Influence of Al2O3 precursors on Cu/ZnO/Al2O3 catalysts for hydrogen production from steam reforming of methanol
-
摘要: 采用共沉淀法制备了一系列Cu/ZnO/Al2O3催化剂,通过XRD、BET、H2-TPR、N2O化学吸附、XPS表征技术,研究了Al2O3前驱体对催化剂结构的影响,同时对其在甲醇重整制氢中的性能进行了考察。结果表明,当Al3+与Cu2+、Zn2+同时共沉淀时,Al3+对碱式碳酸盐中Cu2+-Zn2+部分取代生成类水滑石结构,增强了Zn-Al之间的相互作用。相反,在Cu2+、Zn2+完成共沉淀后,引入Al2O3前驱体对消除Al3+对碱式碳酸盐中Cu-Zn取代的不良影响具有积极作用,有利于促进Cu-ZnO间的相互作用、CuO物种的分散和催化剂的还原,进一步促进表面Cu的分散,有利于其活性的提升。其中,以拟薄水铝石为铝源制备的催化剂呈现出优异的活性。在水醇物质的量比为1.2,反应温度为493 K的条件下,甲醇转化率可达94.8%,H2时空收率可达97.5 mol/(kg·h),并且连续运行25 h其活性仍保持相对稳定。在反应条件下,经过723 K的10 h热处理后,该催化剂的活性损失率仅为5.37%。
-
关键词:
- Al2O3前驱体 /
- Cu/ZnO/Al2O3催化剂 /
- 甲醇水蒸气重整 /
- 热稳定性
Abstract: This research outlines the synthesis of a variety of Cu/ZnO/Al2O3 catalysts utilizing the co-precipitation method, emphasizing an investigation into the impact of various Al2O3 precursors on the catalyst's structure through thorough structural characterization techniques. Furthermore, the catalytic performance of these materials in methanol reforming for hydrogen production was systematically evaluated. The results showed that the simultaneous co-precipitation of Al3+ with Cu2+ and Zn2+ resulted in the partial substitution of Cu2+-Zn2+ in alkali carbonates by Al3+, forming a hydrotalcite-like structure and strengthening the Zn-Al interactions. By contrary, after the coprecipitation of Cu2+ and Zn2+ was completed, the adverse effect of Al3+ on Cu-Zn substitution in alkali carbonates was effectively attenuated by the introduction of the Al2O3 precursor. This promoted Cu-ZnO interaction, facilitated the dispersion of CuO species, catalyst reduction, and further improved the Cu dispersion on the surface, ultimately leading to the improvement of catalytic activity. Notably, the catalyst prepared with pseudo-boehmite as Al2O3 precursor showed the highest activity. Under the conditions of H2O/CH3OH molar ratio of 1.2 and reaction temperature of 493 K, the methanol conversion reached 94.8% and the H2 space-time yield was 97.5 mol/(kg·h). Moreover, its catalytic activity remained relatively stable during a continuous operation for 25 h. Even heat treated at 723 K for 10 h, the activity loss of the catalyst was only 5.37%.-
Key words:
- Al2O3 precursor /
- Cu/ZnO/Al2O3 catalyst /
- methanol steam reforming /
- thermal stability
-
图 7 催化剂的稳定性比较(a)493 K稳定性测试、(b)热处理前后催化剂在493 K时甲醇转化率比较
Figure 7 Comparison of catalysts stability: (a) stability test at 493 K, (b) comparing methanol conversion between catalysts before and after thermal treatedReaction conditions: 493 K, H2O/CH3OH= 1.2, WHSVtotal = 2 h−1; thermal treatment conditions: 723 K, H2O/CH3OH= 1.2, WHSVtotal = 2 h−1,10 h.
表 1 催化剂沉淀前驱体组分信息
Table 1 Component information of catalyst precipitated precursor
samples Main peak location PDF number Malachite 14.715,17.496,24.092,29.470 75−1163 Rosasite 14.637,17.467,24.075,29.591 35−0502 Aurichalcite 13.028,24.032,27.857,32.655 38−0152 HTIC 11.750,23.579,34.617,39.258 38−0487 Al(OH)3 18.502,20.464,21.049,21.343 85−0611 表 2 催化剂的织构性能和晶粒尺寸
Table 2 The crystalline size and texture properties of CZA-x catalysts
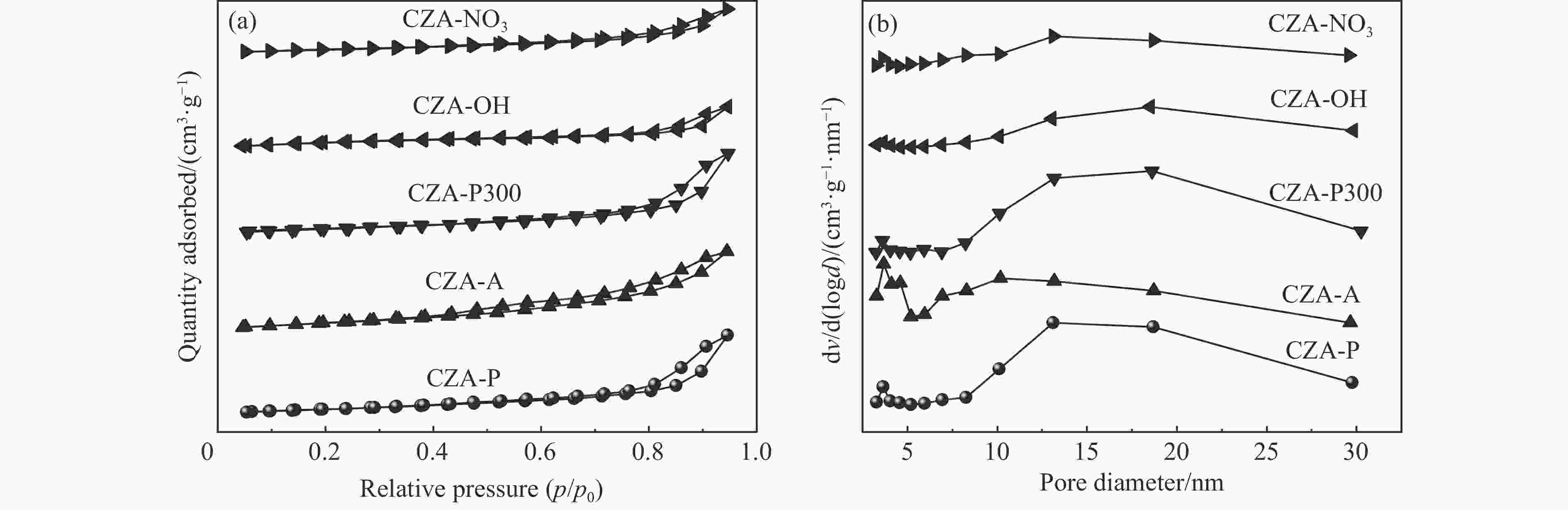
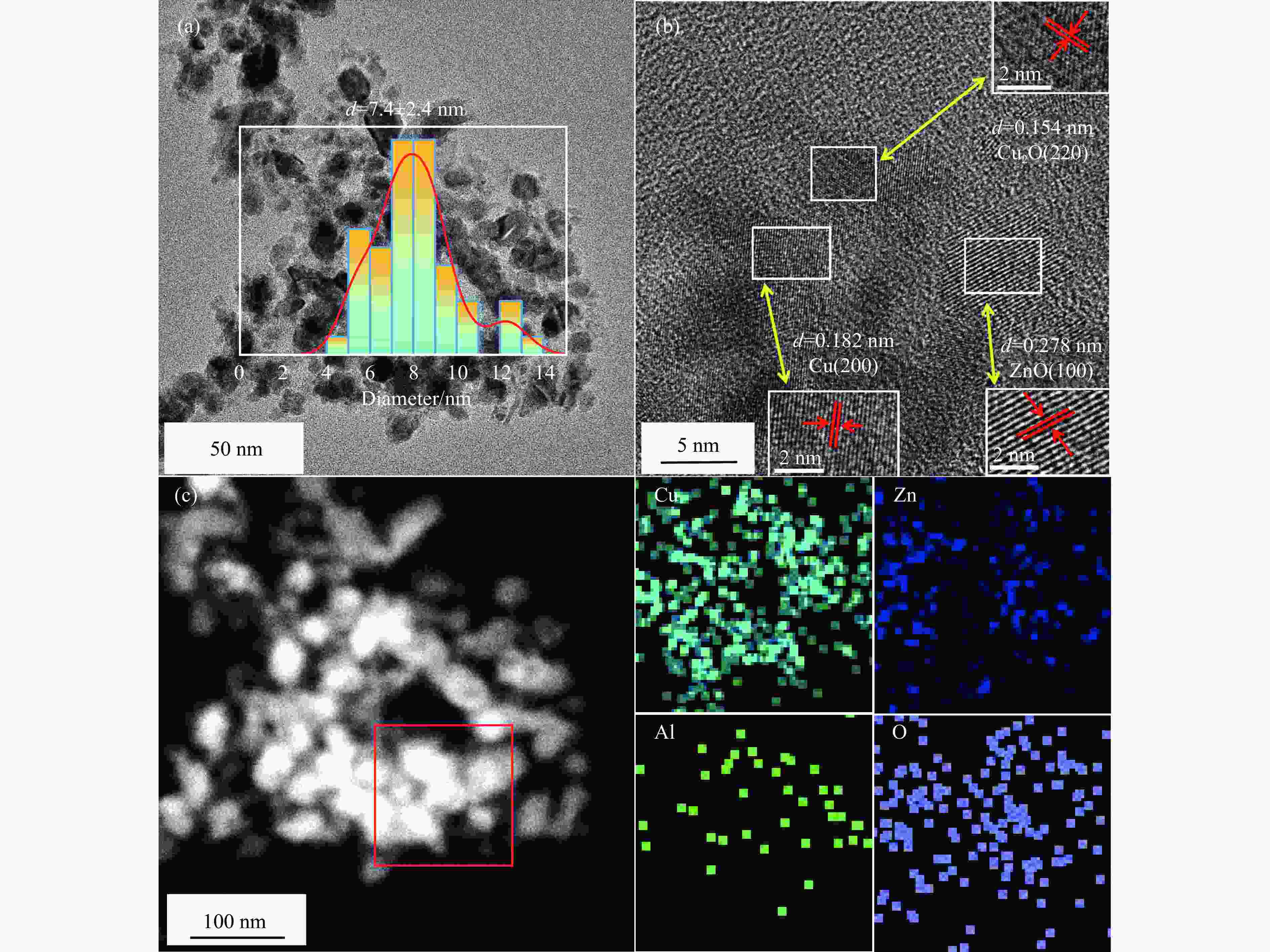
Catalyst Cu content/%a SBET/(m2·g−1)b vpore/(cm3·g−1)c dp/nmc dCuO/nmd dCu/%e CZA-NO3 59.7 60.43 0.146 3.62 9.4 7.4 CZA-OH 59.3 80.14 0.147 3.65 8.6 8.6 CZA-P300 58.9 81.56 0.254 13.18 8.9 8.2 CZA-A 59.1 112.34 0.252 3.67 8.0 9.2 CZA-P 59.7 84.22 0.258 3.64 7.5 10.3 a: Obtained by ICP-OES; b: Calculated by BET method; c: Calculated by BJH method; d: Calculated from Scherrer equation based on CuO (002); e: Calculated from N2O chemisorption. 表 3 催化剂的H2-TPR参数
Table 3 H2-TPR parameters for catalysts
Sample Temperature/℃ H2 consumption/(mmol·g−1) α β γ α β γ total CZA-NO3 209 242 273 0.49 3.02 6.45 9.96 CZA-OH 236 262 − 3.06 6.59 − 9.65 CZA-P300 229 254 − 2.79 6.56 − 9.35 CZA-A 217 242 − 3.24 6.17 − 9.41 CZA-P 209 236 − 4.71 5.76 − 10.47 表 4 催化剂还原后的XPS参数
Table 4 XPS parameters of the reduced catalysts
Catalyst Binding energy/eV Cu+/Cu/% Cu0/Cu/% Cu0/Cu+/% Cu0 Cu+ CZA-P 567.7 569.7 76.1 23.9 31 CZA-OH 568.1 569.8 78.9 21.1 27 CZA-A 568.3 569.9 64.8 35.2 54 CZA-P300 568.5 569.7 30.6 69.2 27 CZA-NO3 568.1 569.8 79.5 20.5 26 表 5 与文献报道催化剂活性对比
Table 5 Comparison with catalyst activity reported in the literature
Catalyst Reaction temperatre
T/Kn(H2O)/
n(MeOH)GHSV/
WHSV(h−1)Methanol conversion
/%After thermal treatment
methanol conversion/%Reference CZA-P 493 1.2 2.0(W) 94.8 89.7 this work 商用催化剂 493 1.2 2.0(W) 95.0 84.3 this work Cu-Ce-A 493 1.2 1760(G) 40 − [34] Ce/Cu/ZnAl 513 1.2 800(G) 92 − [35] 0.38B/CZA 523 3 9000 mL/(g·h) 93 − [36] -
[1] LEBROUHI B E, DJOUPO J J, LAMRANI B, et al. Global hydrogen development-A technological and geopolitical overview[J]. Int J Hydrogen Energy,2022,47(11):7016−7048. doi: 10.1016/j.ijhydene.2021.12.076 [2] GABRIEL K S, EL-EMAM R S, ZAMFIRESCU C. Technoeconomics of large-scale clean hydrogen production-A review[J]. Int J Hydrogen Energy,2022,47(72):30788−30798. doi: 10.1016/j.ijhydene.2021.10.081 [3] RIBEIRINHA P, MATEOS-PEDRERO C, BOAVENTURA M, et al. CuO/ZnO/Ga2O3 catalyst for low temperature MSR reaction: Synthesis, characterization and kinetic model[J]. Appl Catal B: Environ,2018,221:371−379. doi: 10.1016/j.apcatb.2017.09.040 [4] Sá S, SILVA H, BRANDAO L, et al. Catalysts for methanol steam reforming—A review[J]. Appl Catal B: Environ,2010,99(1-2):43−57. doi: 10.1016/j.apcatb.2010.06.015 [5] SUN Z, SUN Z Q. Hydrogen generation from methanol reforming for fuel cell applications: A review[J]. J Cent South Univ,2020,27(4):1074−1103. doi: 10.1007/s11771-020-4352-8 [6] XU X, SHUAI K, XU B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen[J]. Catalysts,2017,7(6):183. doi: 10.3390/catal7060183 [7] PU Y-C, LI S-R, YAN S, et al. An improved Cu/ZnO catalyst promoted by Sc2O3 for hydrogen production from methanol reforming[J]. Fuel,2019,241:607−615. doi: 10.1016/j.fuel.2018.12.067 [8] AJAMEIN H, HAGHIGHI M, SHOKRANI R, et al. On the solution combustion synthesis of copper based nanocatalysts for steam methanol reforming: Effect of precursor, ultrasound irradiation and urea/nitrate ratio[J]. J Mol Catal A: Chem,2016,421:222−234. doi: 10.1016/j.molcata.2016.05.028 [9] CHENG Z, ZHOU W, LAN G, et al. High-performance Cu/ZnO/Al2O3 catalysts for methanol steam reforming with enhanced Cu-ZnO synergy effect via magnesium assisted strategy[J]. J Energy Chem,2021,63:550−557. doi: 10.1016/j.jechem.2021.08.025 [10] YANG X, MENG Q, DING G, et al. Construction of novel Cu/ZnO-Al2O3 composites for furfural hydrogenation: The role of Al components[J]. Appl Catal A-Gen,2018,561:78−86. doi: 10.1016/j.apcata.2018.04.005 [11] BEHRENS M, ZANDER S, KURR P, et al. Performance improvement of nanocatalysts by promoter-induced defects in the support material: methanol synthesis over Cu/ZnO: Al[J]. J Am Chem Soc,2013,135(16):6061−6068. doi: 10.1021/ja310456f [12] FU W, BAO Z, DING W, et al. The synergistic effect of the structural precursors of Cu/ZnO/Al2O3 catalysts for water–gas shift reaction[J]. Catal Commun,2011,12(6):505−509. doi: 10.1016/j.catcom.2010.11.017 [13] KOWALIK P, KONKOL M, ANTONIAK K, et al. The effect of the precursor ageing on properties of the Cu/ZnO/Al2O3 catalyst for low temperature water–gas shift (LT-WGS)[J]. J Mol Catal A: Chem,2014,392:127−133. doi: 10.1016/j.molcata.2014.05.003 [14] ZHANG F, LIU Y, XU X, et al. Effect of Al-containing precursors on Cu/ZnO/Al2O3 catalyst for methanol production[J]. Fuel Process Technol,2018,178:148−155. doi: 10.1016/j.fuproc.2018.04.021 [15] BEHRENS M, KASATKIN I, KÜHL S, et al. Phase-pure Cu, Zn, Al hydrotalcite-like materials as precursors for copper rich Cu/ZnO/Al2O3 catalysts[J]. Chem Mater,2009,22(2):386−397. [16] WANG S, LIU H. Selective hydrogenolysis of glycerol to propylene glycol on hydroxycarbonate-derived Cu-ZnO-Al2O3 catalysts[J]. Chin J Catal,2014,35(5):631−643. doi: 10.1016/S1872-2067(14)60094-2 [17] SEBASTIAN KULD, MAX THORHAUGE, HANNE FALSIG, et al. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis[J]. Science,2016,352(6288):969−974. doi: 10.1126/science.aaf0718 [18] FUJITANI T, NAKAMURA J. The chemical modification seen in the Cu/ZnO methanol synthesis catalysts[J]. Appl Catal A: Gen,2000,191:111−129. doi: 10.1016/S0926-860X(99)00313-0 [19] LEI H, HOU Z, XIE J. Hydrogenation of CO2 to CH3OH over CuO/ZnO/Al2O3 catalysts prepared via a solvent-free routine[J]. Fuel,2016,164:191−198. doi: 10.1016/j.fuel.2015.09.082 [20] WANG L, LIU Y, CHEN M, et al. Production of hydrogen by steam reforming of methanol over Cu/ZnO catalysts prepared via a practical soft reactive grinding route based on dry oxalate-precursor synthesis[J]. J Catal,2007,246(1):193−204. doi: 10.1016/j.jcat.2006.12.006 [21] BAHMANI M, VASHEGHANI FARAHANI B, SAHEBDELFAR S. Preparation of high performance nano-sized Cu/ZnO/Al2O3 methanol synthesis catalyst via aluminum hydrous oxide sol[J]. Appl Catal A: Gen,2016,520:178−187. doi: 10.1016/j.apcata.2016.04.018 [22] KÜHL S, TARASOV A, ZANDER S, et al. Cu-based catalyst resulting from a Cu, Zn, Al hydrotalcite-like compound: a microstructural, thermoanalytical, and in situ XAS study[J]. Chem Eur J,2014,20(13):3782−3792. doi: 10.1002/chem.201302599 [23] WANG X, CHEN X, YE L, et al. Superior performance of Cu/TiO2 catalyst prepared by ice melting method for low-temperature selective catalytic reduction of NOx by NH3[J]. Mol Catal,2020,497:111225. doi: 10.1016/j.mcat.2020.111225 [24] ZHANG G, LI Z, ZHENG H, et al. Influence of the surface oxygenated groups of activated carbon on preparation of a nano Cu/AC catalyst and heterogeneous catalysis in the oxidative carbonylation of methanol[J]. Appl Catal B: Environ,2015,179:95−105. doi: 10.1016/j.apcatb.2015.05.001 [25] GUO Q, LI S, LI J, et al. Enhanced CO2 hydrogenation to methanol on the mesostructured Cu–ZnO/Al2O3–ZrO2 catalyst[J]. ACS Appl Energ Mater,2021,4(8):8311−8321. doi: 10.1021/acsaem.1c01542 [26] LIU C, GUO X, GUO Q, et al. Methanol synthesis from CO2 hydrogenation over copper catalysts supported on MgO-modified TiO2[J]. J Mol Catal A: Chem,2016,425:86−93. doi: 10.1016/j.molcata.2016.09.032 [27] GONG J, YUE H, ZHAO Y, et al. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites[J]. J Am Chem Soc,2012,134(34):13922−13925. doi: 10.1021/ja3034153 [28] CHEN H, CUI H, LV Y, et al. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts: Effects of ZnO morphology and oxygen vacancy[J]. Fuel,2022,314:123035. doi: 10.1016/j.fuel.2021.123035 [29] MA K, TIAN Y, ZHAO Z J, et al. Achieving efficient and robust catalytic reforming on dual-sites of Cu species[J]. Chemical Science,2019,10(9):2578−2584. doi: 10.1039/C9SC00015A [30] ZHANG G, ZHAO J, YANG T, et al. In-situ self-assembled Cu2O/ZnO core-shell catalysts synergistically enhance the durability of methanol steam reforming[J]. Appl Catal A: Gen,2021,616:118072. doi: 10.1016/j.apcata.2021.118072 [31] HOU X, QING S, LIU Y, et al. Enhancing effect of MgO modification of Cu–Al spinel oxide catalyst for methanol steam reforming[J]. Int J Hydrogen Energy,2020,45(1):477−489. doi: 10.1016/j.ijhydene.2019.10.164 [32] ZHANG L, LEI J-T, TIAN Y, et al. Effect of precursor and precipitant concentrations on the catalytic properties of CuO/ZnO/CeO2-ZrO2 for methanol steam reforming[J]. J Fuel Chem Technol,2015,43(11):1366−1374. doi: 10.1016/S1872-5813(15)30041-4 [33] RANJEKAR A M, YADAV G D. Steam reforming of methanol for hydrogen production: a critical analysis of catalysis, processes, and scope[J]. Ind Eng Chem Res,2021,60(1):89−113. doi: 10.1021/acs.iecr.0c05041 [34] 王丽宝, 王东哲, 张磊, 等. 铈源对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J]. 燃料化学学报,2020,48:852−859.WANG Baoli, WANG Dongzhe, ZHANG Lei, et al. Influence of cerium source s on CuO/CeO2 catalysts for hydrogen production from steam reforming of methanol[J]. J Fuel Chem Technol,2020,48:852−859. [35] 杨淑倩, 贺建平, 张娜, 等. 稀土掺杂改性对Cu/ZnAl水滑石衍生物催化剂甲醇水蒸气重整制氢性能的影响[J]. 燃料化学学报,2018,46:179−188. doi: 10.1016/S1872-5813(18)30010-0YANG Shuqian, HE Jianping, ZHANG Na, et al. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol,2018,46:179−188. doi: 10.1016/S1872-5813(18)30010-0 [36] 卢培静, 蔡夫锋, 张军, 等. B改性CuZnAlOx催化剂对甲醇水蒸气重整制氢性能的研究[J]. 燃料化学学报,2019,47:791−798. doi: 10.1016/S1872-5813(19)30035-0LU Peijing, CAI Fufeng, ZHANG Jun, et al. Hydrogen production from methanol steam reforming over B-modified CuZnAlOx catalysts[J]. J Fuel Chem Technol,2019,47:791−798. doi: 10.1016/S1872-5813(19)30035-0 -





 下载:
下载: