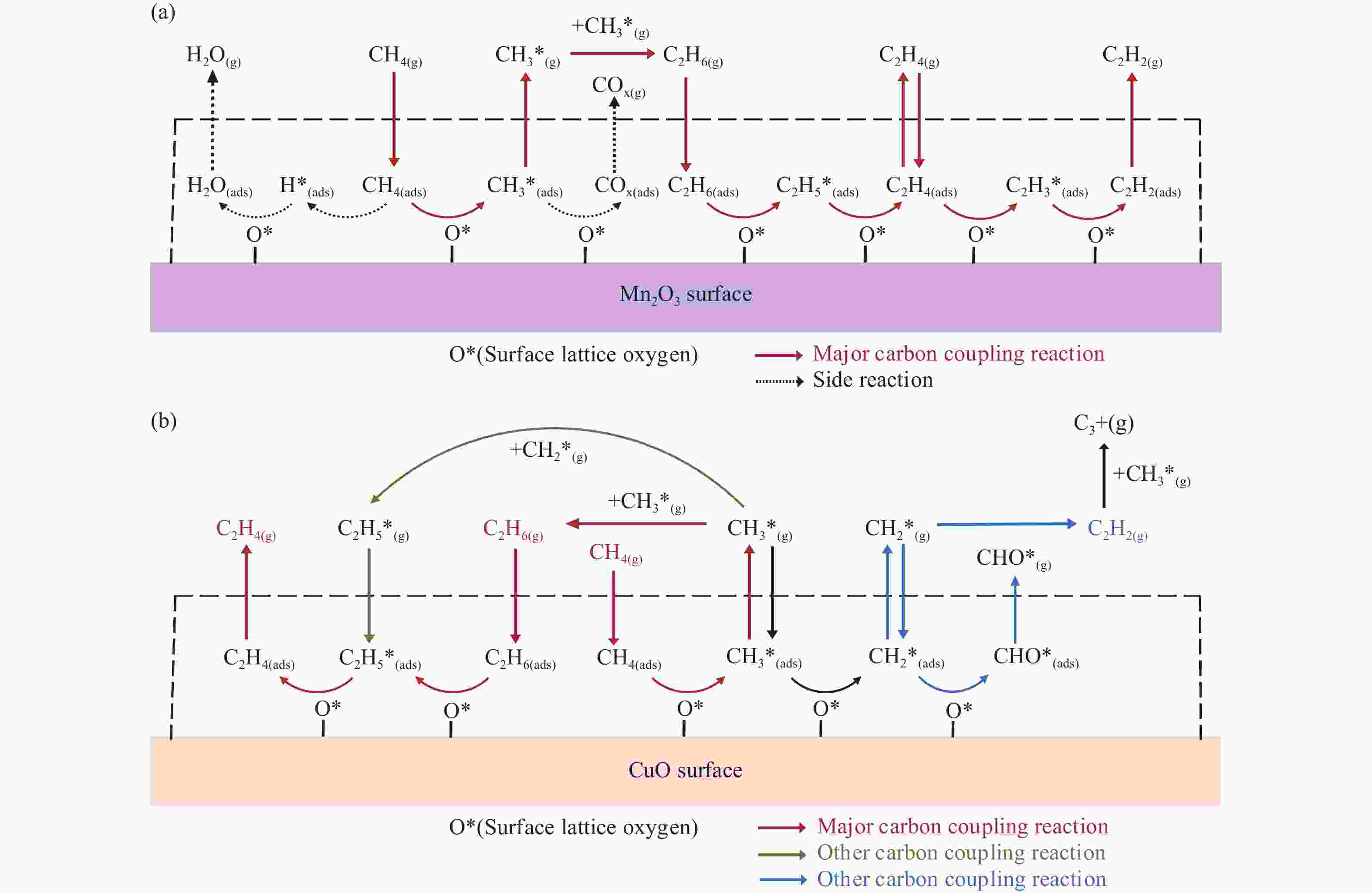
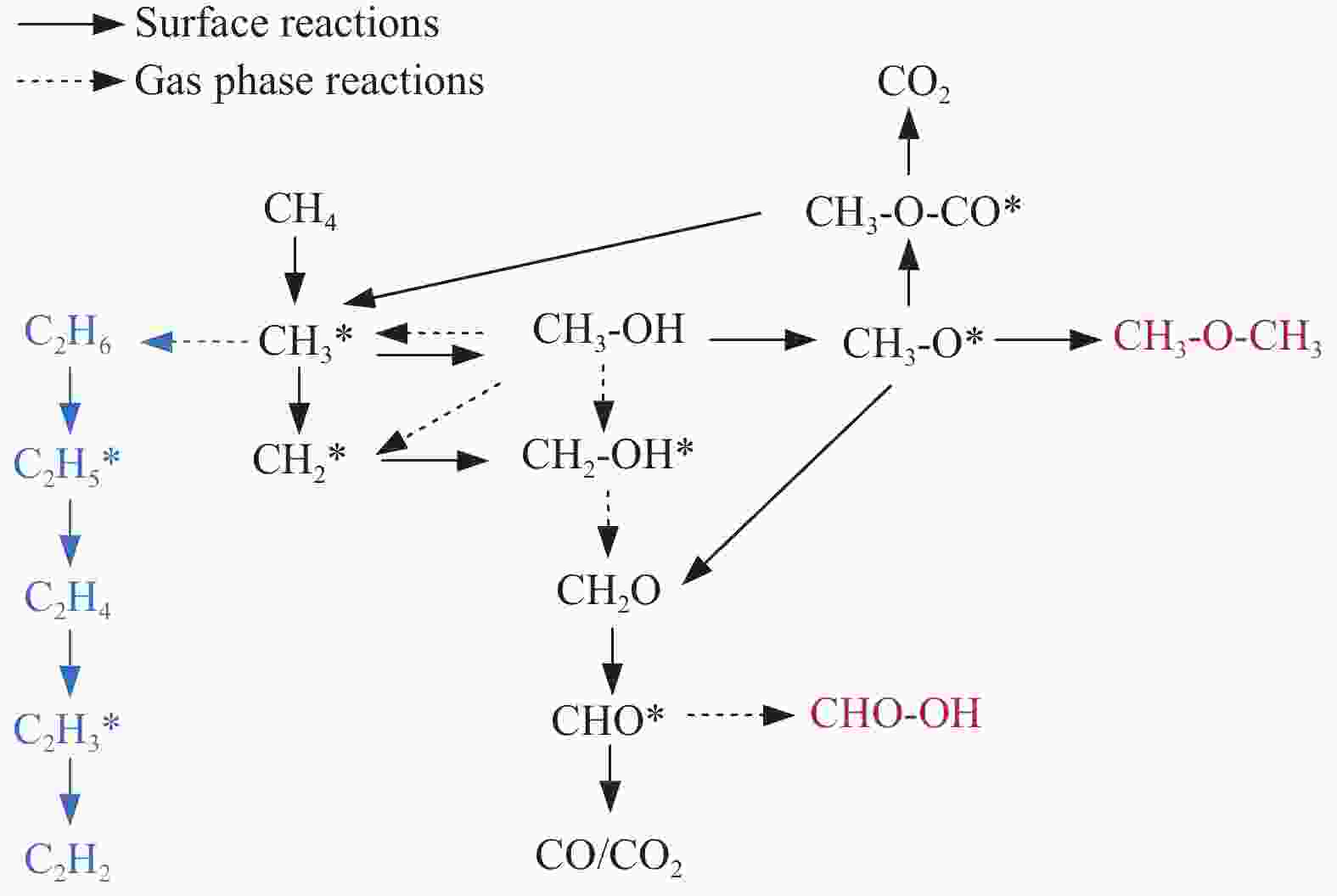
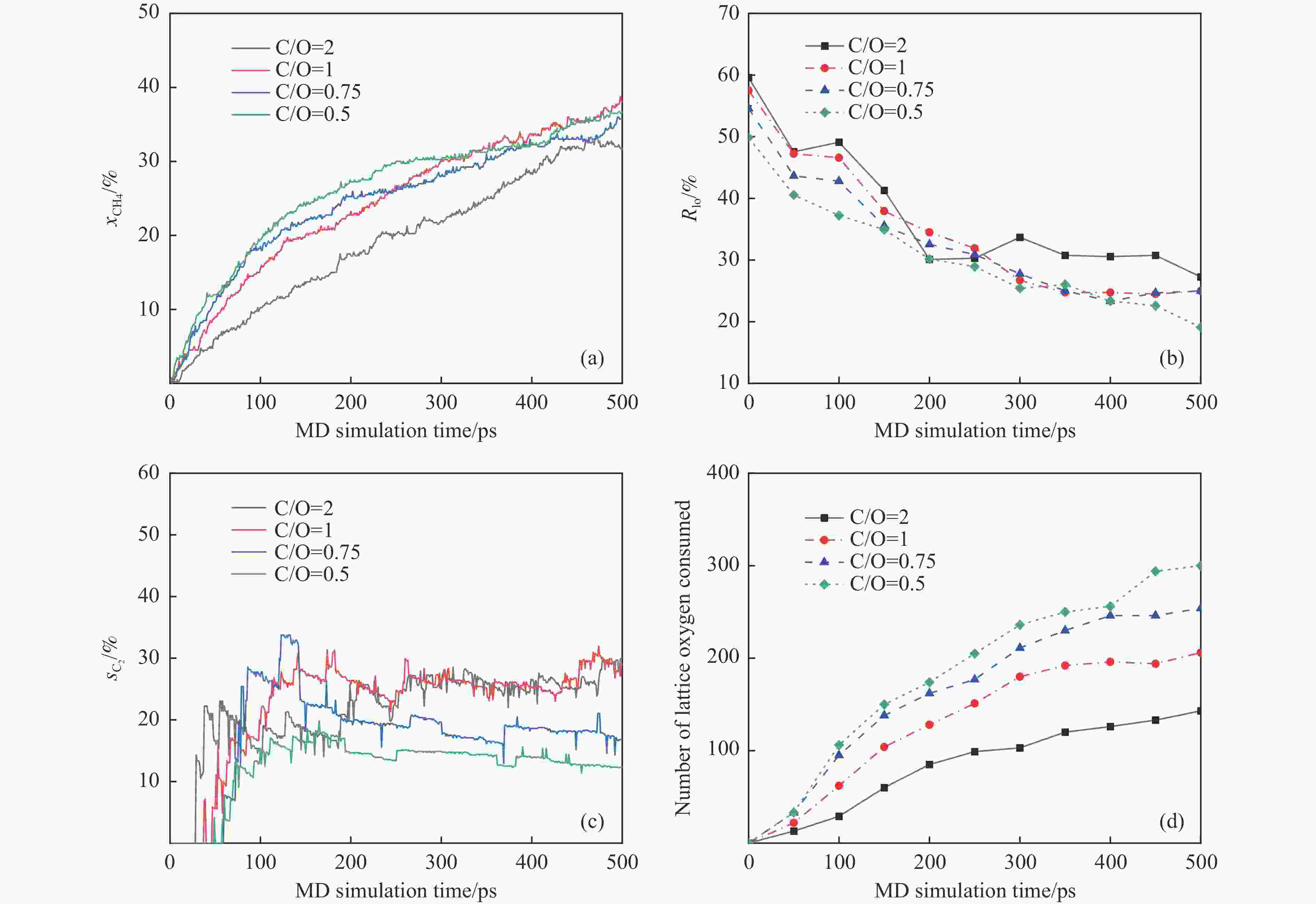
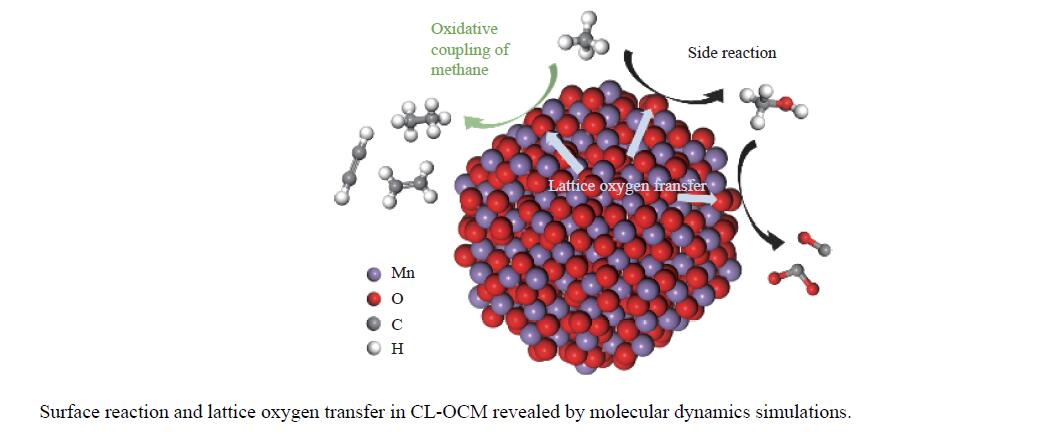
摘要: 本研究采用分子动力学模拟的方法计算八种金属氧化物催化剂-载氧体CL-OCM反应性能,并对性能最优的Mn2O3开展反应时间和颗粒尺寸的研究。结果表明,适当延长反应时间有利于提高 C2H4 选择性; C/O=1 是Mn2O3的理想尺寸。基于以上结果分析了Mn2O3 CL-OCM界面反应路径和晶格氧传递问题,以揭示反应机理。CH3 *气相二聚化生成C2H6的是CL-OCM最主要的碳偶联路径。除此之外,还存在两条碳偶联路径,均由CH2 *引发。CH3 *与OH*表面结合生成甲醇是CL-OCM副反应的先决步骤,抑制甲醇生成是提高CL-OCM反应C2选择性的关键。晶格氧存在转化,表面晶格氧是甲烷活化的活性氧。晶格氧数量差异及体相晶格氧迁移阻力差异是导致CH4转化率和C2选择性不同的主要原因。该研究为CL-OCM催化剂-载氧体的机理探究提供新的方法。Abstract: Chemical looping oxidative coupling of methane (CL-OCM) is a promising methodology for ethylene production from methane. This article utilizes molecular dynamic (MD) simulation to assess the performance of eight metal oxide catalytic oxygen carriers in CL-OCM reactions. It also investigates the impact of reaction time and particle size on the efficiency of the most effective Mn2O3 COC. The results indicate that extending the reaction time appropriately enhances C2H4 selectivity and a C/O ratio of 1 is found to be the optimal size for Mn2O3-based CL-OCM. Furthermore, surface reactions and lattice oxygen transfer are analyzed by MD simulation in Mn2O3-based CL-OCM, providing deeply insights into the reaction mechanism. The findings reveal that the gas-phase dimerization of CH3 * to form C2H6 serves as the primary carbon coupling pathway in CL-OCM. In addition, there are two other carbon coupling pathways, both initiated by CH2 *. Methanol formation through surface combination of CH3 * and OH* represents an initial step in CL-OCM side reactions. Therefore, inhibiting methanol formation is crucial for enhancing C2 selectivity in CL-OCM. There exists a transformation of lattice oxygen and surface lattice oxygen plays a key role in methane activation. The quantity of lattice oxygen and difference in bulk lattice oxygen migration resistance are major factors influencing variations CH4 conversion and C2 selectivity. This study provides a new way to reaction mechanism exploration related to CL-OCM catalytic oxygen carriers.
-
表 1 分子动力学模拟输入条件
Table 1 Molecular dynamic simulation input conditions
Metal oxide Time t/ps Radius r/Å (C/O) No. Different metal oxides Mn2O3 500 12 (1) 1 Mn3O4 2 Fe2O3 3 Fe3O4 4 FeO 5 CuO 6 Cu2O 7 NiO 8 Reaction time Mn2O3 250 12 (1) 9 1000 10 2000 11 Particle size Mn2O3 500 9.5 (2) 12 13.5 (0.75) 13 15.5 (0.5) 14 表 2 Mn2O3体系 CL-OCM反应中间体和产物及其首次出现时间
Table 2 Intermediates and products and their first appearance time for CL-OCM using Mn2O3
Intermediate First appearance
time t/psProduct First appearance
time t/psCH3 * 0.025 C2H6 7.75 CH2 * 9.575 C2H4 45.95 CH* 187.625 C2H2 494.025 H* 5.625 CO 44.225 O* 0.025 CO2 60.275 OH* 5 H2O 15.175 C* 388.95 H2 7.975 CH3O* 23.1 CH2O 6.575 CHO* 36.85 CH3OH 3.325 C2H5 * 39.375 CH3OCH3 145.025 C2H3 * 67.475 CHOOH 59.9 表 3 CuO体系CL-OCM反应中间体和产物及其首次出现时间
Table 3 Intermediates and products and their first appearance time for CL-OCM using CuO
Intermediate First appearance
time t/psProduct First appearance
time t/psCH3 * 0.05 C2H6 184.3 CH2 * 0.025 C2H4 99.15 CH* 29.475 C2H2 93.85 H* 0.7 CO 244.75 O* 2.675 H2O 65.4 OH* 4.6 H2 142.1 CH3O* 32.125 CH2O 14.8 CHO* 72.825 CH3OH 14.45 C2H5 * 93.775 C3H4 408.755 C2H3 * 150.65 − − C3H5 * 404.45 − − 表 4 CL-OCM反应过程中Mn2O3 组分及晶格氧变化
Table 4 Changes of Mn2O3 composition and lattice oxygen during CL-OCM reaction
Time/ps Composition Ro/% Rlo/% 0 Mn2O3 59.84 57.51 50 Mn2O2.85 58.80 47.25 100 Mn2O2.57 56.25 46.60 150 Mn2O2.29 53.41 37.94 200 Mn2O2.12 51.50 34.50 250 Mn2O2.03 49.58 31.91 300 Mn2O1.83 47.80 26.70 350 Mn2O1.74 46.52 24.74 400 Mn2O1.69 45.78 24.74 450 Mn2O1.67 45.49 24.68 500 Mn2O1.63 45.00 25.00 -
[1] MCFARLAND E. Unconventional chemistry for unconventional natural gas[J]. Science,2012,338(6105):340−342. doi: 10.1126/science.1226840 [2] AGARWAL N, FREAKLEY S J, MCVICKER R U, et al. Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions[J]. Science,2017,358(6360):223−227. doi: 10.1126/science.aan6515 [3] WANG P, ZHAO G, WANG Y, et al. MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst[J]. Sci Adv,2017,3(6):e1603180. doi: 10.1126/sciadv.1603180 [4] GAO J, ZHENG Y, JEHNG J-M, et al. Identification of molybdenum oxide nanostructures on zeolites for natural gas conversion[J]. Science,2015,348(6235):686−690. doi: 10.1126/science.aaa7048 [5] JIAO F, LI J, PAN X, et al. Selective conversion of syngas to light olefins[J]. Science,2016,351(6277):1065−1068. doi: 10.1126/science.aaf1835 [6] ZHONG L, YU F, AN Y, et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas[J]. Nature,2016,538(7623):84−87. doi: 10.1038/nature19786 [7] SINGH PAL R, RANA S, KUMAR SHARMA S, et al. Enhancement of oxygen vacancy sites of La2-xMxCe2O7-δ (M = Ca, Ba, Sr) catalyst for the low temperature oxidative coupling of methane: A combined DFT and experimental study[J]. Chem Eng J,2023,458:141379. doi: 10.1016/j.cej.2023.141379 [8] SI J, ZHAO G, LAN T, et al. Insight into the role of Na2WO4 in a low-temperature light-off Mn7SiO12-Na2WO4/cristobalite catalyst for oxidative coupling of methane[J]. ACS Catal,2023,13(2):1033−1044. doi: 10.1021/acscatal.2c04637 [9] NEAL L M, SHAFIEFARHOOD A, LI F. Dynamic methane partial oxidation using a Fe2O3@La0.8Sr0.2FeO3-δ core–shell redox catalyst in the absence of gaseous oxygen[J]. ACS Catal,2014,4(10):3560−3569. doi: 10.1021/cs5008415 [10] DAMASCENO S, TRINDADE F J, FONSECA F C, et al. Oxidative coupling of methane in chemical looping design[J]. Fuel Process Technol,2022,231:107255. doi: 10.1016/j.fuproc.2022.107255 [11] SON N, PARK B H, KIM S, et al. Flexible structural transformation and high oxygen-transfer capacity of mixed inverse spinel magnesium manganese oxides during methane chemical looping combustion[J]. Fuel Process Technol,2022,232:107262. doi: 10.1016/j.fuproc.2022.107262 [12] BASER D S, CHENG Z, FAN J A, et al. Codoping Mg-Mn based oxygen carrier with lithium and tungsten for enhanced C2 yield in a chemical looping oxidative coupling of methane system[J]. ACS Sustainable Chem Eng,2021,9(7):2651−2660. doi: 10.1021/acssuschemeng.0c07241 [13] HUANG J, ZHAO K, JIANG S, et al. Heteroatom-doping regulated Mg6MnO8 for improving C2+ hydrocarbons during chemical looping oxidative coupling of methane[J]. Fuel Process Technol,2022,235:107352. doi: 10.1016/j.fuproc.2022.107352 [14] FLEISCHER V, SIMON U, PARISHAN S, et al. Investigation of the role of the Na2WO4/Mn/SiO2 catalyst composition in the oxidative coupling of methane by chemical looping experiments[J]. J Catal,2018,360:102−117. doi: 10.1016/j.jcat.2018.01.022 [15] SUN W, ZHAO G, GAO Y, et al. An oxygen carrier catalyst toward efficient chemical looping-oxidative coupling of methane[J]. Appl Catal B: Environ,2022,304:120948. doi: 10.1016/j.apcatb.2021.120948 [16] SCHUCKER R C, DERRICKSON K J, ALI A K, et al. Identification of the optimum catalyst and operating conditions for oxidative coupling of methane: Activity and selectivity of alkaline earth-doped lanthanides[J]. Ind Eng Chem Res,2020,59(41):18434−18446. doi: 10.1021/acs.iecr.0c03005 [17] JIANG S, DING W, ZHAO K, et al. Enhanced chemical looping oxidative coupling of methane by Na-doped LaMnO3 redox catalysts[J]. Fuel,2021,299:120932. doi: 10.1016/j.fuel.2021.120932 [18] LI Z, HE L, WANG S, et al. Fast optimization of LiMgMnOx/La2O3 catalysts for the oxidative coupling of methane[J]. ACS Comb Sci,2017,19(1):15−24. doi: 10.1021/acscombsci.6b00108 [19] ZAVYALOVA U, HOLENA M, SCHLÖGL R, et al. Statistical analysis of past catalytic data on oxidative methane coupling for new insights into the composition of high-performance catalysts[J]. ChemCatChem,2011,3(12):1935−1947. doi: 10.1002/cctc.201100186 [20] FLEISCHER V, STEUER R, PARISHAN S, et al. Investigation of the surface reaction network of the oxidative coupling of methane over Na2WO4/Mn/SiO2 catalyst by temperature programmed and dynamic experiments[J]. J Catal,2016,341:91−103. doi: 10.1016/j.jcat.2016.06.014 [21] LOMONOSOV V I, SINEV M Y. Oxidative coupling of methane: Mechanism and kinetics[J]. Kinet Catal,2016,57(5):647−676. doi: 10.1134/S0023158416050128 [22] DUAN X, ZHAO T, YANG Z, et al. Oxygen activation-boosted manganese oxide with unique interface for chlorobenzene oxidation: Unveiling the roles and dynamic variation of active oxygen species in heterogeneous catalytic oxidation process[J]. Appl Catal B: Environ,2023,331:122719. doi: 10.1016/j.apcatb.2023.122719 [23] ZHAO K, ZHENG A, LI H, et al. Exploration of the mechanism of chemical looping steam methane reforming using double perovskite-type oxides La1.6Sr0.4FeCoO6[J]. Appl Catal B: Environ,2017,219:672−682. doi: 10.1016/j.apcatb.2017.08.027 [24] HUANG Z, GAO N, LIN Y, et al. Exploring the migration and transformation of lattice oxygen during chemical looping with NiFe2O4 oxygen carrier[J]. Chem Eng J,2022,429:132064. doi: 10.1016/j.cej.2021.132064 [25] CHEN D, HE D, LU J, et al. Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization[J]. Appl Catal B: Environ,2017,218:249−259. doi: 10.1016/j.apcatb.2017.06.053 [26] FU Q, LI W-X, YAO Y, et al. Interface-confined ferrous centers for catalytic oxidation[J]. Science,2010,328(5982):1141−1144. doi: 10.1126/science.1188267 [27] YANG L, ZHU D, WU X, et al. Lattice oxygen migration of iron-based oxygen carrier during chemical looping reaction process[J]. Fuel Process Technol,2023,251:107929. doi: 10.1016/j.fuproc.2023.107929 [28] BURGER C M, ZHU W, MA G, et al. Experimental and computational investigations of ethane and ethylene kinetics with copper oxide particles for chemical looping combustion[J]. Proc Combust Inst,2021,38(4):5249−5257. doi: 10.1016/j.proci.2020.06.006 [29] YANG Y, LIN Y-A, YAN X, et al. Cooperative atom motion in Ni-Cu nanoparticles during the structural evolution and the implication in the high-temperature catalyst design[J]. ACS Appl Energy Mater,2019,2(12):8894−8902. doi: 10.1021/acsaem.9b01923 [30] SUN C, SU R, CHEN J, et al. Carbon formation mechanism of C2H2 in Ni-based catalysts revealed by in situ electron microscopy and molecular dynamics simulations[J]. ACS Omega,2019,4(5):8413−8420. doi: 10.1021/acsomega.9b00958 [31] ZHAO H, GUI J, CAO J, et al. Molecular dynamics simulation of the microscopic sintering process of CuO nanograins inside an oxygen carrier particle[J]. J Phys Chem C,2018,122(44):25595−25605. doi: 10.1021/acs.jpcc.8b04253 [32] TAN Q, QIN W, CHEN Q, et al. Synergetic effect of ZrO2 on the oxidation-reduction reaction of Fe2O3 during chemical looping combustion[J]. Appl Surf Sci,2012,258(24):10022−10027. doi: 10.1016/j.apsusc.2012.06.067 [33] LIU W, ZHU Y, WU Y, et al. Molecular dynamics and machine learning in catalysts[J]. Catalysts,2021,11(9):1129. doi: 10.3390/catal11091129 [34] LI X, ZHENG M, REN C, et al. ReaxFF molecular dynamics simulations of thermal reactivity of various fuels in pyrolysis and combustion[J]. Energy Fuels,2021,35(15):11707−11739. doi: 10.1021/acs.energyfuels.1c01266 [35] LI G, ZHENG F, HUANG Q, et al. Molecular insight into pyrolysis processes via reactive force field molecular dynamics: A state-of-the-art review[J]. J Anal Appl Pyrolysis,2022,166:105620. doi: 10.1016/j.jaap.2022.105620 [36] SENFTLE T P, HONG S, ISLAM M M, et al. The ReaxFF reactive force-field: development, applications and future directions[J]. npj Comput Mater,2016,2(1):15011. doi: 10.1038/npjcompumats.2015.11 [37] MENG L, ZHU Y, ZHU M, et al. Exploring depolymerization mechanism and complex reaction networks of aromatic structures in chemical looping combustion via ReaxFF MD simulations[J]. J Energy Inst,2023,107:101180. doi: 10.1016/j.joei.2023.101180 [38] WANG Y, GU M, ZHU Y, et al. Analysis of soot formation of CH4 and C2H4 with H2 addition via ReaxFF molecular dynamics and pyrolysis-gas chromatography/mass spectrometry[J]. J Energy Inst,2022,100:177−188. doi: 10.1016/j.joei.2021.11.007 [39] HONG D, LIU L, WANG C, et al. Construction of a coal char model and its combustion and gasification characteristics: Molecular dynamic simulations based on ReaxFF[J]. Fuel,2021,300:120972. doi: 10.1016/j.fuel.2021.120972 [40] REDDIVARI S, LASTOSKIE C, WU R, et al. Chemical composition and formation mechanisms in the cathode-electrolyte interface layer of lithium manganese oxide batteries from reactive force field (ReaxFF) based molecular dynamics[J]. Front Energy,2017,11(3):365−373. doi: 10.1007/s11708-017-0500-8 [41] ARYANPOUR M, VAN DUIN A C T, KUBICKI J D. Development of a reactive force field for iron-oxyhydroxide systems[J]. J Phys Chem A,2010,114(21):6298−6307. doi: 10.1021/jp101332k [42] ZHU W, GONG H, HAN Y, et al. Development of a reactive force field for simulations on the catalytic conversion of C/H/O molecules on Cu-metal and Cu-oxide surfaces and application to Cu/CuO-based chemical looping[J]. J Phys Chem C,2020,124(23):12512−12520. doi: 10.1021/acs.jpcc.0c02573 [43] SHIN Y K, GAI L, RAMAN S, et al. Development of a ReaxFF reactive force field for the Pt-Ni alloy catalyst[J]. J Phys Chem A,2016,120(41):8044−8055. doi: 10.1021/acs.jpca.6b06770 [44] XIA X-R, XIONG G-X, MIAO Q, et al. Deactivation of NaCl/B2O3/Fe2O3 catalysts and their improvement for the oxidative coupling of methane[J]. Catal Lett,1995,31(2):183−195. [45] LEE S H, JUNG D W, KIM J B, et al. Effect of altervalent cation-doping on catalytic activity of neodymium sesquioxide for oxidative coupling of methane[J]. Appl Catal A: Gen,1997,164(1):159−169. [46] KELLER G E, BHASIN M M. Synthesis of ethylene via oxidative coupling of methane: I. Determination of active catalysts[J]. J Catal,1982,73(1):9−19. doi: 10.1016/0021-9517(82)90075-6 [47] CHUNG E Y, WANG W K, NADGOUDA S G, et al. Catalytic oxygen carriers and process systems for oxidative coupling of methane using the chemical looping technology[J]. Ind Eng Chem Res,2016,55(50):12750−12764. doi: 10.1021/acs.iecr.6b03304 [48] DRISCOLL D J, LUNSFORD J H. Gas-phase radical formation during the reactions of methane, ethane, ethylene, and propylene over selected oxide catalysts[J]. J Phys Chem,1985,89(21):4415−4418. doi: 10.1021/j100267a002 [49] DONG M, HAN B, YE R, et al. Boosting the generation of key intermediate methyl radical (CH3·) in OCM reaction on magnesium oxide catalysts by regulating the electronic state of the active site[J]. Mol Catal,2023,542:113125. doi: 10.1016/j.mcat.2023.113125 [50] ORTIZ-BRAVO C A, CHAGAS C A, TONIOLO F S. Oxidative coupling of methane (OCM): An overview of the challenges and opportunities for developing new technologies[J]. J Nat Gas Sci Eng,2021,96:104254. doi: 10.1016/j.jngse.2021.104254 [51] MARTIN G-A, MIRODATOS C. Evidence of carbene formation in oxidative coupling of methane over lithium promoted magnesium oxide[J]. J Chem Soc, Chem Commun,1987, (18):1393−1394. -




 下载:
下载: