A thermodynamic consideration on the synthesis of methane from CO, CO2, and their mixture by hydrogenation
-
摘要: 由CO和CO2加氢制甲烷是目前氢能储存及燃料和化学品可持续生产的有效途径之一,但目前对该反应过程(尤其是针对CO和CO2混合物加氢)的一些细节尚不明晰。为此,作为前期有关CO和CO2加氢制烯烃和醇研究工作的补充,本工作对CO和CO2,尤其是两者混合物的加氢制甲烷反应过程进行了热力学分析。结果证实,与单独CO或CO2相比,二者混合物加氢制甲烷更为合适,总碳基甲烷收率可作为评估甲烷合成反应过程效率的重要指标。CO加氢的甲烷平衡收率比CO2加氢的高,而CO和CO2混合物加氢的总碳基甲烷平衡收率位于两者之间;对于CO和CO2混合物加氢,尽管CO和CO2的平衡转化率随进料组成不同会有很大的变化,但其总碳基甲烷平衡收率随着原料中CO2/(CO+CO2)物质的量比的增大而线形降低。整体上看,在温度低于400 ℃和压力高于0.1 MPa时,无论是CO、CO2、还是两者混合物的化学计量比加氢,其总碳基甲烷平衡收率均高于85%。这些结果无疑对高效CO和CO2加氢制甲烷催化剂研制及反应过程的设计和操作优化有重要的参考价值。Abstract: The synthesis of methane from CO and CO2 by hydrogenation is now considered as a promising route in effectively storing hydrogen energy as well as sustainably producing fuels and chemicals, while many reaction details involved in such processes, in particular for the hydrogenation of the CO and CO2 mixture, are not yet adequately understood. As a supplement to our previous works on the hydrogenation of CO and CO2 into alcohols and hydrocarbons, a thermodynamic consideration is made in this work to evaluate the potential and limit for the synthesis of methane from CO, CO2, and their mixture in particular. The results consolidate that in comparison with single CO or CO2, their mixture is probably more credible in practice for the production of methane by hydrogenation, where the overall C-based methane yield can be used as the major index to evaluate the process efficiency. The hydrogenation of CO shows a higher equilibrium yield of methane than the hydrogenation of CO2, while the overall C-based equilibrium yield of methane for the hydrogenation of the CO and CO2 mixture just lies in between and decreases almost lineally with the increase of the CO2/(CO+CO2) molar ratio in the feed, despite the great change in the equilibrium conversions of CO and CO2 with the feed composition. Nevertheless, an adequate overall C-based equilibrium yield of methane (> 85%) can be achieved at a temperature lower than 400 ℃ and a pressure higher than 0.1 MPa for the stoichiometric hydrogenation of CO, CO2, or their mixture whichever. These results should be beneficial to the design of more efficient catalysts and processes for the hydrogenation of CO and CO2 to methane.1) #:共同第一作者
-
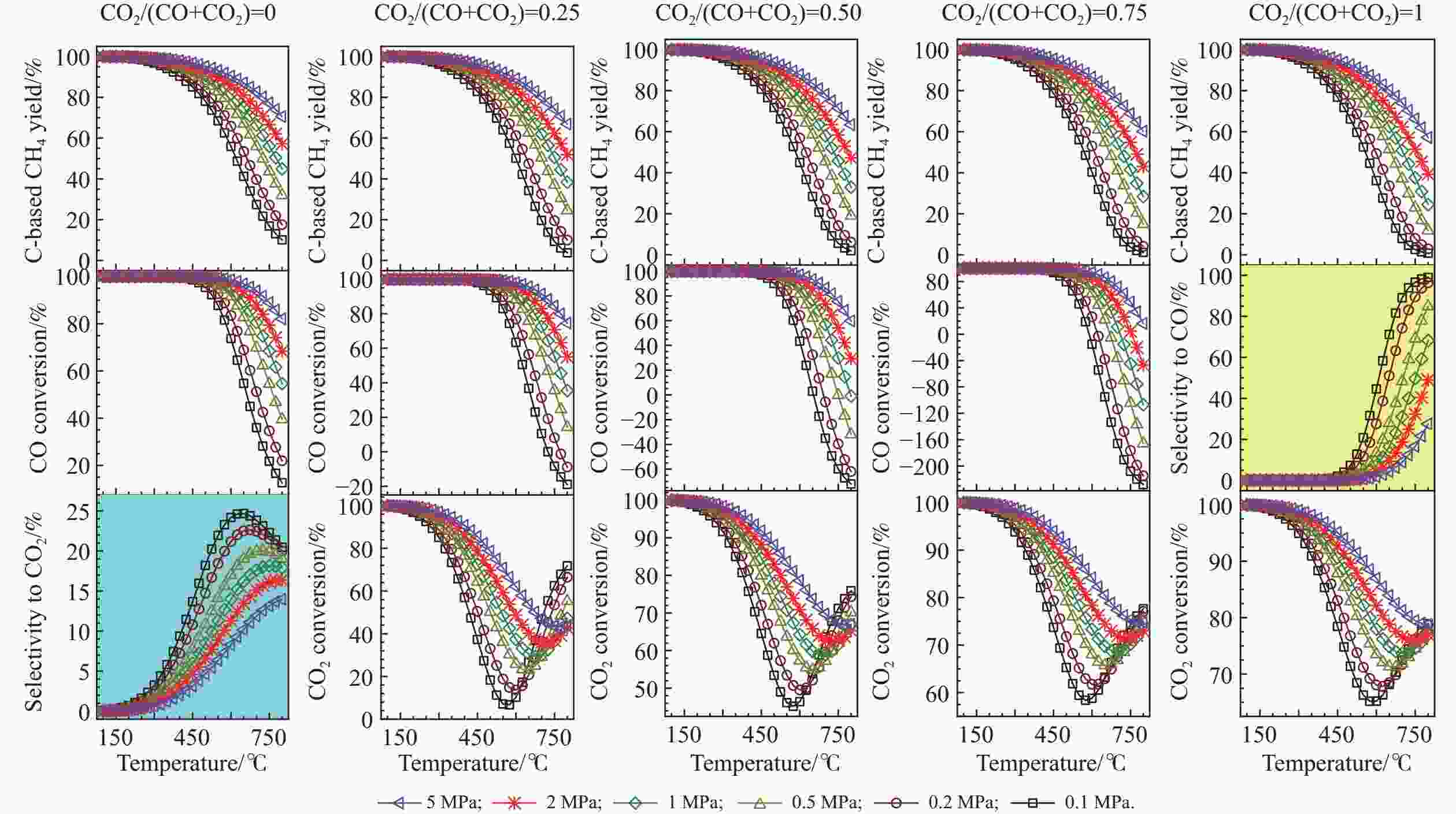
图 2 化学计量比的氢气存在下,CO和CO2混合物加氢制甲烷反应过程中,甲烷的总碳基平衡收率、CO转化率和CO2转化率随温度、压力和进料组成的变化
Figure 2 Overall C-based equilibrium methane yield, CO conversion, and CO2 conversion for the hydrogenation of the CO and CO2 mixture to methane by using stoichiometric amount of H2 in the feed, via the reactions of R1 and R2, where the WGS reaction of R4 or the reverse one (RWGS) as a nonindependent reaction occurs inevitably. Depending on the CO2/(CO+CO2) molar ratio (z), the initial reaction mixture has a H2∶CO∶CO2 molar ratio of (3+z)∶(1−z)∶z. For the hydrogenation of single CO (CO2/(CO+CO2) = 0), the equilibrium selectivity to CO2 is displayed instead of the equilibrium CO2 conversion, whereas for the hydrogenation of single CO2 (CO2/(CO+CO2) = 1), the equilibrium selectivity to CO is shown instead of the equilibrium CO conversion
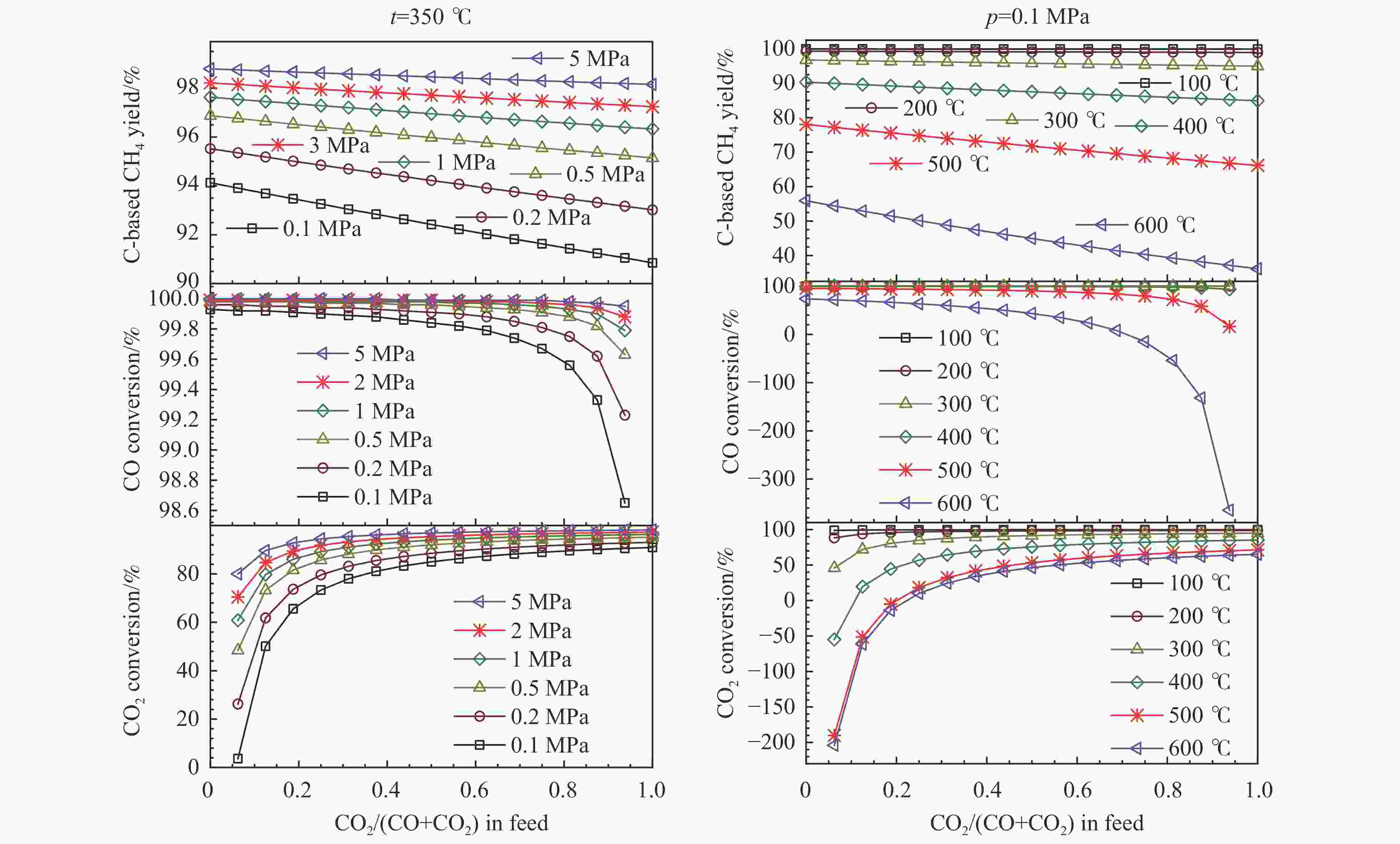
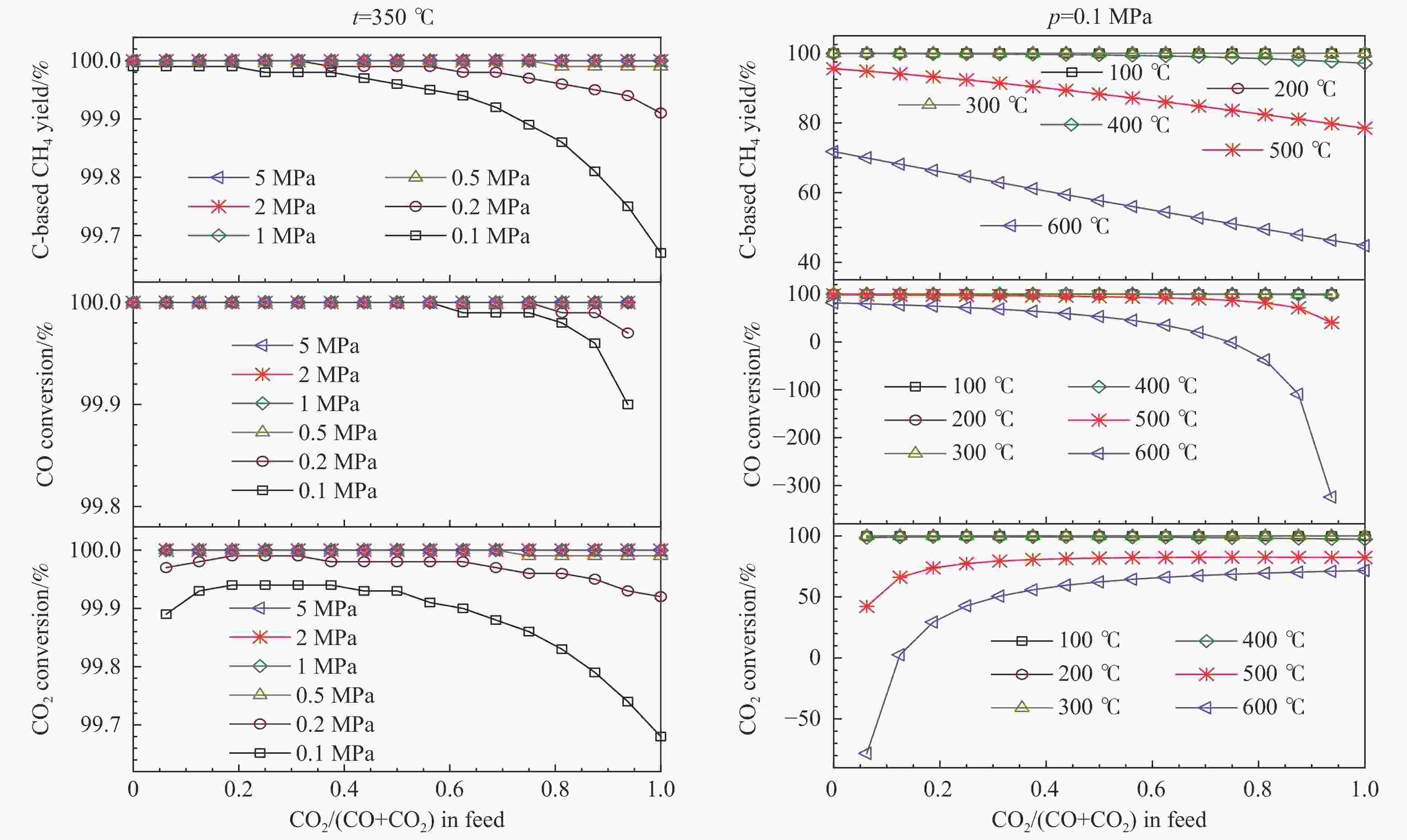
图 3 化学计量比的氢气存在下,CO和CO2混合物加氢制甲烷反应过程中,甲烷的总碳基平衡收率、CO转化率和CO2转化率随反应混合物进料组成的变化
Figure 3 Overall C-based equilibrium methane yield, CO conversion, and CO2 conversion varied with the feed CO2/(CO+CO2) molar ratio (z) for the hydrogenation of CO and CO2 mixture into methane by using stoichiometric amount of H2 in the feed, at 350 ℃ and different pressures (left) and at 0.1 MPa and different temperatures (right), via the reactions of R1 and R2, where the WGS reaction of R4 or the reverse one (RWGS) as a nonindependent reaction occurs inevitably; the initial reaction mixture has a H2∶CO∶CO2 molar ratio of (3+z)∶(1−z)∶z
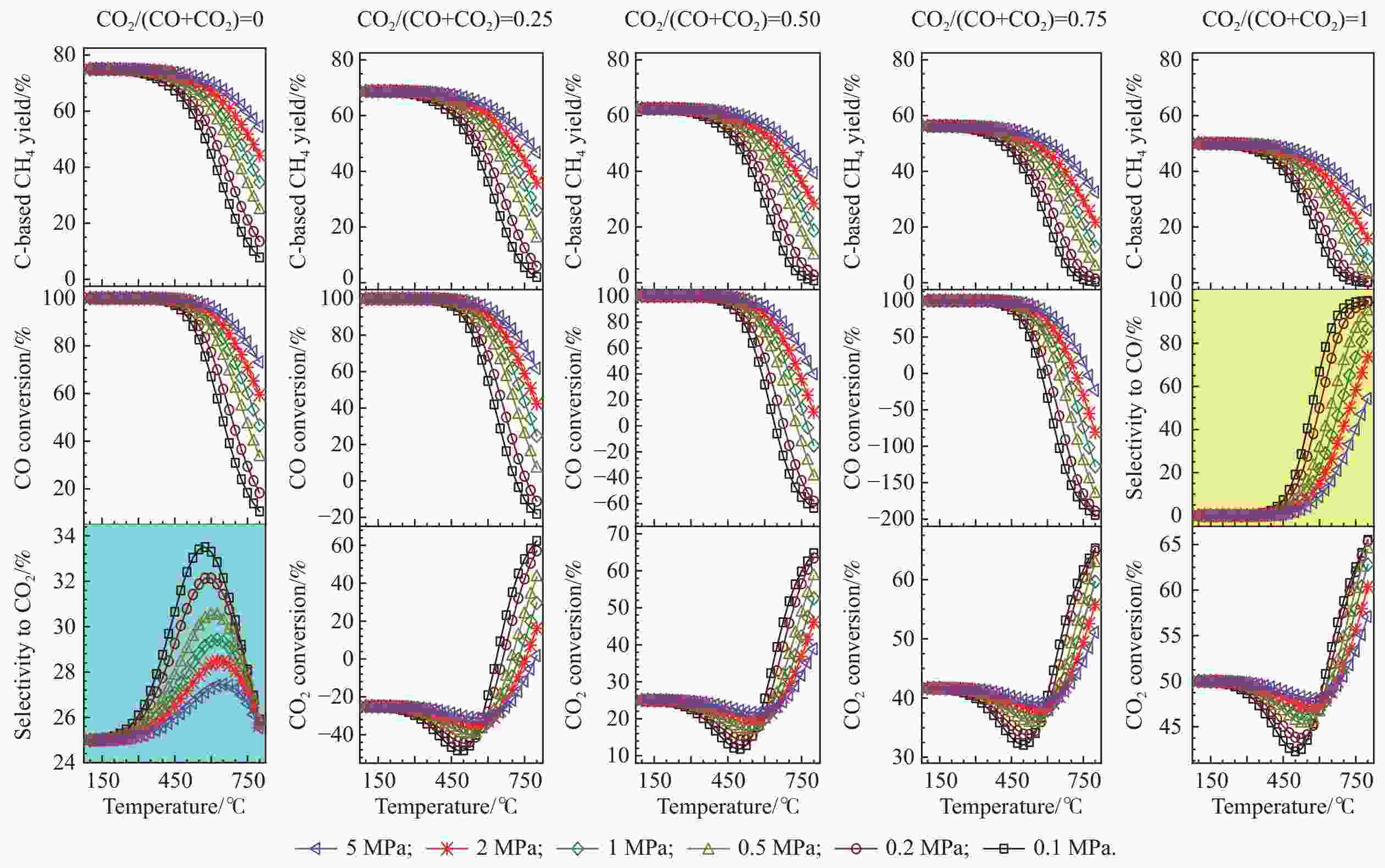
图 4 低H/C比下,CO和CO2混合物加氢制甲烷反应过程中,甲烷的总碳基平衡收率、CO转化率和CO2转化率随温度、压力和进料组成的变化
Figure 4 Overall C-based equilibrium methane yield, CO conversion, and CO2 conversion for the hydrogenation of the CO and CO2 mixture to methane by using H2-deficient feed, via the reactions of R1 and R2, where the WGS reaction of R4 or the reverse one (RWGS) as a nonindependent reaction occurs inevitably. The initial reaction mixture has a H2/(CO+CO2) molar ratio of 2. For the hydrogenation of single CO (CO2/(CO+CO2) = 0),the equilibrium selectivity to CO2 is displayed instead of the equilibrium CO2 conversion, whereas for the hydrogenation of single CO2 (CO2/(CO+CO2) = 1), the equilibrium selectivity to CO is shown instead of the equilibrium CO conversion.
图 5 低H/C比下,CO和CO2混合物加氢制甲烷反应过程中,甲烷的总碳基平衡收率、CO转化率和CO2转化率随反应混合物进料组成的变化
Figure 5 Overall C-based equilibrium methane yield, CO conversion, and CO2 conversion varied with the feed CO2/(CO+CO2) molar ratio (z) for the hydrogenation of CO and CO2 mixture into methane by using H2-deficient feed, at 350 ℃ and different pressures (left) and at 0.1 MPa and different temperatures (right), via the reactions of R1 and R2, where the WGS reaction of R4 or the reverse one (RWGS) as a nonindependent reaction occurs inevitably. The initial reaction mixture has a H2/(CO+CO2) molar ratio of 2.
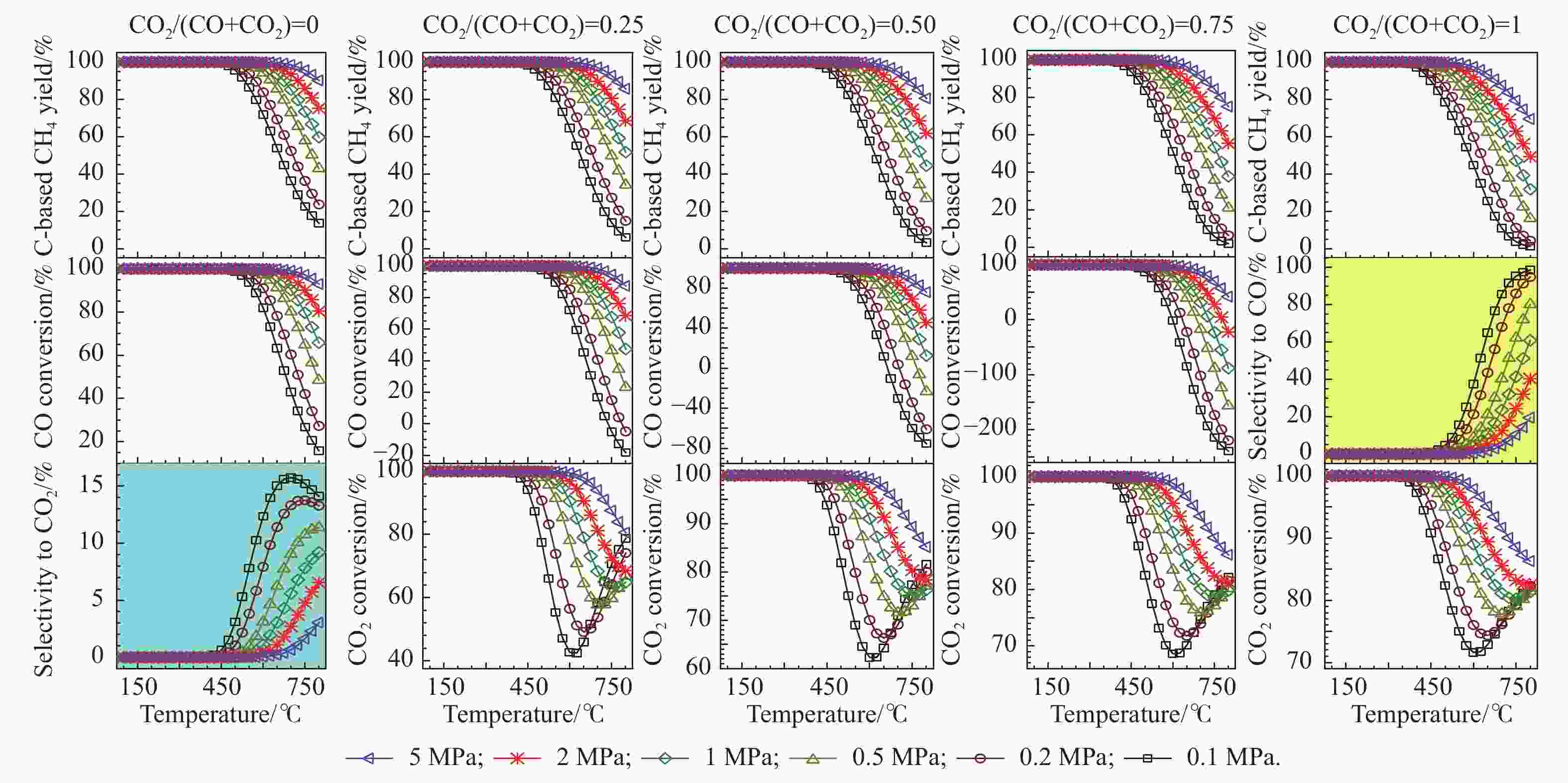
图 6 高H/C比下,CO和CO2混合物加氢制甲烷反应过程中,甲烷的总碳基平衡收率、CO转化率和CO2转化率随温度、压力和进料组成的变化
Figure 6 Overall C-based equilibrium methane yield, CO conversion, and CO2 conversion for the hydrogenation of the CO and CO2 mixture to methane by using the feed with H2 in surplus, via the reactions of R1 and R2, where the WGS reaction of R4 or the reverse one (RWGS) as a nonindependent reaction occurs inevitably. The initial reaction mixture has a H2/(CO+CO2) molar ratio of 5. For the hydrogenation of single CO (CO2/(CO+CO2) = 0), the equilibrium selectivity to CO2 is displayed instead of the equilibrium CO2 conversion, whereas for the hydrogenation of single CO2 (CO2/(CO+CO2) = 1), the equilibrium selectivity to CO is presented instead of the equilibrium CO conversion.
图 7 高H/C比下,CO和CO2混合物加氢制甲烷反应过程中,甲烷的总碳基平衡收率、CO转化率和CO2转化率随反应混合物进料组成的变化
Figure 7 Overall C-based equilibrium methane yield, CO conversion, and CO2 conversion varied with the feed CO2/(CO+CO2) molar ratio (z) for the hydrogenation of CO and CO2 mixture into methane by using the feed with H2 in surplus, at 350 ℃ and different pressures (left) and at 0.1 MPa and different temperatures (right), via the reactions of R1 and R2, where the WGS reaction of R4 or the reverse one (RWGS) as a nonindependent reaction occurs inevitably. The initial reaction mixture has a H2/(CO+CO2) molar ratio of 5.
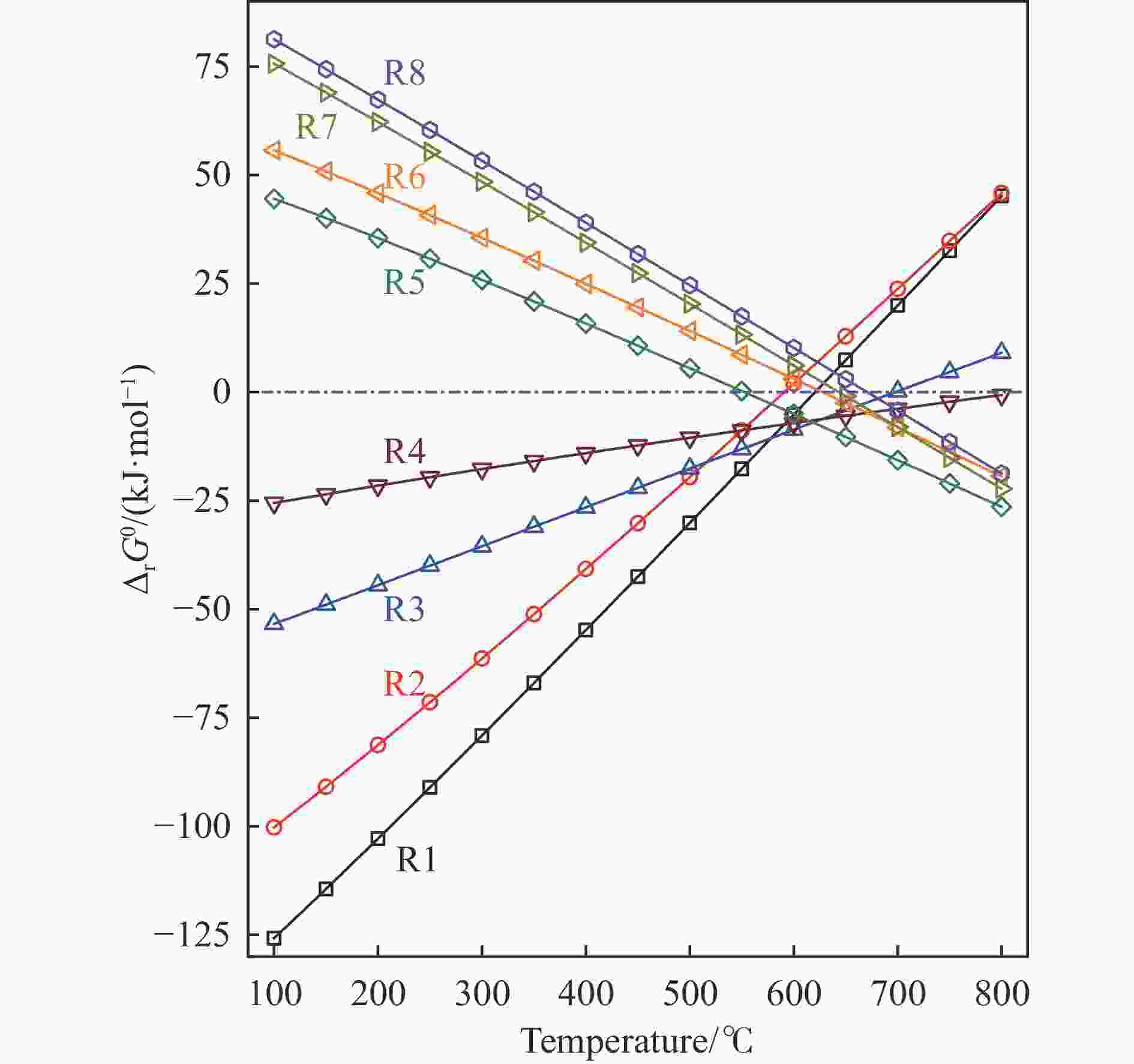
表 1 CO和CO2加氢制甲烷过程中涉及的主要反应及其相关的基本热力学数据
Table 1 Thermodynamic data of various reactions involved in the hydrogenation of CO and CO2 to methane
Entry Reaction ΔrH0298K/(kJ·mol−1) ΔrS0298K/(J K−1·mol−1) ΔrG0298K/(kJ·mol−1) n(H2)/n(COx) Rv R1 CO + 3H2 = CH4 + H2O −206.14 −214.63 −142.15 3 0.5 R2 CO2 + 4H2 = CH4 + 2H2O −164.98 −172.56 −113.53 4 0.6 R3 CO = 1/2C + 1/2CO2 −86.23 −87.93 −60.01 − 0.5 R4 CO + H2O = CO2 + H2 −41.17 −42.08 −28.62 1 1 R5 CH4 = C + 2H2 74.85 80.84 50.75 − 2 R6 C + 2H2O = CO2 + 2H2 90.13 91.72 62.78 2 1.5 R7 1/2CO2 + 1/2CH4 = CO + H2 123.65 128.35 85.39 1 2 R8 C + H2O = CO + H2 131.29 133.79 91.40 1 2 Notes: n(H2)/n(COx) is the stoichiometric H2/CO or H2/CO2 molar ratio in the reactants or products; Rv is the stoichiometric volume-expanding or molecule-increasing factor, defined as the number of gaseous product molecules divided by the number of gaseous. reactant molecules for the corresponding reaction. -
[1] ZHOU W, CHENG K, KANG J C, et al. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels[J]. Chem Soc Rev,2019,48(12):3193−3228. doi: 10.1039/C8CS00502H [2] WANG H, FAN S, WANG S, et al. Research progresses in the hydrogenation of carbon dioxide to certain hydrocarbon products[J]. J Fuel Chem Technol,2021,49(11):1609−1619. doi: 10.1016/S1872-5813(21)60122-6 [3] MEMON M A, JIANG Y, HASSAN M A, et al. Heterogeneous catalysts for carbon dioxide methanation: A view on catalytic performance[J]. Catalysts,2023,13(12):1514. doi: 10.3390/catal13121514 [4] BUSHUYEV O S, de LUNA P, DINH C T, et al. What should we make with CO2 and how can we make it?[J]. Joule,2018,2(5):825−832. doi: 10.1016/j.joule.2017.09.003 [5] RA E C, KIM K Y, KIM E H, et al. Recycling carbon dioxide through catalytic hydrogenation: recent key developments and perspectives[J]. ACS Catal,2020,10(19):11318−11345. doi: 10.1021/acscatal.0c02930 [6] GAO J, WANG Y, PING Y, et al. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas[J]. RSC Adv,2012,2(6):2358−2368. doi: 10.1039/c2ra00632d [7] GUO S, WANG H, QIN Z, et al. Feasibility, limit, and suitable reaction conditions for the production of alcohols and hydrocarbons from CO and CO2 through hydrogenation, a thermodynamic consideration[J]. Ind Eng Chem Res,2022,61(46):17027−17038. doi: 10.1021/acs.iecr.2c02898 [8] GUO S, WANG H, QIN Z, et al. Conversion of the CO and CO2 mixture to alcohols and hydrocarbons by hydrogenation under the influence of the water-gas shift reaction, a thermodynamic consideration[J]. J Fuel Chem Technol,2023,51(4):482−491. doi: 10.1016/S1872-5813(23)60346-9 [9] POLING B E, PRAUSNITZ J M, O’CONNELL J P. The Properties of Gases and Liquids [M]. Fifth Edition. McGraw-Hill: New York, 2004. [10] YAWS C L. Chemical Properties Handbook [M]. McGraw-Hill: Beijing, 1999. [11] LIU J, QIN Z, WANG J. Methanol synthesis under supercritical conditions: calculations of equilibrium conversions by using the Soave-Redlich-Kwong equation of state[J]. Ind Eng Chem Res,2001,40(17):3801−3805. doi: 10.1021/ie0100479 [12] SOAVE G. Equilibrium constants from a modified Redlich-Kwong equation of state[J]. Chem Eng Sci,1972,27(6):1197−1203. doi: 10.1016/0009-2509(72)80096-4 [13] GRAAF G H, SIJTSEMA P J J M, STAMHUIS E J, et al. Chemical equilibria in methanol synthesis[J]. Chem Eng Sci,1986,41(11):2883−2890. doi: 10.1016/0009-2509(86)80019-7 -




 下载:
下载: