Ethanol production from syngas over RhnNin/TiO2(n = 1, 2, 3, 4) catalysts: probing into the roles of RhnNin alloy clusters size in tuning catalytic performance
-
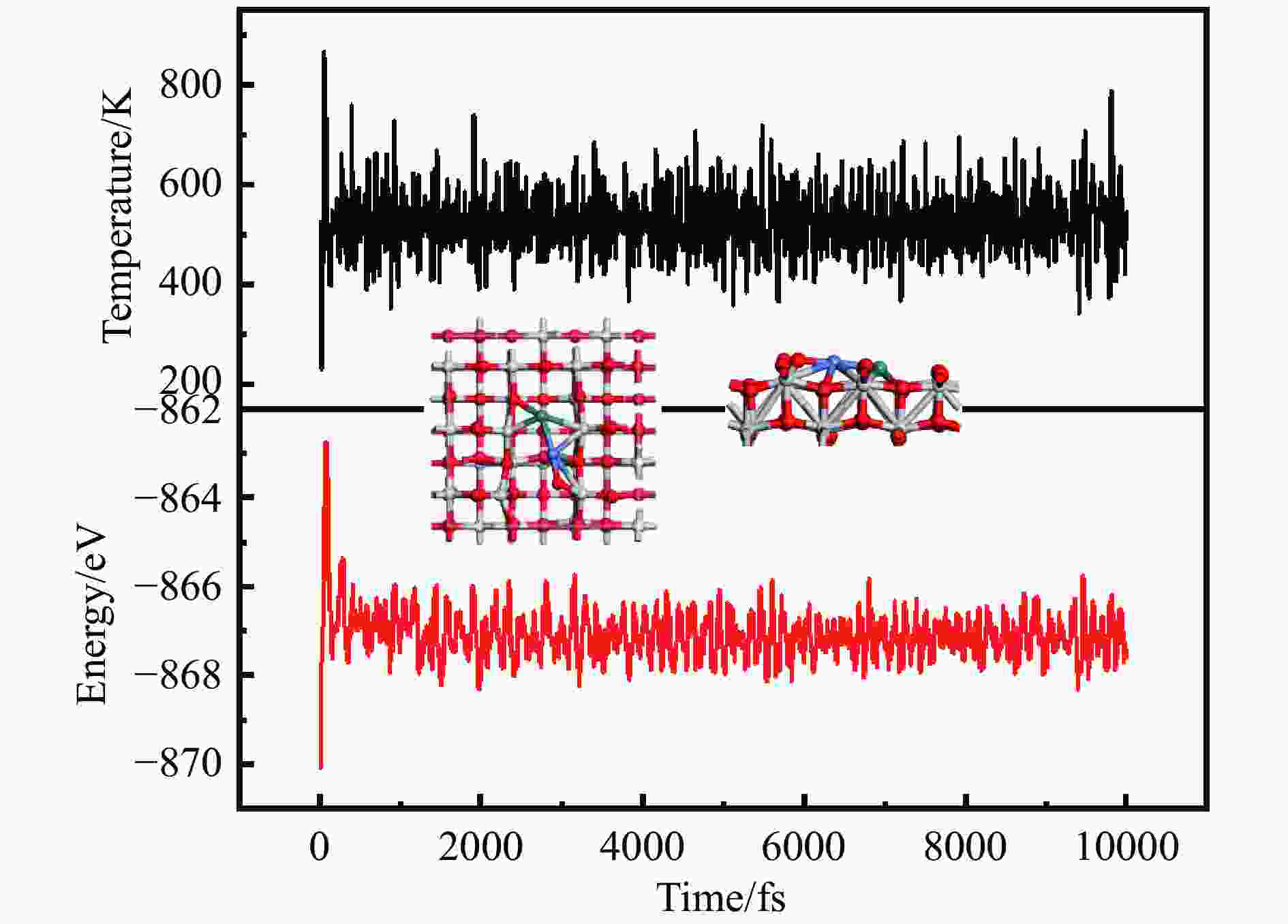
摘要: 为明确RhnNin合金团簇尺寸诱导的金属-载体相互作用对合成气制乙醇反应性能的调控机制,本工作采用密度泛函理论(DFT)和微观动力学方法研究了不同Rh-Ni合金团簇尺寸RhnNin/TiO2(n = 1、2、3、4)上合成气制乙醇反应。结果表明,Rh1Ni1/TiO2和Rh3Ni3/TiO2能够显著促进CO活化转化及C−C链的形成,并抑制甲烷的生成。其中,Rh1Ni1/TiO2表现出最高的乙醇生成活性和相对选择性。电子性质分析表明,在Rh1Ni1/TiO2催化剂上,合金团簇上Ni原子及载体上Ti和O原子向Rh原子转移的电荷最多,合金团簇上Rh-Ni间相互作用最强,且合金团簇与TiO2载体间的相互作用最强,催化剂的催化活性最高。在525 K下,从头算分子动力学模拟(AIMD)模拟显示Rh1Ni1/TiO2催化剂具有较高的热稳定性。
-
关键词:
- 合成气制乙醇 /
- Rh-Ni合金团簇尺寸 /
- 金属-载体间相互作用 /
- 密度泛函理论
Abstract: The direct production of ethanol from syngas on RhnNin/TiO2 (n = 1, 2, 3, 4) has been investigated by using density functional theory (DFT) and micro-kinetic methods, in order to elucidate the regulatory mechanism of RhnNin alloy cluster size-induced metal-support interactions on the performance of ethanol synthesis. The results showed that Rh1Ni1/TiO2 and Rh3Ni3/TiO2 can significantly enhance the CO conversion and C−C chain formation, while inhibiting the methane generation. Among them, Rh1Ni1/TiO2 exhibits the highest ethanol generation activity and relative selectivity. Electronic property analyses revealed that Ni atoms on the alloy clusters and Ti and O atoms on the supports transferred the most of charge to the Rh atoms on the Rh1Ni1/TiO2 catalysts. The Rh-Ni interactions on the alloy clusters were the strongest, and the alloy clusters exhibited the strongest interactions with the TiO2 supports, resulting in the highest catalytic activity among the catalysts. Ab-initio molecular dynamics (AIMD) simulations at 525 K showed that the Rh1Ni1/TiO2 catalyst exhibited high thermal stability. -
表 1 RhnNin(n = 1、2、3、4)合金团簇同分异构体构型及结合能
Table 1 Configuration and binding energy of isomers of RhnNin(n = 1, 2, 3, 4) alloy clusters
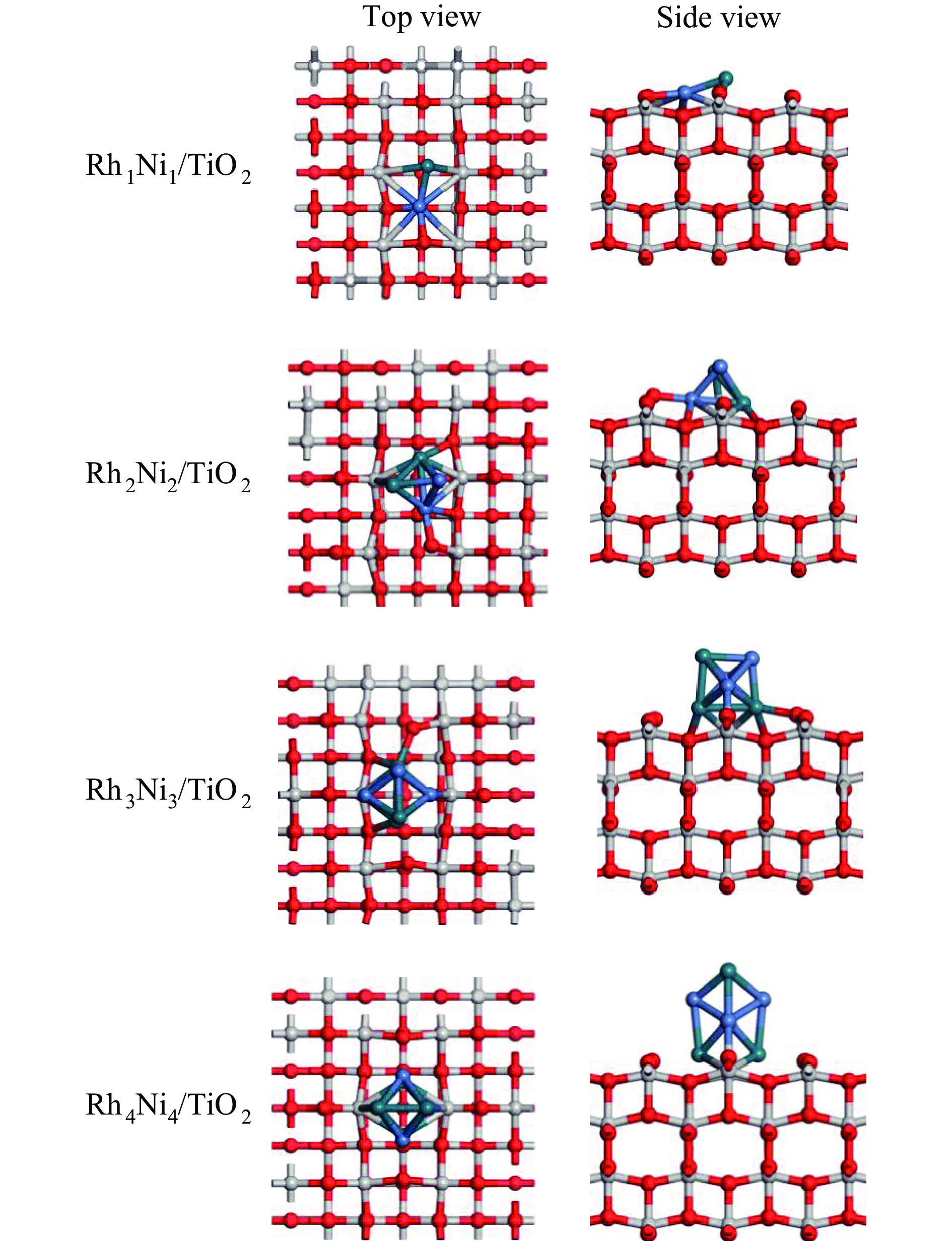
Cluster Structure EB/(kJ·mol−1) Cluster Structure EB/(kJ·mol−1) Rh1Ni1 
157.4 Rh4Ni4-1 
328.5 Rh2Ni2 
251.2 Rh4Ni4-2 
329.4 Rh3Ni3-1 
301.7 Rh4Ni4-3 
328.0 Rh3Ni3-2 
300.2 Rh4Ni4-4 
326.9 Rh4Ni4-5 
327.1 表 2 TiO2载体与RhnNin(n = 1、2、3、4)团簇之间的结合能Eb(n)和平均原子结合能$\overline E_{\mathrm{b}} $(n)
Table 2 Binding energy Eb(n) between TiO2 support and RhnNin(n = 1, 2, 3, 4) cluster and average atomic binding energy $\overline E_{\mathrm{b}} $ (n)
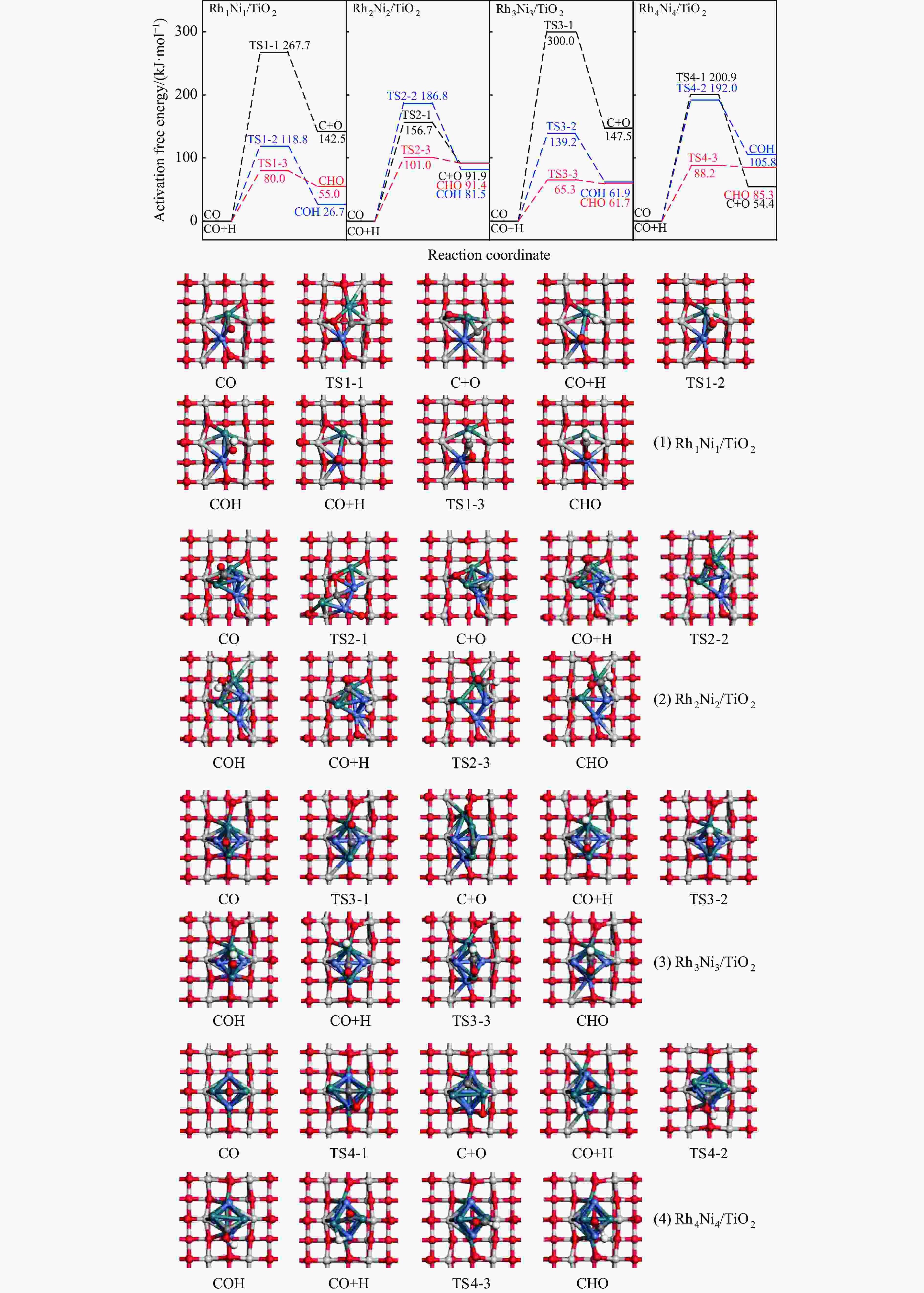
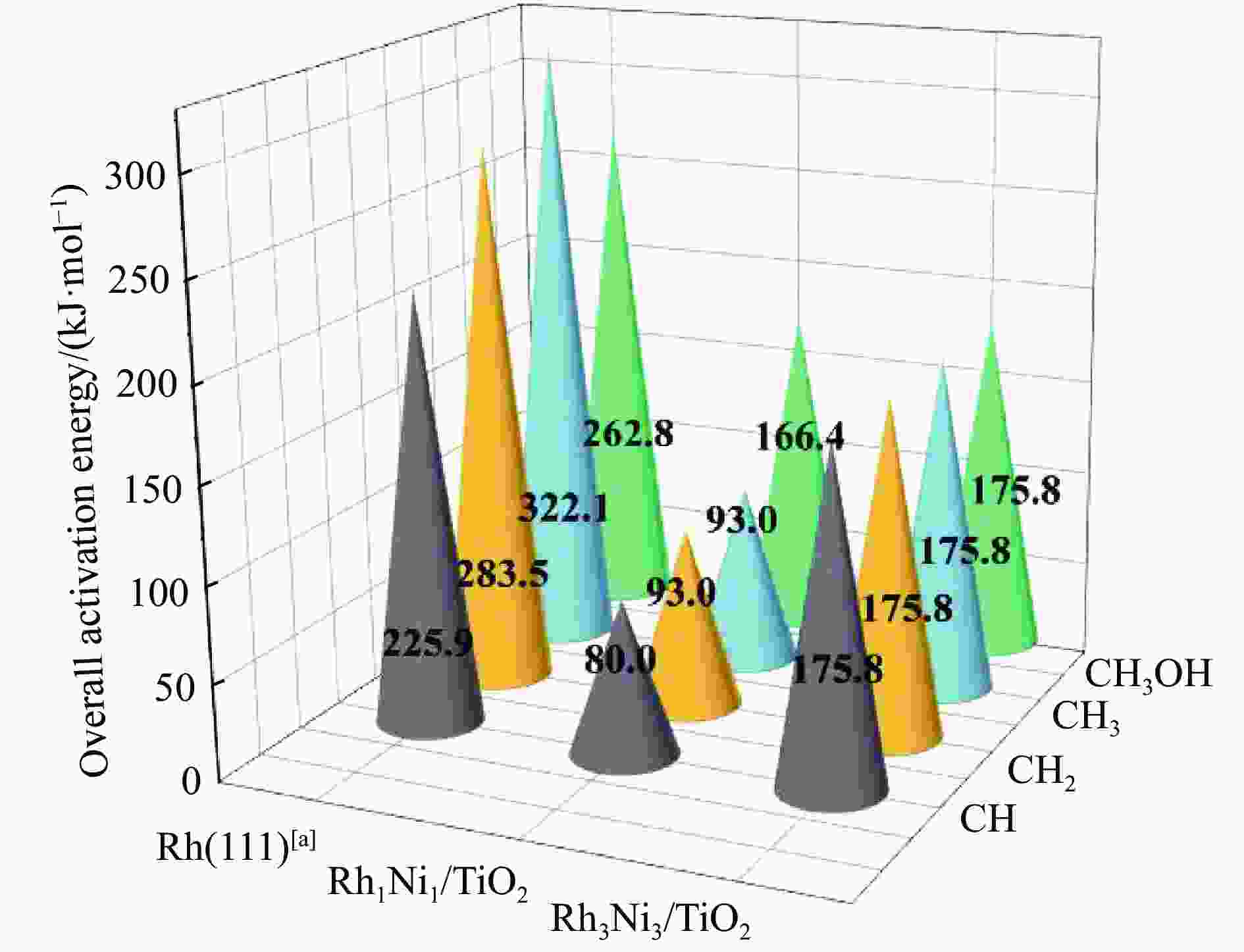
Catalyst E/(kJ·mol−1) Eb(n) $\overline E_{\mathrm{b}} $(n) Rh1Ni1/TiO2 −368.7 −184.3 Rh2Ni2/TiO2 −531.1 −132.8 Rh3Ni3/TiO2 −346.5 −57.7 Rh4Ni4/TiO2 −449.9 −56.2 表 3 由CO活化生成CHO过程中(1) Rh1Ni1/TiO2、(2) Rh2Ni2/TiO2、(3) Rh3Ni3/TiO2和(4) Rh4Ni4/TiO2催化剂上C−H键形成的活化能(Ea)及从反应物到过渡态上的C−H键缩短量(ΔDC−H)
Table 3 The activation energy (Ea) for C−H bond formation on (1) Rh1Ni1/TiO2、(2) Rh2Ni2/TiO2、(3) Rh3Ni3/TiO2 and (4) Rh4Ni4/TiO2 catalysts during CHO formation from CO activation and the C−H bond shortening (ΔDC−H) from reactant to transition state
Catalyst Rh1Ni1/TiO2 Rh2Ni2/TiO2 Rh3Ni3/TiO2 Rh4Ni4/TiO2 Ea/(kJ·mol−1) 80.0 101.0 65.3 88.2 △DC−H/Å 1.386 1.886 1.082 1.627 表 4 525K下Rh1Ni1/TiO2和Rh3Ni3/TiO2催化剂表面上乙醇生成中所涉及基元反应的活化自由能(Ga kJ/mol)、反应自由热(ΔG kJ/mol)及基元反应对应的唯一虚频
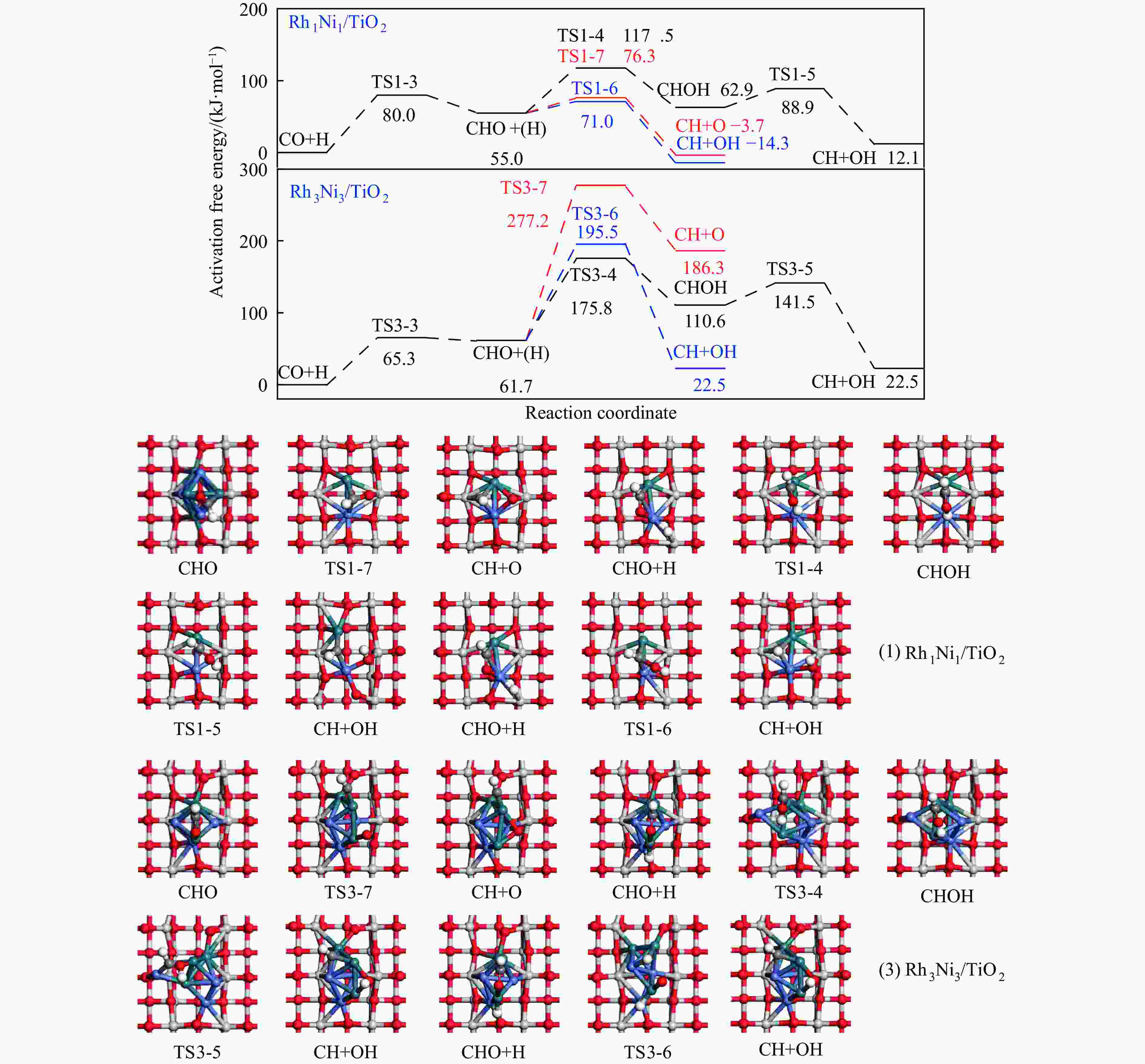
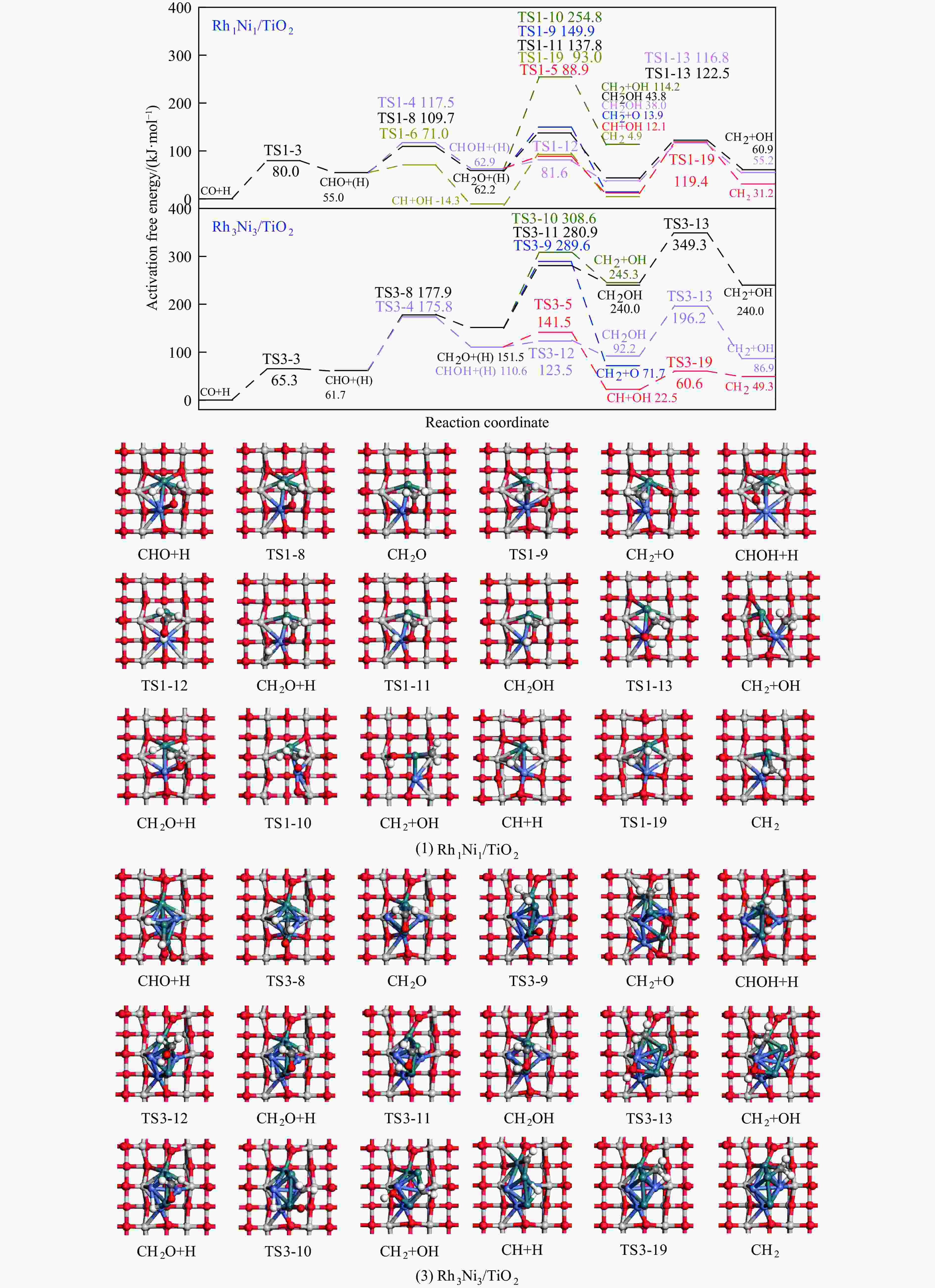
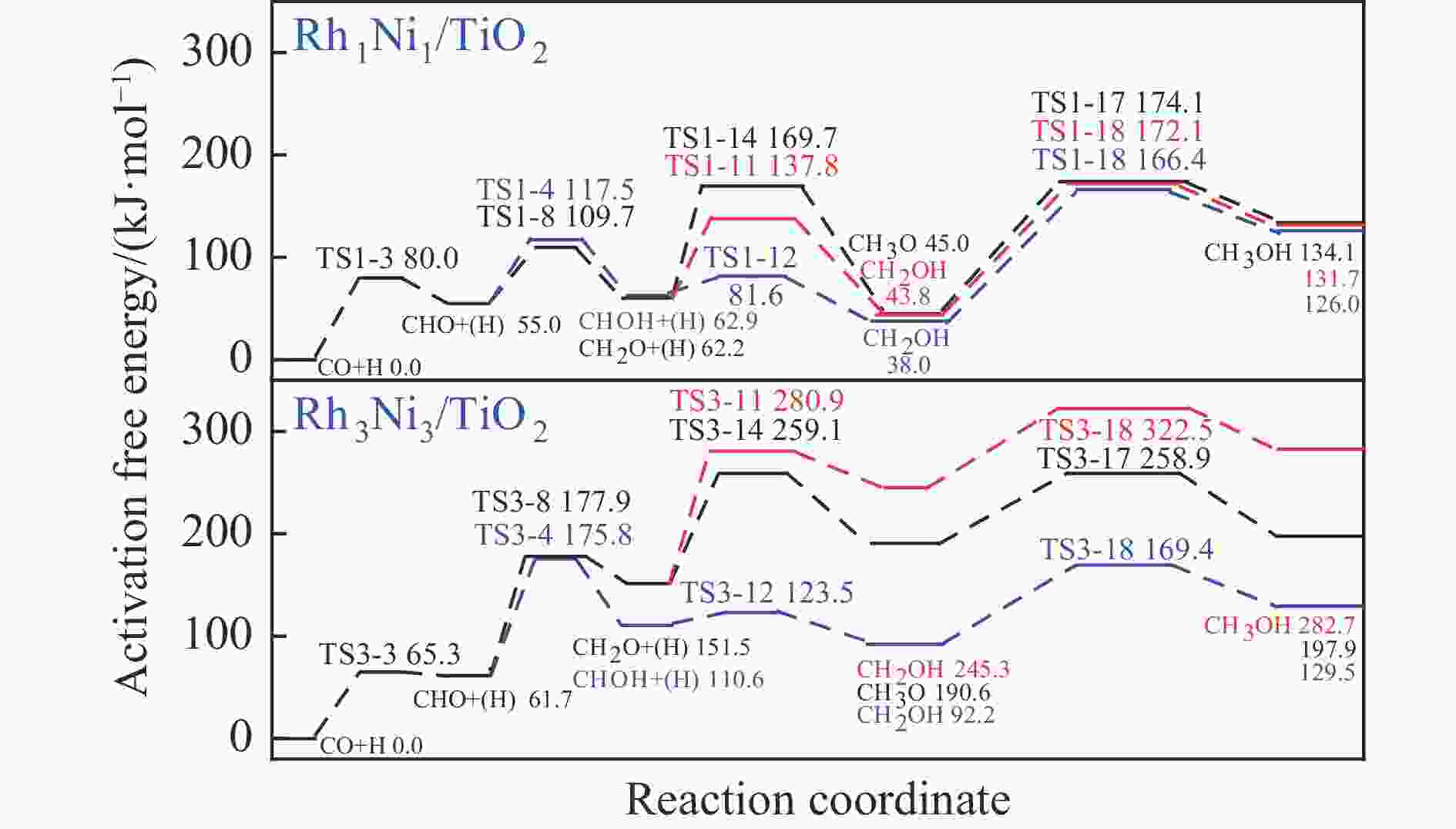
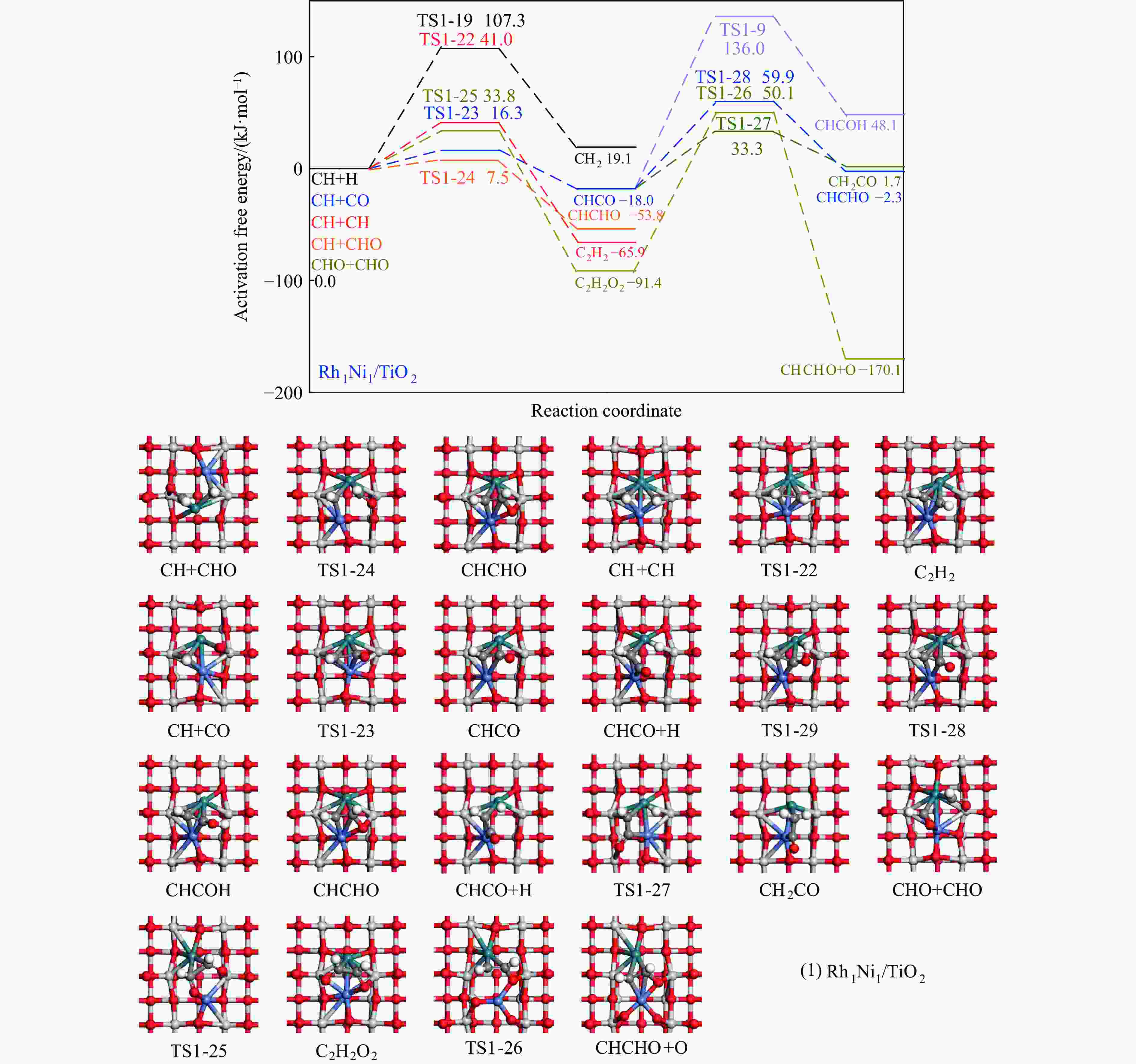
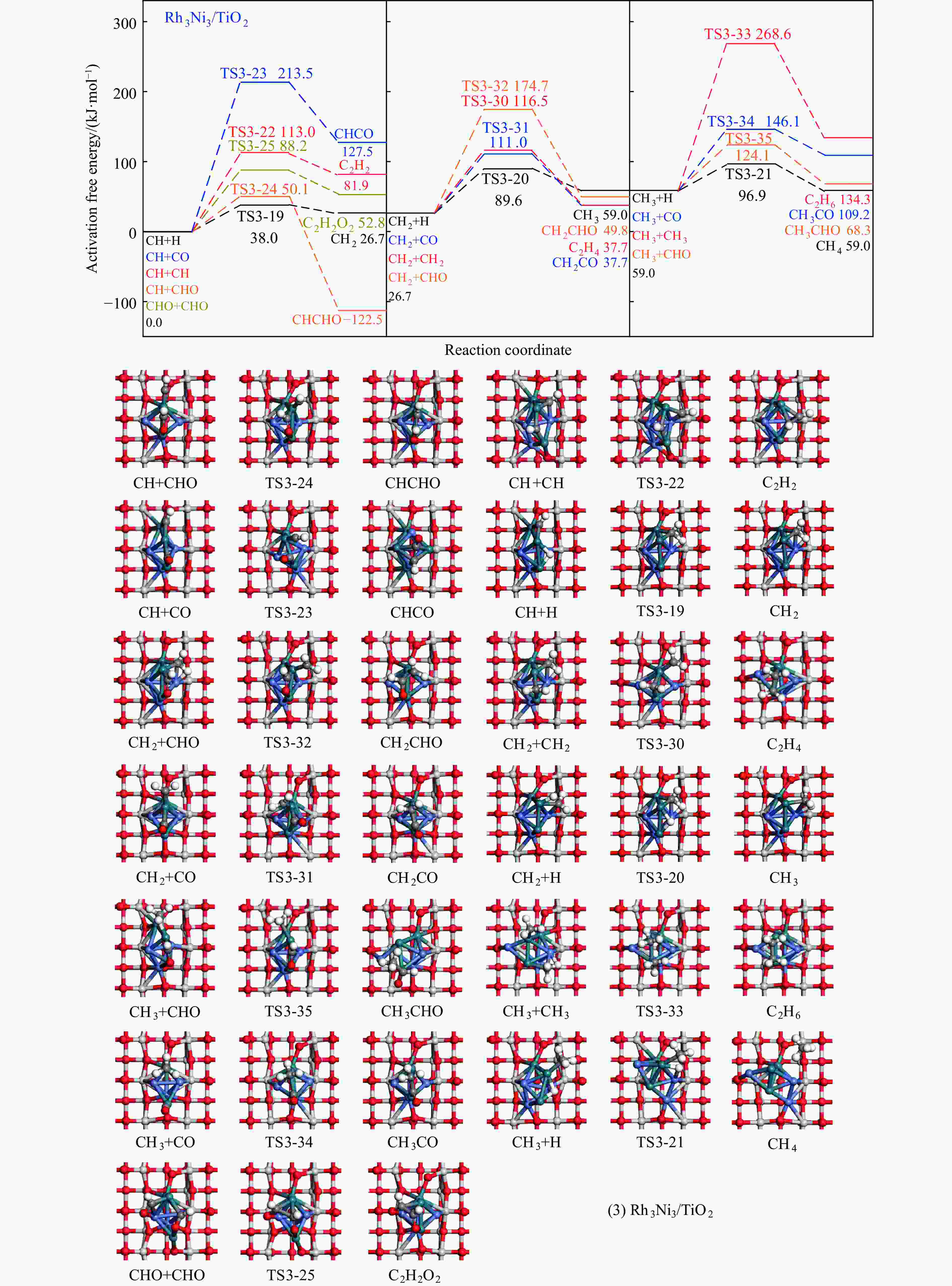
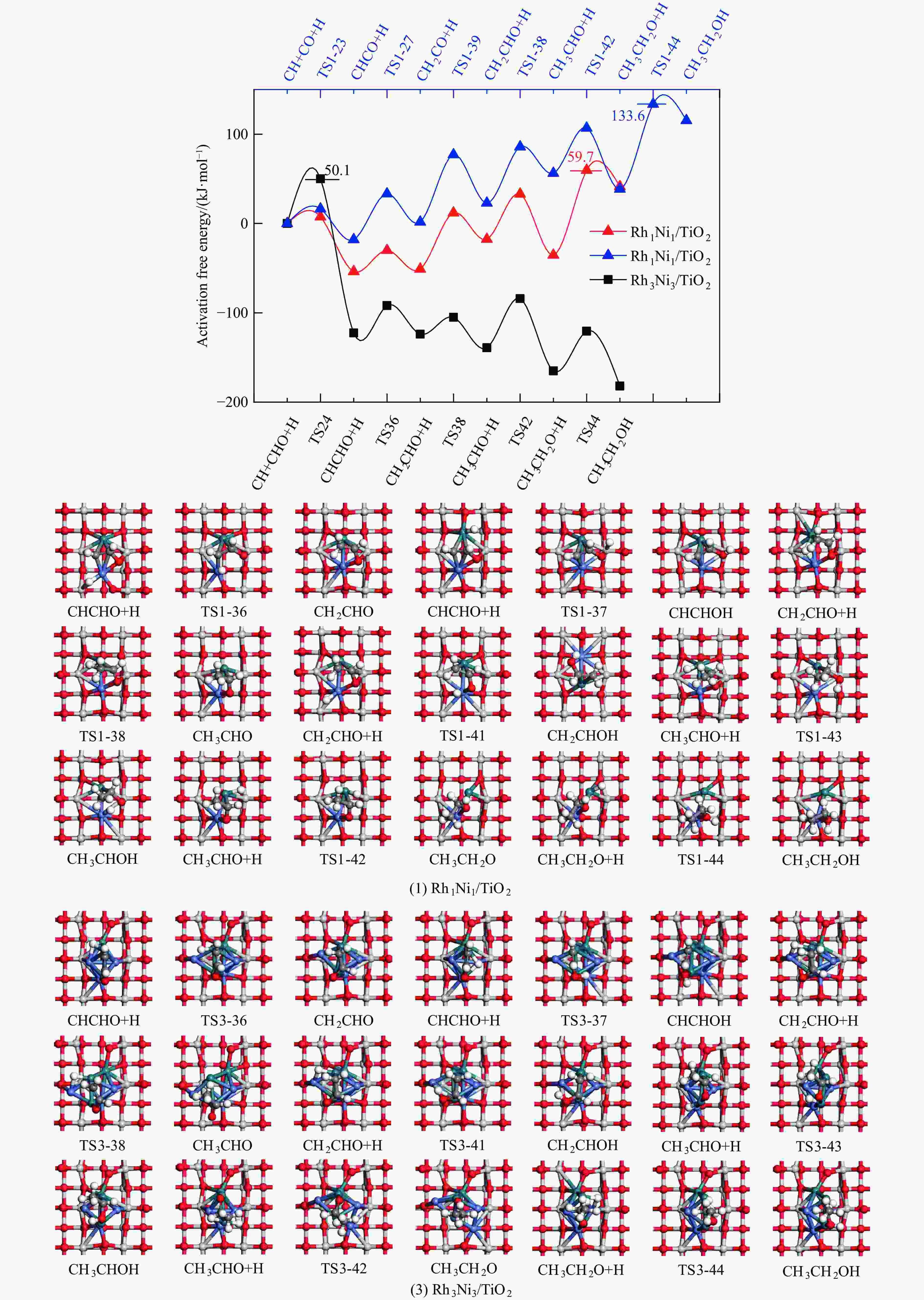
Table 4 Activation free energies (Ga kJ/mol), reaction free energies (ΔG kJ/mol) and the unique imaginary frequency of the elementary reactions involved in the formation of C2H5OH over Rh1Ni1/TiO2 and Rh3Ni3/TiO2 catalysts at 525 K
Elementary step Rh1Ni1/TiO2 Rh3Ni3/TiO2 Ga ΔG f/i Ga ΔG f/i R-1 CO→C+O 267.7 142.5 95.9i 300.0 147.5 299.8i R-2 CO+H→COH 118.8 26.7 1502.2i 139.2 61.9 1468.2i R-3 CO+H→CHO 80.0 55.0 243.6i 65.3 61.7 214.8i R-4 CHO+H→CHOH 62.5 8.0 500.3i 114.1 49.0 861.6i R-5 CHOH→CH+OH 26.0 −50.9 186.9i 30.9 −88.1 312.9i R-6 CHO+H→CH+OH 16.0 −69.3 329.5i 133.8 −39.1 299.0i R-7 CHO→CH+O 21.3 −58.7 370.8i 215.6 124.7 181.4i R-8 CHO+H→CH2O 54.8 7.3 735.7i 116.2 89.8 750.2i R-9 CH2O→CH2+O 87.6 −48.4 191.2i 90.3 −29.7 166.8i R-10 CH2O+H→CH2+OH 192.5 51.9 127.0i 157.1 28.8 391.7i R-11 CH2O+H→CH2OH 75.5 −18.5 952.9i 129.4 93.9 1041.8i R-12 CHOH+H→CH2OH 18.7 −24.9 383.3i 12.9 −18.4 669.0i R-13 CH2OH→CH2+OH 78.7 17.2 254.2i 103.9 −5.3 157.2i R-14 CH2O+H→CH3O 107.5 −17.2 668.0i 107.6 39.1 625.4i R-15 CH3O→CH3+O 79.0 −135.7 552.3i 140.2 −29.2 125.0i R-16 CH3O+H→CH3+OH 162.2 47.0 971.0i 160.9 −144.9 570.4i R-17 CH3O+H→CH3OH 129.1 89.1 901.1i 68.3 7.3 1038.9i R-18 CH2OH+H→CH3OH 128.3 88.0 368.1i 77.1 37.3 710.4i R-19 CH+H→CH2 107.3 19.1 617.7i 38.0 26.7 496.3i R-20 CH2+H→CH3 70.7 14.5 692.0i 62.9 32.3 722.7i R-21 CH3+H→CH4 149.7 71.0 1189.9i 37.9 0.0 781.4i R-22 CH+CH→C2H2 41.0 −65.9 343.9i 113.0 81.9 64.5i R-23 CH+CO→CHCO 16.3 −18.0 119.0i 213.5 127.5 405.5i R-24 CH+CHO→CHCHO 7.5 −53.8 175.6i 50.1 −122.5 219.2i R-25 CHO+CHO→C2H2O2 33.8 −91.4 329.3i 88.2 52.8 477.9i R-26 C2H2O2→CHCHO+O 141.6 −78.7 93.1i 219.2 18.2 421.9i R-27 CHCO+H→CH2CO 51.3 19.7 672.7i 63.5 −40.9 830.6i R-28 CHCO+H→CHCHO 77.9 15.7 168.8i 129.6 −22.7 214.2i R-29 CHCO+H→CHCOH 154.0 66.1 1382.0i 132.3 −14.8 566.4i R-30 CH2+CH2→C2H4 — — — 89.8 −67.7 70.9 i R-31 CH2+CO→CH2CO — — — 84.3 11.0 302.7i R-32 CH2+CHO→CH2CHO — — — 148.0 23.1 168.9i R-33 CH3+CH3→C2H6 — — — 209.6 75.3 542.8i R-34 CH3+CO→CH3CO — — — 87.0 50.1 497.2i R-35 CH3+CHO→CH3CHO — — — 65.1 9.3 138.3i R-36 CHCHO+H→CH2CHO 23.8 2.7 417.2i 30.6 −1.4 647.5i R-37 CHCHO+H→CHCHOH 257.9 48.0 895.6i 86.9 29.9 1107.7i R-38 CH2CHO+H→CH3CHO 63.0 33.5 337.4i 18.8 −15.2 269.3i R-39 CH2CO+H→CH2CHO 75.4 21.2 311.6i — — — R-40 CH2CO+H→CH2COH 143.9 57.8 971.5i — — — R-41 CH2CHO+H→CH2CHOH 107.7 3.0 786.4i 104.8 60.9 1069.5i R-42 CH3CHO+H→CH3CH2O 50.6 −17.7 395.1i 54.9 −25.9 489.0i R-43 CH3CHO+H→CH3CHOH 164.3 108.5 516.3i 132.0 106.3 1089.7i R-44 CH3CH2O+H→C2H5OH 95.0 76.7 424.2i 44.4 −17.0 1147.5i 表 5 525 K下Rh1Ni1/TiO2和Rh3Ni3/TiO2催化剂上乙醇生成有利路径中涉及中间体覆盖度及产物形成速率
Table 5 Intermediate coverage and product formation rates involved in favorable pathways for the formation of ethanol on Rh1Ni1/TiO2 and Rh3Ni3/TiO2 catalysts at 525 K
Parameter Rh1Ni1/TiO2 Rh3Ni3/TiO2 Coverage θCO 5.44×10−2 1.39×10−5 θH 2.23×10−5 1.29×10−8 θCHO 1.19×10−8 1.00 θCH 3.15×10−6 1.53×10−21 θCHOH 5.18×10−13 8.46×10−11 $\theta_{{\mathrm{CH_2 OH}}} $ 4.24×10−2 2.09×10−4 θCHCHO 6.94×10−8 1.38×10−15 $\theta_{{\mathrm{CH_2CHO}}} $ 5.54×10−4 9.27×10−17 $\theta_{{\mathrm{CH_3CHO}}} $ 3.27×10−5 3.63×10−13 $\theta_{{\mathrm{CH_3CH_2O}}} $ 8.51×10−1 3.26×10−14 $\theta_{{\mathrm{CH_2}}} $ 7.21×10−10 4.55×10−19 $\theta_{{\mathrm{CH_3}}} $ 5.13×10−2 1.48×10−21 θ* 4.73×10−10 2.22×10−13 Formation rate/s−1 $r_{{\mathrm{CH_3OH}}} $ 1.76×10−6 6.21×10−7 $r_{{\mathrm{CH_4}}} $ 1.62×10−8 3.53×10−20 $r_{{\mathrm{C_2H_2}}} $ 9.11×10−3 1.47×10−40 $r_{{\mathrm{C_2H_5OH}}} $ 7.35×10−2 1.75×10−13 Relative selectivity/% $s_{{\mathrm{CH_3OH}}} $ 0.00% 100.00% $s_{{\mathrm{CH_4}}} $ 0.00% 0.00% $s_{{\mathrm{C_2H_2}}} $ 11.03% 0.00% $s_{{\mathrm{C_2H_5OH}}} $ 88.97% 0.00% 表 6 RhnNin/TiO2(n = 1、2、3、4)上Ni、Ti及O原子平均失去电荷量及Rh原子平均得到电荷量
Table 6 The amount of average charge loss of Ni, Ti and O atoms and average amount in charge gain of Rh atoms over RhnNin/TiO2(n = 1, 2, 3, 4)
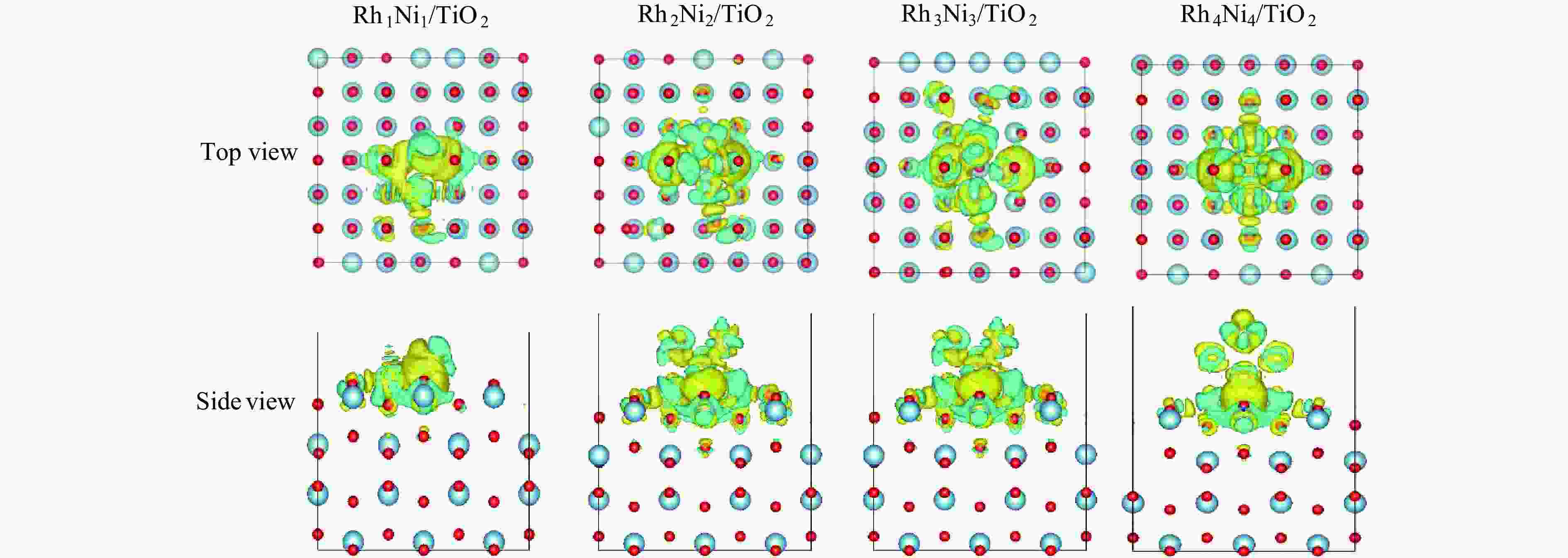
Catalyst △qRh/e △qNi/e △qTi/e △qO/e Rh1Ni1/TiO2 0.627 −0.389 −2.268 −0.801 Rh2Ni2/TiO2 0.198 −0.232 −2.188 −0.805 Rh3Ni3/TiO2 0.064 −0.132 −2.169 −0.805 Rh4Ni4/TiO2 0.088 −0.039 −2.224 −0.795 -
[1] 王占慧, 凌丽霞, 王俊刚, 等. 合成气制乙醇RhCu双金属催化剂活性位点的作用机制研究[J]. 燃料化学学报(中英文),2021,49(3):358−365.WANG Z H, LING L X, WANG J G, et al. Role of study on the effect of active sites of ethanol synthesis from syngas over RhCu bimetallic catalyst[J]. J Fuel Chem Technol,2021,49(3):358−365. [2] 陈维苗, 丁云杰, 薛飞, 等. CO加氢制C2含氧化合物 Rh基催化剂中常见助剂的作用[J]. 物理化学学报,2015,31(1):1−10. doi: 10.3866/PKU.WHXB201411054CHEN W M, DING Y J, XUE F, et al. Role of common promoters in Rh-based catalysts for CO hydrogenation to C2-oxygenates[J]. Acta Phys-Chim Sin,2015,31(1):1−10. doi: 10.3866/PKU.WHXB201411054 [3] BURCH R, PETCH M I. Investigation of the synthesis of oxygenates from carbon monoxide/hydrogen mixtures on supported rhodium catalysts[J]. Appl Catal A: Gen,1992,88(1):39−60. doi: 10.1016/0926-860X(92)80195-I [4] YIN H M, DING Y J, LUO H Y, et al. Influence of iron promoter on catalytic properties of Rh-Mn-Li/SiO2 for CO hydrogenation[J]. Appl Catal A: Gen,2003,243(1):155−164. doi: 10.1016/S0926-860X(02)00560-4 [5] UNDERWOOD R P, BELL A T. Lanthana-promoted Rh/SiO2: ІІ. Studies of CO hydrogenation[J]. J Catal,1988,111(2):325−335. doi: 10.1016/0021-9517(88)90091-7 [6] WILSON T P, KASAI P H, ELLGEN P C. The state of manganese promoter in rhodium-silica gel catalysts[J]. J Catal,1981,69(1):193−201. doi: 10.1016/0021-9517(81)90141-X [7] KATZER J R, SLEIGHT A W, GAJARDO P, et al. The role of the support in CO hydrogenation selectivity of supported rhodium[J]. Faraday Discuss Chem Soc,1981,72:121−133. doi: 10.1039/dc9817200121 [8] 陈维苗, 丁云杰, 薛飞, 等. 合成气制C2含氧化合物Rh基催化剂中的载体效应[J]. 化工进展,2014,33(7):1753−1762. doi: 10.3969/j.issn.1000-6613.2014.07.017CHEN W M, DING Y J, XUE F, et al. Support effect of Rh-based catalyst for CO hydrogenation to C2-oxygenates[J]. Chem Ind Eng Prog,2014,33(7):1753−1762. doi: 10.3969/j.issn.1000-6613.2014.07.017 [9] KANG L, ZHANG Y, MA L X, et al. The roles of Rh crystal phase and facet in syngas conversion to ethanol[J]. Chem Eng Sci,2022,248:117186. doi: 10.1016/j.ces.2021.117186 [10] ZHANG R G, PENG M, WANG B J. Catalytic selectivity of Rh/TiO2 catalyst in syngas conversion to ethanol: probing into the mechanism and functions of TiO2 support and promoter[J]. Catal Sci Technol,2017,7:1073−1085. doi: 10.1039/C6CY02350A [11] HAIDER M A, GOGATE M R, DAVIS R J. Fe-promotion of supported Rh catalysts for direct conversion of syngas to ethanol[J]. J Catal,2009,261(1):9−16. doi: 10.1016/j.jcat.2008.10.013 [12] ZHONG H X, WANG J M, GUO S X, et al. Mutual tailored bimetallic Rh–Co supported on La modified SiO2 for direct ethanol synthesis from syngas[J]. Ind Eng Chem Res,2019,58(8):2631−2643. doi: 10.1021/acs.iecr.8b05474 [13] OJEDA M, GRANADOS M L, ROJAS S, et al. Manganese-promoted Rh/Al2O3 for C2-oxygenates synthesis from syngas effect of manganese loading[J]. Appl Catal A: Gen,2004,261(1):47−55. doi: 10.1016/j.apcata.2003.10.033 [14] KRISHNAMURTHY R, CHUANG S S C, GHOSAL K. Carbon monoxide adsorption and hydrogenation on Cu-Rh/SiO2 catalysts[J]. Appl Catal A-Gen,1994,114(1):109−125. doi: 10.1016/0926-860X(94)85111-5 [15] LI F, MA H F, ZHANG H T, et al. Ethanol synthesis from syngas on Mn- and Fe-promoted Rh/g-Al2O3[J]. C R Chimie,2014,17:1109−1115. doi: 10.1016/j.crci.2014.01.015 [16] LUK H T, MONDELLI C, FERRE D C, et al. Status and prospects in higher alcohols synthesis from syngas[J]. Chem Soc Rev,2017,46:1358−1426. doi: 10.1039/C6CS00324A [17] HU J, WANG Y, CAO C, et al. Conversion of biomass-derived syngas to alcohols and C2 oxygenates using supported Rh catalysts in a microchannel reactor[J]. Catal Today,2007,120:90−95. doi: 10.1016/j.cattod.2006.07.006 [18] YU J, MAO D, HAN L, et al. CO hydrogenation over Fe-promoted Rh–Mn–Li/SiO2 catalyst: The effect of sequences for introducing the Fe promoter[J]. Fuel Process Technol,2013,112:100−105. doi: 10.1016/j.fuproc.2013.03.004 [19] YU J, MAO D, HAN L, et al. The effect of Fe on the catalytic performance of Rh–Mn–Li/SiO2 catalyst: A DRIFTS study[J]. Catal Commun,2012,27:1−4. doi: 10.1016/j.catcom.2012.06.010 [20] XIANG M L, LI D B, LI W H, et al. Potassium and nickel doped β-Mo2C catalysts for mixed alcohols synthesis via syngas[J]. Catal Commun,2007,8(3):513−518. doi: 10.1016/j.catcom.2006.07.028 [21] ZHAO L H, FANG K G, JIANG D, et al. Sol-gel derived Ni-Mo bimetallic carbide catalysts and their performance for CO hydrogenation[J]. Catal Today,2010,158(3):490−495. [22] FANG K G, LI D B, LIN M G, et al. A short review of heterogeneous catalytic process for mixed alcohols synthesis via syngas[J]. Catal Today,2009,147(2):133−138. doi: 10.1016/j.cattod.2009.01.038 [23] MOZAMMEL T, DUMBRE D, HUBESCH R, et al. Carbon dioxide reforming of methane over mesoporous alumina supported Ni(Co), Ni(Rh) bimetallic, and Ni(CoRh) trimetallic catalysts: Role of nanoalloying in improving the stability and nature of coking[J]. Energy Fuels,2020,34(12):16433−16444. doi: 10.1021/acs.energyfuels.0c03249 [24] BIZKARRA K, BERMUDEZ J M, ARCELUS-ARRILLAGA P, et al. Nickel based monometallic and bimetallic catalysts for synthetic and real bio-oil steam reforming[J]. Int J Hydrogen Energ,2018,43(26):11706−11718. doi: 10.1016/j.ijhydene.2018.03.049 [25] GUO F, RAN J Y, NIU J T, et al. Comparative density functional theory study of carbon formation and removal mechanism on Rh modified Ni-based catalyst in the CH4/CO2 reforming[J]. Int J Energy Res,2021,45(7):10100−10111. doi: 10.1002/er.6501 [26] MAO Y R, ZHANG L Z, ZHENG X J, et al. Coke-resistance over Rh–Ni bimetallic catalyst for low temperature dry reforming of methane[J]. Int J Hydrogen Energ,2023,48(37):13890−13901. doi: 10.1016/j.ijhydene.2022.12.299 [27] TANG L, HUANG X, RAN J Y, et al. Density functional theory studies on direct and oxygen assisted activation of C–H bond for dry reforming of methane over Rh–Ni catalyst[J]. Int J Hydrogen Energ,2022,47(91):30391−30403. [28] NING X, WANG H M, ZHOU G H, et al. Selective catalytic hydrodechlorination of 1, 2-Dichloroethane to ethylene over Ni−Rh nanoparticle catalysts supported on γ−Al2O3[J]. ACS Appl Nano Mater,2023,6:390−397. doi: 10.1021/acsanm.2c04520 [29] GHOSH T, LIU X W, SUN W M, et al. Revealing the origin of low-temperature activity of Ni–Rh nanostructures during CO oxidation reaction with operando TEM[J]. Adv Sci,2022,9:2105599. doi: 10.1002/advs.202105599 [30] XUE Q Q, YI H L, LI Z W, et al. Experimental and DFT studies on diesel-steam-reforming to hydrogen over a bimetallic Rh-Ni-based MgO-Al2O3 microsphere catalyst[J]. Fuel,2022,318:123632. doi: 10.1016/j.fuel.2022.123632 [31] JEFFREY C S, WU H C. Bimetallic Rh–Ni/BN catalyst for methane reforming with CO2[J]. Chem Eng J,2009,148(2-3):539−545. doi: 10.1016/j.cej.2009.01.011 [32] ARANDIYAN H, WANG Y, SCOTT J, et al. In situ exsolution of bimetallic Rh–Ni nanoalloys: a highly efficient catalyst for CO2 methanation[J]. ACS Appl Mater Interfaces,2018,10(19):16352−16357. doi: 10.1021/acsami.8b00889 [33] JUNG Y G, LEE D H, KIM Y M, et al. Rh-Ni and Rh-Co Catalysts for autothermal reforming of gasoline[J]. Bull Korean Chem Soc,2014,35(1):231−235. doi: 10.5012/bkcs.2014.35.1.231 [34] LIU H L, ZHOU C H, Li W Q, et al. Rh bimetallic catalysts with high catalytic activity for the hydrogenation of N-Ethylcarbazole[J]. ACS Sustainable Chem Eng,2021,9(15):5260−5267. doi: 10.1021/acssuschemeng.0c08270 [35] VALERO M C, RAYNAUD P, SSUTET P. Interplay between molecular adsorption and metal-support interaction for small supported metal clusters: CO and C2H4 adsorption on Pd4/γ-Al2O3[J]. J Catal,2007,247(2):339−355. doi: 10.1016/j.jcat.2007.02.014 [36] SHETTY S, VAN SANTENA R A, STEVENS P A, et al. Molecular steps for the syngas conversion on the Rh6 cluster[J]. J Mol Catal A-Chem,2010,330(1):73−87. [37] ZHANG R G, PENG M, DUAN T, et al. Insight into size dependence of C2 oxygenate synthesis from syngas on Cu cluster: The effect of cluster size on the selectivity[J]. Appl Surf Sci,2017,407(15):282−296. [38] DONG C Y, LI Y L, CHENG D Y, et al. Supported metal clusters: fabrication and application in heterogeneous catalysis[J]. ACS Catal,2020,10(15):11011−11045. [39] ZHANG R G, DUAN T, WANG B J, et al. Unraveling the role of support surface hydroxyls and its effect on the selectivity of C2 species over Rh/γ-Al2O3 catalyst in syngas conversion: A theoretical study[J]. Appl Surf Sci,2016,379(30):384−394. [40] LING L X, CAO Y T, HAN M, et al. Catalytic performance of Pd n (n = 1, 2, 3, 4 and 6) clusters supported on TiO2-V for the formation of dimethyl oxalate via the CO catalytic coupling reaction: A theoretical study[J]. Phys Chem Chem Phys,2020,22(8):4549−4560. doi: 10.1039/C9CP06773F [41] SHAO X X, GUO X Y, SHI X F, et al. C2H2 semi-hydrogenation over the TiO2 supported Pd n and Pd nCO cluster catalysts: Influences of cluster size and CO on the catalytic performance[J]. Fuel,2024,358:130053. doi: 10.1016/j.fuel.2023.130053 [42] SONDON T, GUEVARA J. Magnetic properties of Ni–Rh clusters: Behavior in the Ni-rich region[J]. Physica B,2004,354(4):303−306. [43] SPIVEY J J, EGBEBI A, Heterogeneous catalytic synthesis of ethanol from biomass-derived syngas[J]. Chem Soc Rev, 2007, 36, 1514–1528. [44] WANG J J, ZHANG Q H, WANG Y, et al. Rh-catalyzed syngas conversion to ethanol: Studies on the promoting effect of FeOx[J], Catal Today, 2011, 171, 257–265. [45] MA J L, YIN L F, LING L X, et al. The formation of high energy density fuel via the hydrogenation of naphthalene over Ni catalyst: The combined DFT and microkinetic analysis[J]. Fuel,2023,333:126307. doi: 10.1016/j.fuel.2022.126307 [46] WU Y Y, GUO X Y, SHI X F, et al. C2H2 Semi-hydrogenation over S-modified PdM IMCs: Tuning catalytic performance by surface S Atom, and metal M type and ratio[J]. Appl Surf Sci,2023,637:157906. doi: 10.1016/j.apsusc.2023.157906 [47] WANG V, XU N, LIU J C, et al. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code[J]. Comput Phys Commun,2021,267:108033−108051. doi: 10.1016/j.cpc.2021.108033 [48] MARTYNA G J, KLEIN M L, TUCKEMAN M. Nose–Hoover chains: the canonical ensemble via continuous dynamics[J]. J Chem Phys,1992,97(4):2635−2643. doi: 10.1063/1.463940 [49] 董超, 段海明. Rh nCr(n = 1-7)团簇结构和电子性质的密度泛函理论研究[J]. 新疆大学学报(自然科学版),2011,28(1):65−70.DONG C, DUAN H M. Structural and electronic properties of Rh nCr(n = 1-7) clusters studied by density–functional theory[J]. Xinjiang Univ, Nat Sci Ed Chin Engl,2011,28(1):65−70. [50] SUN C, SMITH S C. Strong interaction between gold and anatase TiO2(001) predicted by first principle studies[J]. J Phys Chem C,2012,116(5):3524−3531. doi: 10.1021/jp208948x [51] ZHANG Y S, LIU J X, QIAN K, et al. Structure sensitivity of Au-TiO2 strong metal–support interactions[J]. Angew Chem Int Ed,2021,133:12181−12188. doi: 10.1002/ange.202101928 [52] SAQIAIN M A, HUSSAIN A, SIDDIQ M, et al. Thermally activated surface oxygen defects at the perimeter of Au/TiO2: a DFT + U study[J]. Phys Chem Chem Phys,2015,17(38):25403−25410. doi: 10.1039/C5CP04113A [53] FINAZZI E, VALENTIN C D, PACCHIONI G, et al. Excess electron states in reduced bulk anatase TiO2: comparison of standard GGA, GGA + UGGA + U, and hybrid DFT calculations[J]. J Chem Phys,2008,129:154113. doi: 10.1063/1.2996362 [54] MORGAN B J, WATSON G W. A DFT + U description of oxygen vacancies at the TiO2 rutile (110) surface[J]. Surf Sci,2007,601:5034−5041. doi: 10.1016/j.susc.2007.08.025 [55] HUYGH S and NEYTS E C. Adsorption of C and CH x radicals on anatase (001) and the influence of oxygen vacancies[J]. J Phys Chem C,2015,119(9):4908−4921. doi: 10.1021/jp5127249 [56] LINH N H, NGUYEN T Q, DINO W A, et al. Effect of oxygen vacancy on the adsorption of O2 on anatase TiO2(001): A DFT-based study[J]. Surf Sci,2015,633:38−45. doi: 10.1016/j.susc.2014.11.015 [57] CHENG H Z and SELLONI A. Surface and subsurface oxygen vacancies in anatase TiO2 and differences with rutile[J]. Phys Rev B, 009, 79: 092101. [58] WANG B J, GUO W S, ZHANG R G, et al. C2 Oxygenates formation from syngas over the Cu-rich and Rh-rich surfaces of Rh-Cu bimetallic catalysts: probing into the effects of the surface structure and composition on the catalytic performance[J]. J Phys Chem C,2019,123:19528−19539. doi: 10.1021/acs.jpcc.9b03731 [59] CHOI Y M, LIU P. Mechanism of ethanol synthesis from syngas on Rh(111)[J]. J Am Chem Soc,2009,131:13054−13061. doi: 10.1021/ja903013x [60] ZHANG Y R, SU X, LI L, et al. Ru/TiO2 catalysts with size-dependent metal/support interaction for tunable reactivity in Fischer–Tropsch synthesis[J]. ACS Catal,2020,10(21):12967−12975. doi: 10.1021/acscatal.0c02780 [61] MENG H, YANG Y S, SHEN T Y, et al. A strong bimetal-support interaction in ethanol steam reforming[J]. Nat Commun,2023,14:3189. doi: 10.1038/s41467-023-38883-x [62] 侯志全, 郭萌, 刘雨溪, 等. 金属间化合物的合成及其催化应用[J]. 材料研究学报,2020,34(2):1−10. doi: 10.11901/1005.3093.2019.334HOU Z Q, GUO M, LIU Y X, et al. Synthesis of intermetallic compounds and theircatalytic applications[J]. Chin J Mater Res,2020,34(2):1−10. doi: 10.11901/1005.3093.2019.334 [63] HAIDER M A, GOGATE M R, DAVIS R J. Fe-promotion of supported Rh catalysts for direct conversion of syngas to ethanol[J]. J Catal,2009,261(1):9−16. doi: 10.1016/j.jcat.2008.10.013 [64] CHEN W M, DING Y J, SONG X G, et al. Promotion effect of support calcination on ethanol production from CO[J]. Appl Catal A-Gen,2011,407(1-2):231−237. doi: 10.1016/j.apcata.2011.08.044 -




 下载:
下载: