Cr-MIL-101 derived nano-Cr2O3 for highly efficient dehydrogenation of n-hexane
-
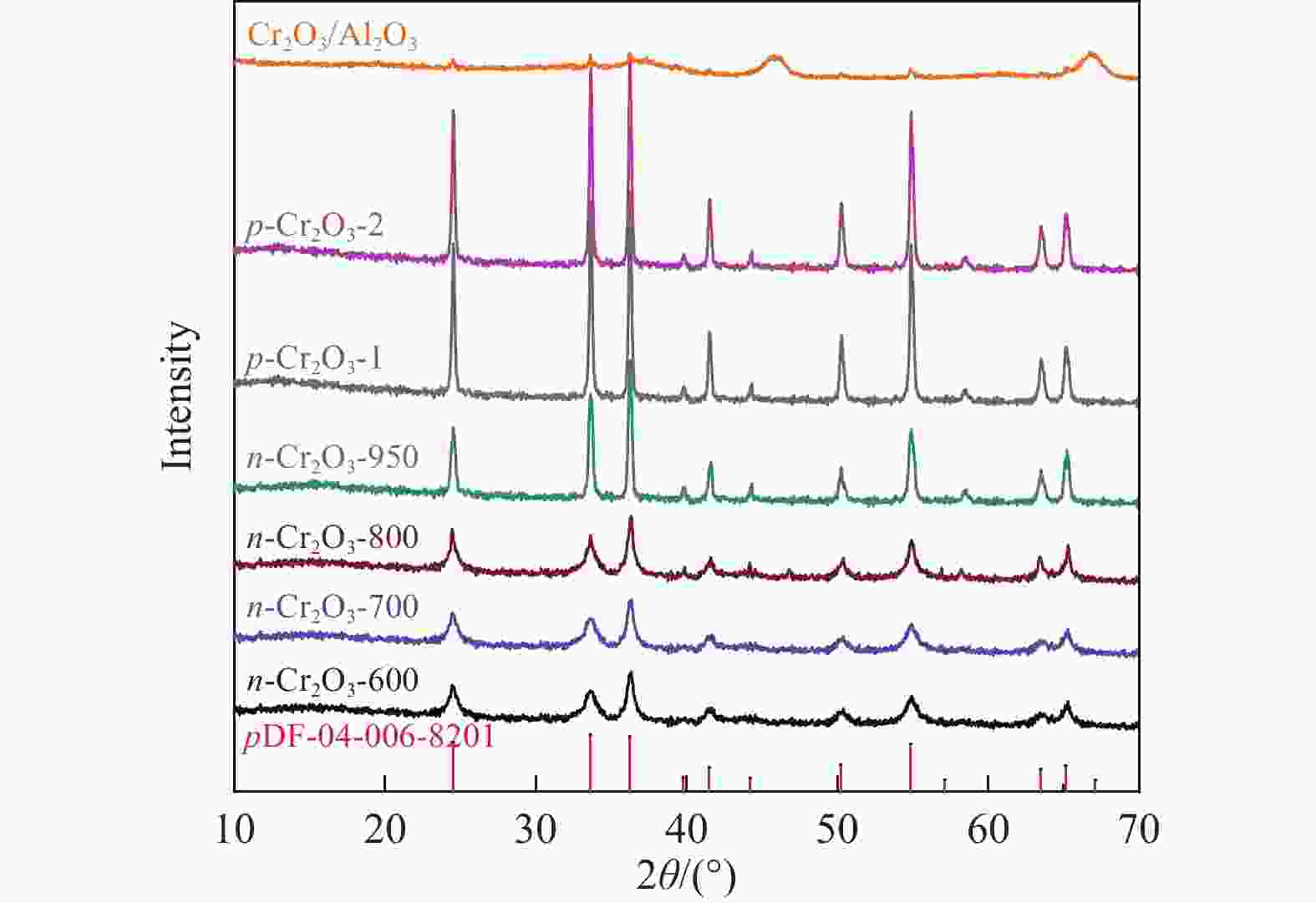
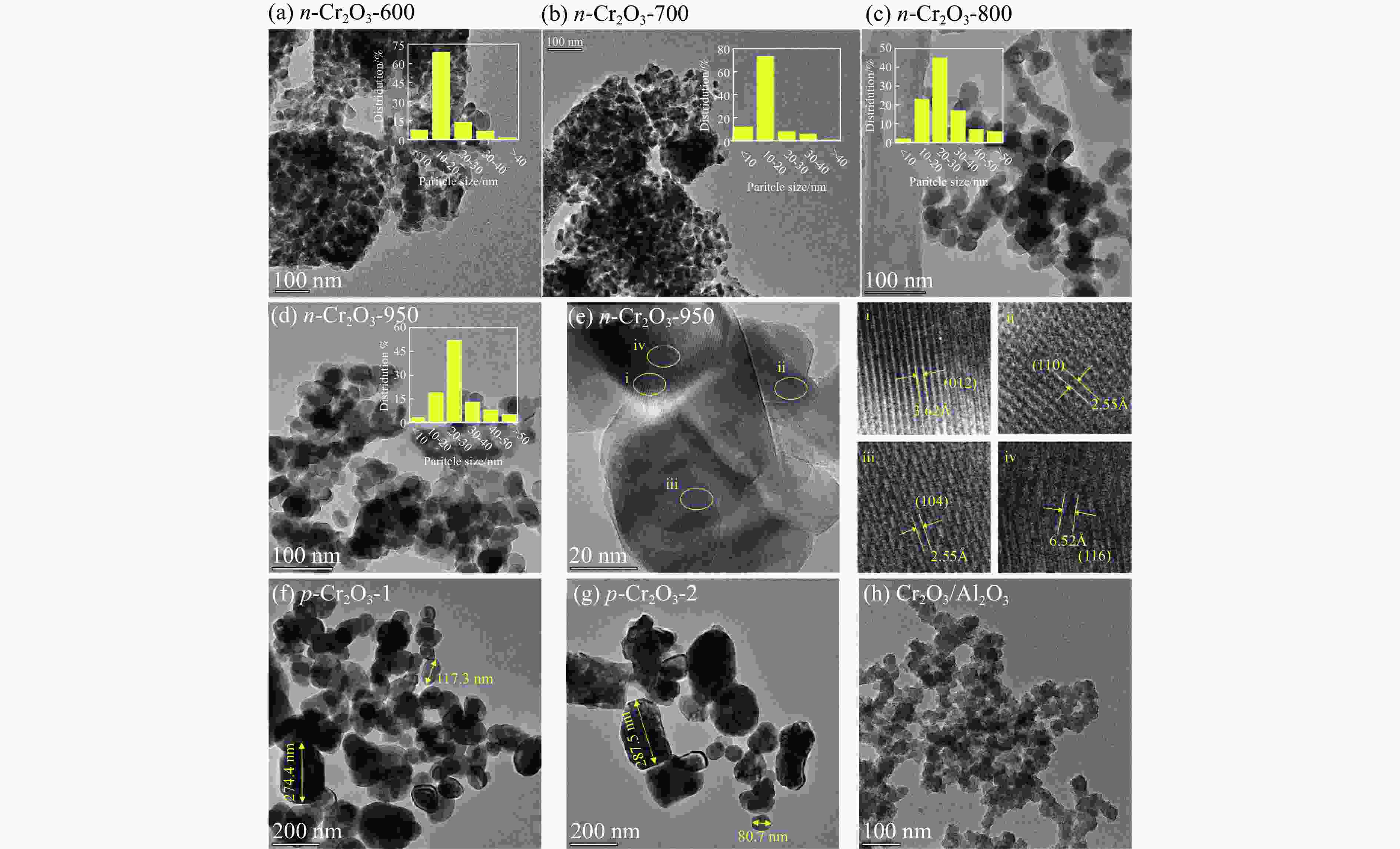
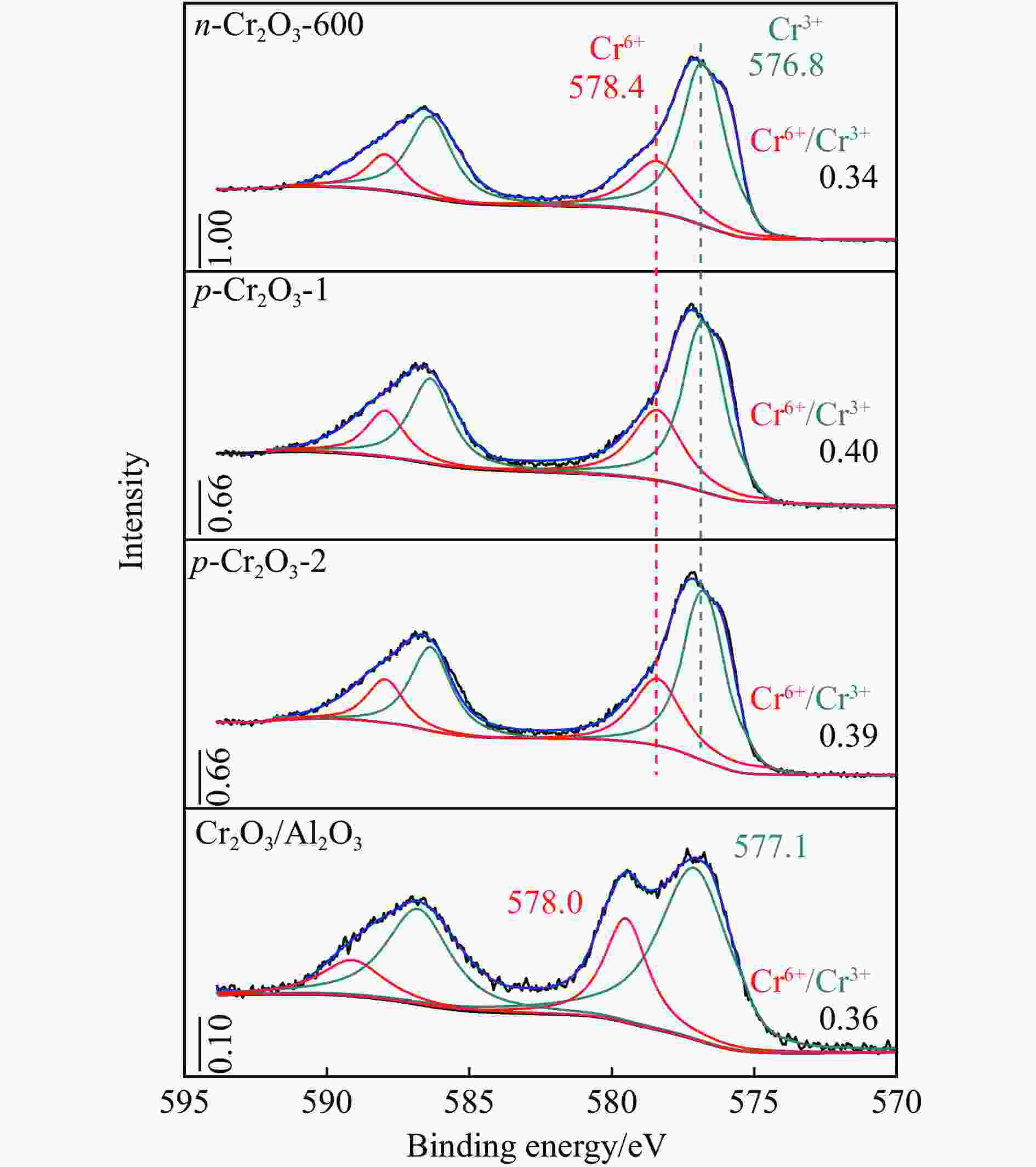
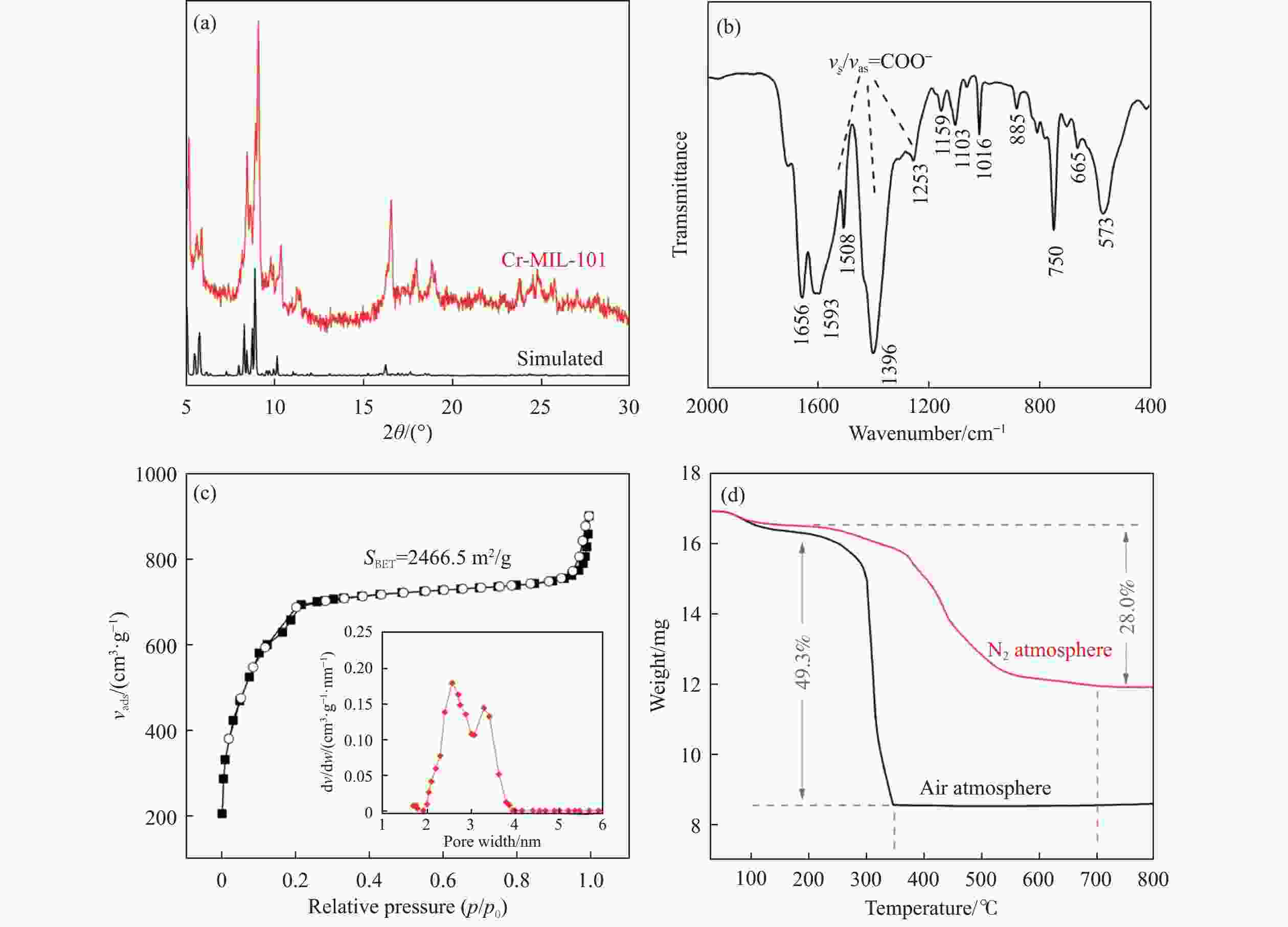
摘要: 通过热解大比表面Cr-MIL-101制备纳米Cr2O3(n-Cr2O3),考察其催化正己烷脱氢反应性能,并比较与沉淀法p-Cr2O3-1、焙烧铬盐得到的p-Cr2O3-2以及工业Cr2O3/Al2O3催化正己烷脱氢活性差异。n-Cr2O3能够催化正己烷高效脱氢为己烯和苯,并且其催化脱氢活性与焙烧温度有关。600 ℃焙烧的n-Cr2O3催化正己烷脱氢转化率最高40.6%,对产物己烯和苯的选择性分别为20.1%和69.3%。提高焙烧温度,n-Cr2O3催化正己烷脱氢活性下降但稳定性增强,催化剂积炭量减少。p-Cr2O3-1和p-Cr2O3-2催化正己烷脱氢转化率很低(<7.5%),比活性分别为1.5和1.7 g/(m2·h),低于n-Cr2O3-600的(2.0 g/(m2·h))。通过BET、XRD、TEM和FT-IR等表征发现,n-Cr2O3为具有较大比表面的纳米颗粒(10−20 nm),多暴露晶面和脱氢活性位,而p-Cr2O3是比表面非常小的大颗粒,所暴露脱氢活性位少。相比之下,Cr2O3/Al2O3催化剂由于大比表面Al2O3的分散作用,催化正己烷脱氢效率更高(2.4 g/(m2·h))。因此,由Cr-MIL-101焙烧得到的n-Cr2O3催化正己烷脱氢的高活性源于这种纳米Cr2O3所具有的独特性质:小颗粒,大比表面,多暴露活性位。Abstract: Nano-Cr2O3 (n-Cr2O3) was prepared by calcining the mesoporous Cr-MIL-101, and the catalytic performance for n-hexane dehydrogenation was investigated. It was found that dehydrogenation of n-hexane on n-Cr2O3 can produce n-hexenes and benzene efficiently, and the catalytic performance is related to the calcination temperature. The optimal n-hexane conversion can be obtained on n-Cr2O3-600, is 40.6%, and the selectivities to n-hexenes and benzene are 20.1% and 69.3%, respectively. Increasing the calcination temperature, the conversion of n-hexane is decreased while the stability is enhanced. The n-hexane conversion of p-Cr2O3-1 (obtained by precipitation method) and p-Cr2O3-2 (obtained by calcinating Cr(NO3)·9H2O directly) catalysts are relative low (<7.5%), and their specific activity for n-hexane dehydrogenation are 1.5 and 1.7 g/(m2·h), respectively, lower than that of n-Cr2O3-600 (2.0 g/(m2·h)). The results of BET、XRD、TEM and FT-IR reveal that n-Cr2O3 is the nanoparticle with large specific surface area that more crystal planes and dehydrogenation active sites are exposed, while p-Cr2O3 is the large particle with extremely low surface area that the dehydrogenation active sites are less exposed. By contrast, industrial Cr2O3/Al2O3 catalyst possesses high specific activity of 2.4 g/(m2·h) due to the dispersion effect of Al2O3. Therefore, the highly catalytic activity of n-Cr2O3 for n-hexane dehydrogenation is attributed to the unique properties of n-Cr2O3: small particle, large specific surface area and more exposed active sites. This work not only explains the highly dehydrogenation performance of nano-Cr2O3 derived by Cr-MIL-101, but also provides guidance for the precise design and synthesis of high-performance CrOx-based catalyst for the dehydrogenation of alkanes.
-
Key words:
- n-hexane /
- dehydrogenation /
- n-hexenes /
- benzene /
- nano-Cr2O3
-
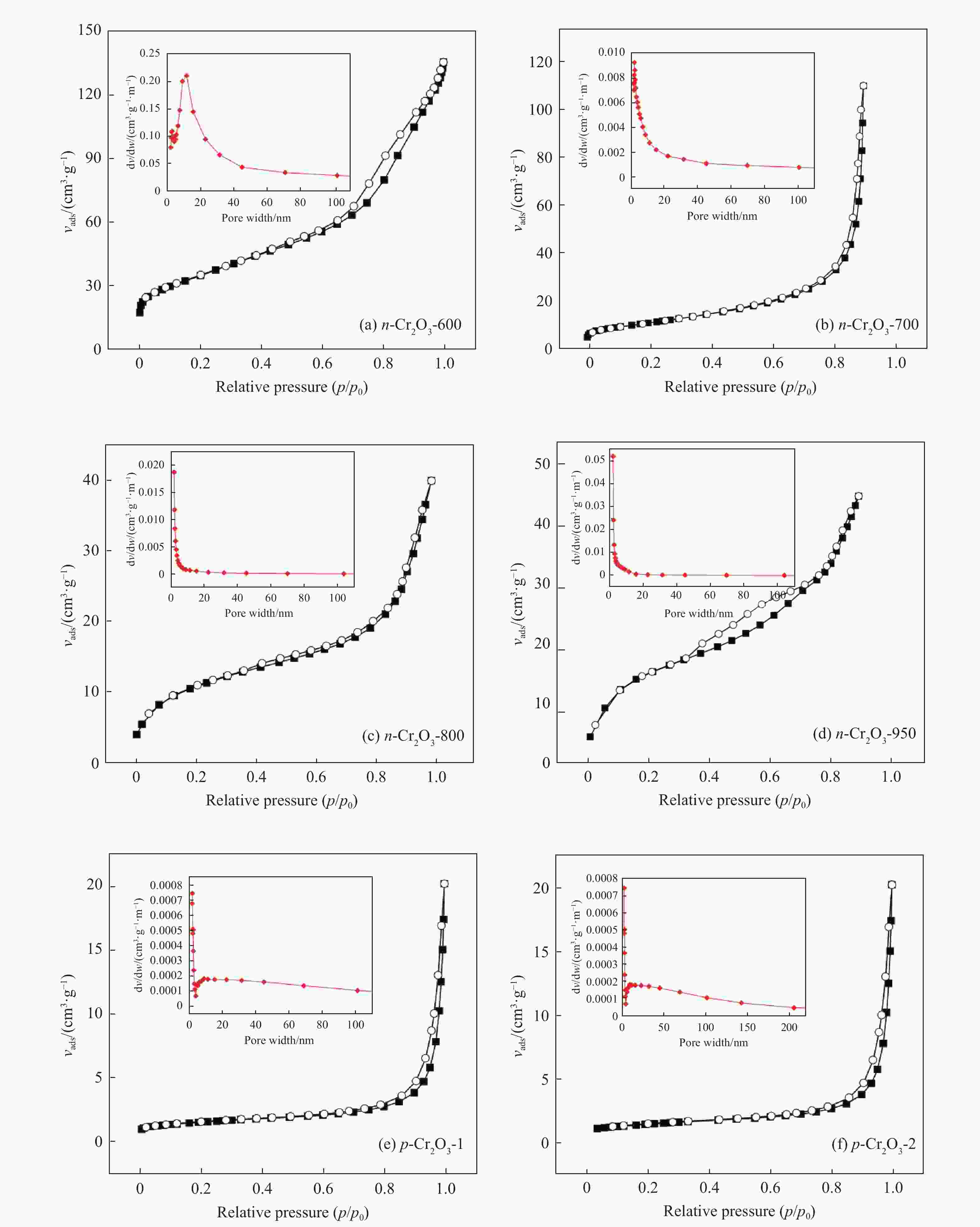
表 1 Cr2O3催化剂的织构性质
Table 1 Textural properties of Cr2O3 catalysts
Sample SBET/(m2·g−1) vpore/(cm3·g−1) Dpore/nm n-Cr2O3-600 62.5 0.28 16.4 n-Cr2O3-700 57.0 0.22 18.3 n-Cr2O3-800 39.1 0.14 17.4 n-Cr2O3-950 13.4 0.09 19.7 p-Cr2O3-1 5.2 0.03 26.1 p-Cr2O3-2 6.1 0.03 33.5 Cr2O3/Al2O3 117.5 0.21 4.9 表 2 常压、不同温度下正己烷脱氢为单己烯热力学平衡数据
Table 2 Thermodynamic equilibrium data for n-hexane dehydrogenation to n-hexenes at different temperatures
t/℃ △G/(kJ·mol−1) Ke Equilibrium conversion/% 400 165.75 1.57×10−2 11.76 450 133.55 6.64×10−2 22.66 500 96.85 0.27 40.36 550 66.65 0.80 57.88 600 30 2.33 75.51 表 3 反应1 h时不同焙烧温度n-Cr2O3催化正己烷脱氢反应
Table 3 Catalytic results of n-Cr2O3 catalyst for n-hexane dehydrogenation at TOS of 1 h
Sample n-Cr2O3-600 n-Cr2O3-700 n-Cr2O3-800 n-Cr2O3-950 Conversion of n-C6H14/% 40.6 37.8 22.8 11.3 Selectivity to product/% n-C6H12 20.1 21.5 25.1 28.2 C6H6 69.3 68.1 64.4 60.6 2,4-C6H10 2.9 3.0 3.1 3.2 Cracking products 3.2 2.9 2.8 2.9 Isomerization products 2.8 2.9 2.9 3.0 Others 1.7 1.6 1.7 2.1 -
[1] 任杰, 张怀科, 李永旺. F-T合成油品加工技术的研究进展[J]. 燃料化学学报,2009,37(6):769−776.REN Jie, ZHANG Huaike, LI Yongwang. Research progress of the processing technology for Fischer-Tropsch syncrude[J]. J Fuel Chem Technol,2009,37(6):769−776. ). [2] 王清俊, 孙来芝, 陈雷, 等. 合成气经费托路线直接制芳烃进展[J]. 燃料化学学报,2023,51(1):52−66.WANG Qingjun, SUN Laizhi, CHEN Lei, et al. Recent advance in directing synthesis of aromatic hydrocarbons from syngas via Fischer-Tropsch route[J]. J Fuel Chem Technol,2023,51(1):52−66. [3] 于飞, 李正甲, 安芸蕾, 等. 合成气催化转化直接制备低碳烯烃研究进展[J]. 燃料化学学报,2016,44(7):801−814.YU Fei, LI Zhengjia, AN Yunlei, et al. Research progress in the direct conversion of syngas to lower olefins[J]. J Fuel Chem Technol,2016,44(7):801−814. [4] 张方方, 张新宽, 于中伟. 提高石脑油综合利用效率的措施及优化方案[J]. 石油炼制与化工,2021,52(5):16−21. doi: 10.3969/j.issn.1005-2399.2021.05.003ZHANG Fangfang, ZHANG Xinkuan, YU Zhongwei. Measures and optimization scheme for improving comprehensive utilization efficiency of naphtha[J]. Petroleum Processing and Petrochemicals,2021,52(5):16−21. ). doi: 10.3969/j.issn.1005-2399.2021.05.003 [5] 吴冰峰, 于中伟, 王子健, 等. 多产芳烃并兼产小分子烷烃的正己烷转化机理[J]. 石油学报(石油加工),2023,39(1):35−44.WU Bingfeng, YU Zhongwei, WANG Zijian, et al. Conversion mechanism of n-hexane for high yield of aromatics and concurrent yield of micromolecular alkanes[J]. Acta Petrolei Sinica (Petroleum Processing Section),2023,39(1):35−44. [6] 相宏伟, 唐宏青, 李永旺. 煤化工工艺技术评述与展望 Ⅳ. 煤间接液化技术[J]. 燃料化学学报,2021,29(4):289−298.XIANG Hongwei, TANG Hongqing, LI Yongwang. Perspectives on R&D in coal chemical industry IV. synthesis of fuels from coal via Fischer-Tropsch reaction[J]. J Fuel Chem Technol,2021,29(4):289−298. [7] 谭晓林, 马波, 张喜文, 等. Cr系丙烷脱氢催化剂研究进展[J]. 化工进展,2010,29(1):51−57.TAN Xiaolin, MA Bo, ZHANG Xiwen, et al. Advances in Cr-based catalysts for propane dehydrogenation[J]. Chem Ind Eng Prog,2010,29(1):51−57. [8] WECKHUYSEN B M, SCHOONHEYDT R A. Alkane dehydrogenation over supported chromium oxide catalysts[J]. Catal Today,1999,5:223−232. [9] 李春义, 王国玮. 丙烷和丁烷气固相催化脱氢制烯烃[J]. 中国科学(化学),2018,48(4):342−361. doi: 10.1360/N032017-00155LI Chunyi, WANG Guowei. Gas-solid catalytic dehydrogenation of propane and butanes to olefins[J]. Scienta Sinica Chimica,2018,48(4):342−361. doi: 10.1360/N032017-00155 [10] FRIDMAN V Z, XING R. Investigating the CrO x/Al2O3 dehydrogenation catalyst model: II. relative activity of the chromium species on the catalyst surface[J]. Appl Catal A: Gen,2017,530:154−165. doi: 10.1016/j.apcata.2016.11.024 [11] FRIDMAN V Z, XING R, Severance M. Investigating the CrO x/Al2O3 dehydrogenation catalyst model: I. identification and stability evaluation of the Cr species on the fresh and equilibrated catalysts[J]. Appl Catal A: Gen,2016,523:39−53. doi: 10.1016/j.apcata.2016.05.008 [12] SANTHOSHKUMAR M, HAMMER N, RONNING M, et al. The nature of active chromium species in Cr-catalysts for dehydrogenation of propane: new insights by a comprehensive spectroscopic study[J]. J Catal,2009,261:116−128. doi: 10.1016/j.jcat.2008.11.014 [13] 解则安, 曹奇, 曾敬, 等. Cr基催化剂的丙烷脱氢活性位结构及反应机理的研究进展[J]. 沈阳师范大学学报(自然科学版),2021,39(2):125−131.XIE Zean, CAO Qi, ZENG Jing, et al. Research progress on active site structure and reaction mechanism of propane dehydrogenation over Cr-based catalysts[J]. Journal of Shenyang Normanl University (Natural Science Edition),2021,39(2):125−131. [14] FRIDMAN V Z, XING R. Deactivation studies of the CrO x/Al2O3 dehydrogenation catalysts under cyclic redox conditions[J]. Ind Eng Chem Res,2017,56:7937−7947. doi: 10.1021/acs.iecr.7b01638 [15] 牛鑫善. Cr系异丁烷脱氢催化剂的制备与反应性能研究[D]. 青岛: 青岛科技大学, 2017.NIU Xinshan. Study on preparation and reaction performance of Cr-based isobutane dehydrogenation catalysts[D]. Qingdao: Qingdao University of Science and Technology, 2017.) [16] 李宁, 季生福, 史雪君, 等. 溶胶-凝胶法制备Cr-基催化剂及其CO2氧化乙烷制乙烯[J]. 燃料化学学报,2009,37(3):339−345.LI Ning, JI Fusheng, SHI Xuejun, et al. Oxidative dehydrogenation of ethane with carbon dioxide over the Cr-based catalysts prepared by sol-gel method[J]. J Fuel Chem Technol,2009,37(3):339−345. [17] 邓双, 李会泉, 张懿. 纳米Cr2O3系列催化剂上CO2氧化乙烷脱氢制乙烯反应[J]. 催化学报,2003,24(10):744−750. doi: 10.3321/j.issn:0253-9837.2003.10.007DENG Shuang, LI Huiquan, ZHANG Yi. Oxidative dehydrogenation of ethane with carbon dioxide to Ethylene over nanosized Cr2O3 catalysts[J]. Chin J Catal,2003,24(10):744−750. doi: 10.3321/j.issn:0253-9837.2003.10.007 [18] 邓双, 李会泉, 张懿. 纳米Cr2O3的制备、表征及催化性能[J]. 无机化学学报,2003,19(8):825−830. doi: 10.3321/j.issn:1001-4861.2003.08.006DENG Shuang, LI Huiquan, ZHANG Yi. Preparation, characterization and catalytic activity of nanosized chromiun oxide[J]. J Inorg Chem,2003,19(8):825−830. doi: 10.3321/j.issn:1001-4861.2003.08.006 [19] GONG X, SHU Y, JIANG Z, et al. Metal-Organic frameworks for the exploitation of distance between active sites in efficient photocatalysis[J]. Angew Chem Int Ed,2020,59:5326−5331. doi: 10.1002/anie.201915537 [20] 刘淑芝, 赵福临, 郭齐, 等. 金属有机骨架MIL-101的合成、改性及在催化反应中的应用进展[J]. 化工进展,2017,36(3):918−925.LIU Shuzhi, ZHAO Fulin, GUO Qi, et al. Progress in synthesis, modification and catalytic application of metal-organic frameworks MIL-101[J]. Chem Ind Eng Prog,2017,36(3):918−925. [21] 梁方方, 周凌云, 李想, 等. 乙二胺改性金属有机骨架材料MIL-101(Cr)常压下吸附CO2[J]. 过程工程学报,2015,15(6):1069−1074. doi: 10.12034/j.issn.1009-606X.215310LIANG Fangfang, ZHOU Lingyun, LI Xiang, et al. Adsorption of CO2 on ethylenediamine modified metal-organic framework material MIL-101(Cr) under atmospheric pressure[J]. Chin J Process Eng,2015,15(6):1069−1074. doi: 10.12034/j.issn.1009-606X.215310 [22] KIM J, LEE Y, AHN W. Dry-gel conversion synthesis of Cr-MIL-101 aided by grinding: high surface area and high yield synthesis with minimum purification[J]. Chem Commun,2013,49:7647. doi: 10.1039/c3cc44559c [23] NASHY E H A, OSMAN O, MAHMOUD A A, et al. Molecular spectroscopic study for suggested mechanism of chrome tanned leather[J]. Spectrochimica Acta,2012,88:171−176. doi: 10.1016/j.saa.2011.12.024 [24] WANG D, HE S, SHAN C, et al. Chromium speciation in tannery effluent after alkaline precipitation: isolation and characterization[J]. J Hazard Mater,2016,316:169−177. doi: 10.1016/j.jhazmat.2016.05.021 [25] 孙聆东, 付雪峰, 钱程, 等. 水热法合成CdS/ZnO核壳结构纳米微粒[J]. 高等学校化学学报,2001,22(6):879−882. doi: 10.3321/j.issn:0251-0790.2001.06.001SUN Lindong, FU Xuefeng, QIAN Chen, et al. Synthesis of core/shell structural CdS/ZnO nanoparticles by hydrothermal method[J]. Chem J Chin U,2001,22(6):879−882. doi: 10.3321/j.issn:0251-0790.2001.06.001 [26] 杨剑, 滕凤恩. 纳米材料综述[J]. 材料导报,1997,11(2):6−10. doi: 10.3321/j.issn:1005-023X.1997.02.002YANG Jian, TENG Fengen. The summary of nanocrystalline materials[J]. Mater Rep,1997,11(2):6−10. doi: 10.3321/j.issn:1005-023X.1997.02.002 [27] CAVANI F, KOUTRYREV M, TRIFIRO F, et al. Chemical and physical characterization of alumina-supported chromia-based catalysts and their activity in dehydrogenation of isobutane[J]. J Catal,1996,158:236−250. doi: 10.1006/jcat.1996.0023 [28] ZHANG T, GUO X, SONG C, et al. Fabrication of isolated VO x sites on alumina for highly active and stable non-oxidative dehydrogenation[J]. J Phy Chem C,2021,125:19229−19237. doi: 10.1021/acs.jpcc.1c04737 [29] LI X, FAN R, SHEN H, et al. A site distance dependence of product selectivity in n-hexane dehydrogenation over alumina-supported chromium catalyst[J]. Chem Eng J,2024,488:150842. doi: 10.1016/j.cej.2024.150842 -




 下载:
下载: