Mechanism of Methanol Synthesis from CO2 Hydrogenation over Rh16/In2O3 Catalysts: A Combined Study on Density Functional Theory and Microkinetic Modeling
-
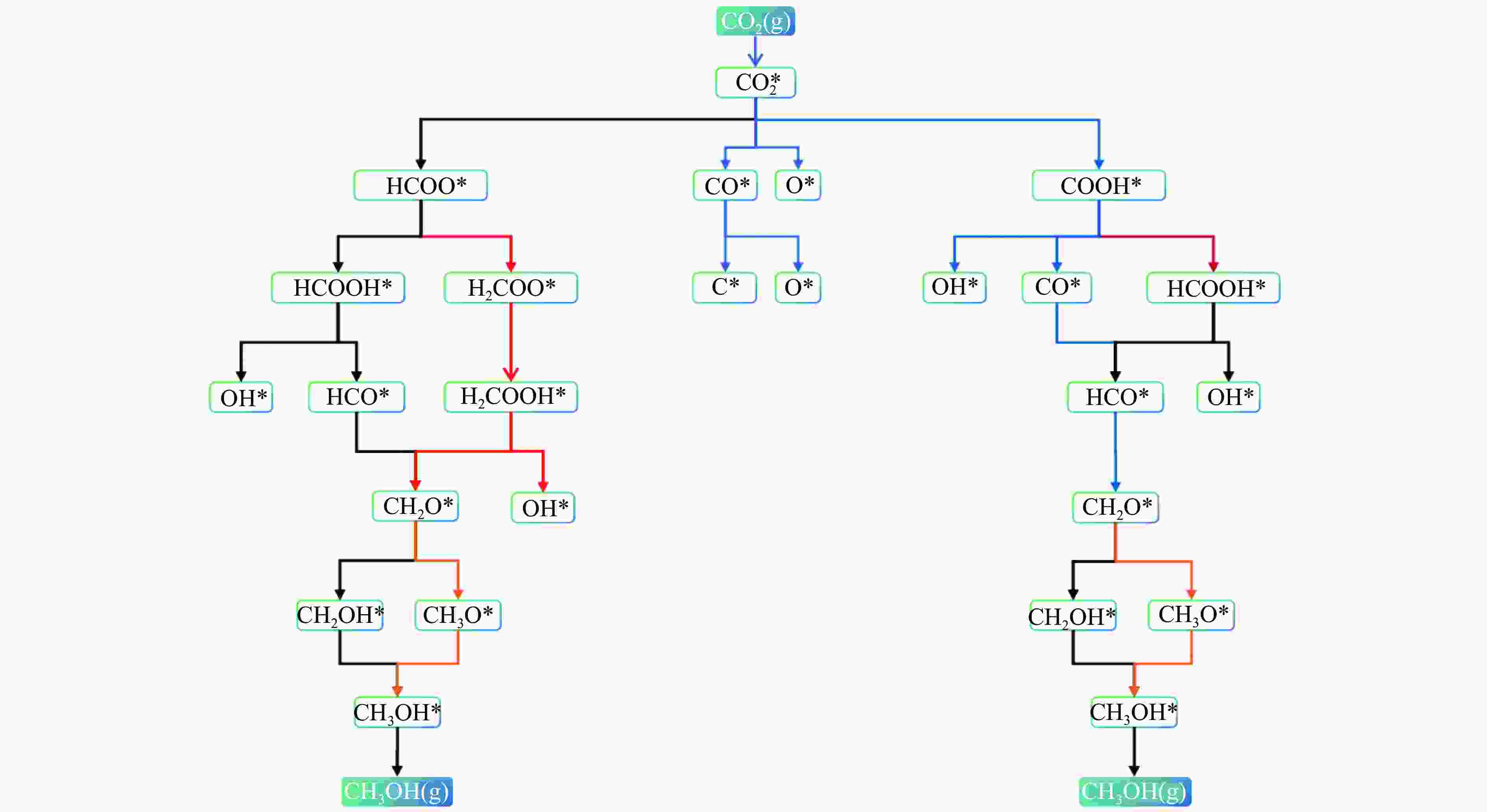
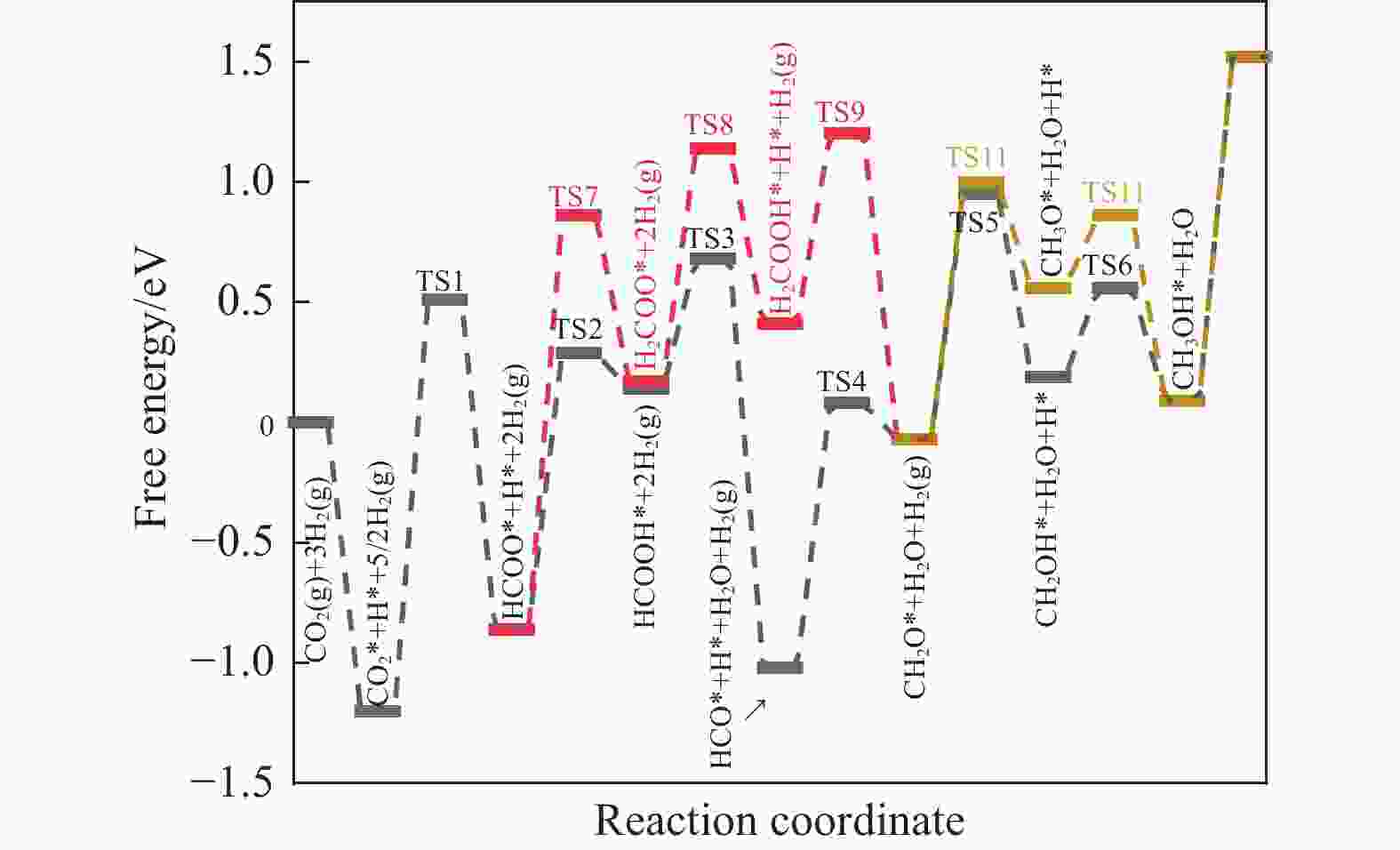
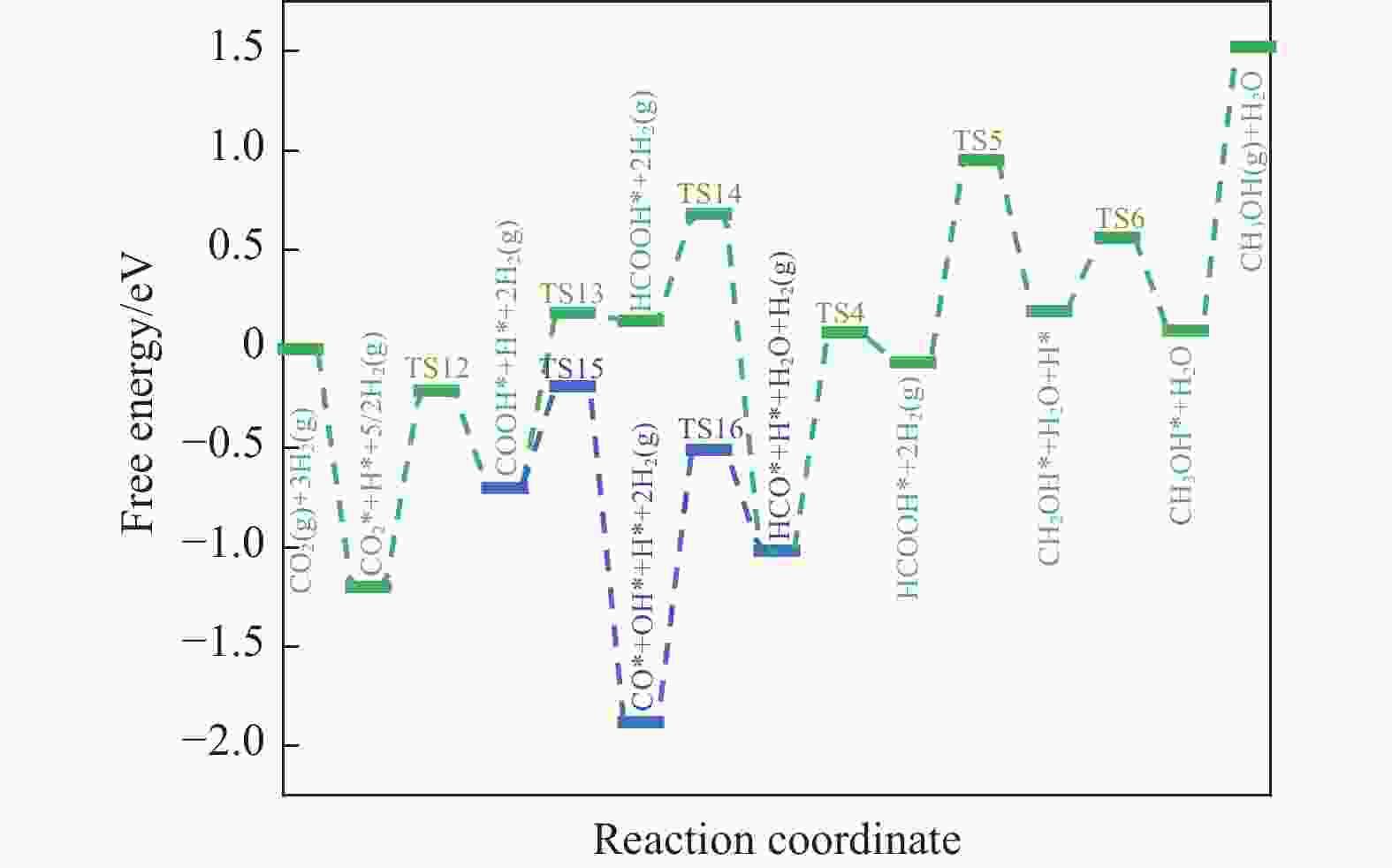
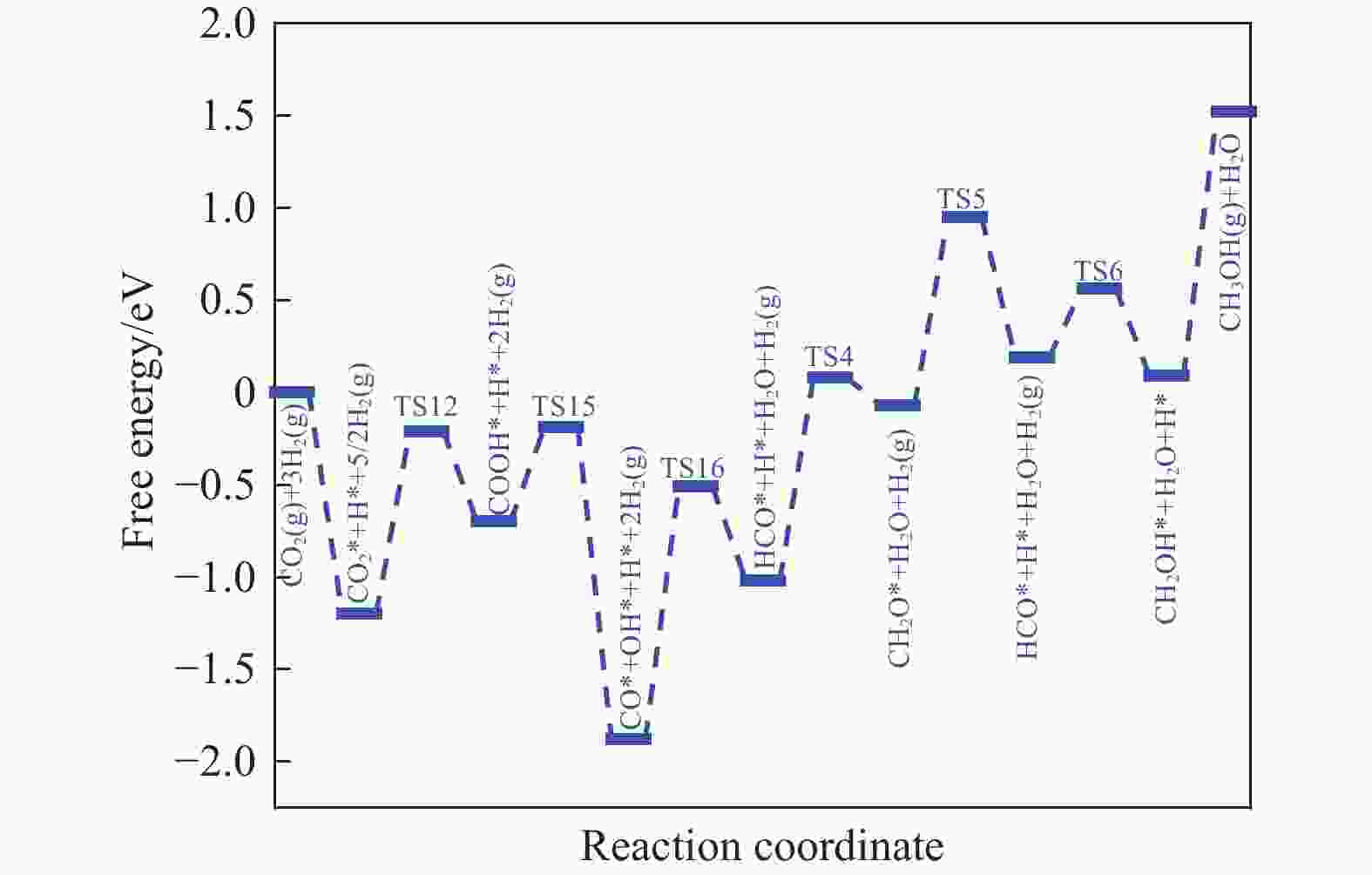
摘要: 本研究采用密度泛函理论 (DFT) 和微动力学模型分析了 Rh16/In2O3 催化剂上二氧化碳 (CO2) 氢化成甲醇 (CH3OH) 的情况;研究了 Rh16/In2O3 界面上 H2 的自发解离和 CO2 的有效吸附,其中, In2O3 中的氧空位提供了有利的效果。此外,Bader 电荷分析显示 Rh16 上带有轻微的正电荷,这对于理解催化剂的电子特性和活性非常重要。证实了RWGS+CO-Hydro 途径是甲醇合成的主要途径,其特点是经过一系列中间转化:CO2*→COOH*→CO*+OH*→HCO*→CH2O*→CH2OH*→ CH3OH*。在不同温度 (373−873K) 和压力 (10−2−103 bar) 下进行的反应速率控制程度分析 (DRC) 揭示了两个关键的动力学现象:在较低温度和较高压力下,转化步骤 CO* + H* → HCO * 显着影响总体反应速率;而在较高温度下,CH2O* + H* → CH3O* 的步骤占主导地位。Abstract: In this investigation, the hydrogenation of carbon dioxide (CO2) to methanol (CH3OH) over a Rh16/In2O3 catalyst is meticulously analyzed through the application of Density Functional Theory (DFT) and microdynamics modeling. The research focuses on the spontaneous dissociation mechanisms of H2 and CO2 at the Rh16/In2O3 interface, with a special emphasis on the role of oxygen vacancies in In2O3 which enhance adsorption processes. Bader charge analysis revealed a marginal positive charge on Rh16, elucidating critical insights into the electronic characteristics and catalytic activity of the system. The study establishes the RWGS+CO-Hydro pathway as the predominant mechanism for methanol synthesis, characterized by a sequential transformation of intermediates: CO2*→COOH*→CO*+OH*→HCO*→CH2O*→CH2OH*→ CH3OH*. Further, Degree of Reaction Rate Control (DRC) analysis conducted across a range of temperatures (373−873K) and pressures (10−2−103 bar) identified two principal kinetic phenomena: at lower temperatures coupled with higher pressures, the conversion of CO* + H* to HCO* significantly impacts the overall rate of reaction; conversely, at higher temperatures, the step from CH2O* + H* to CH3O* is found to dominate.
-
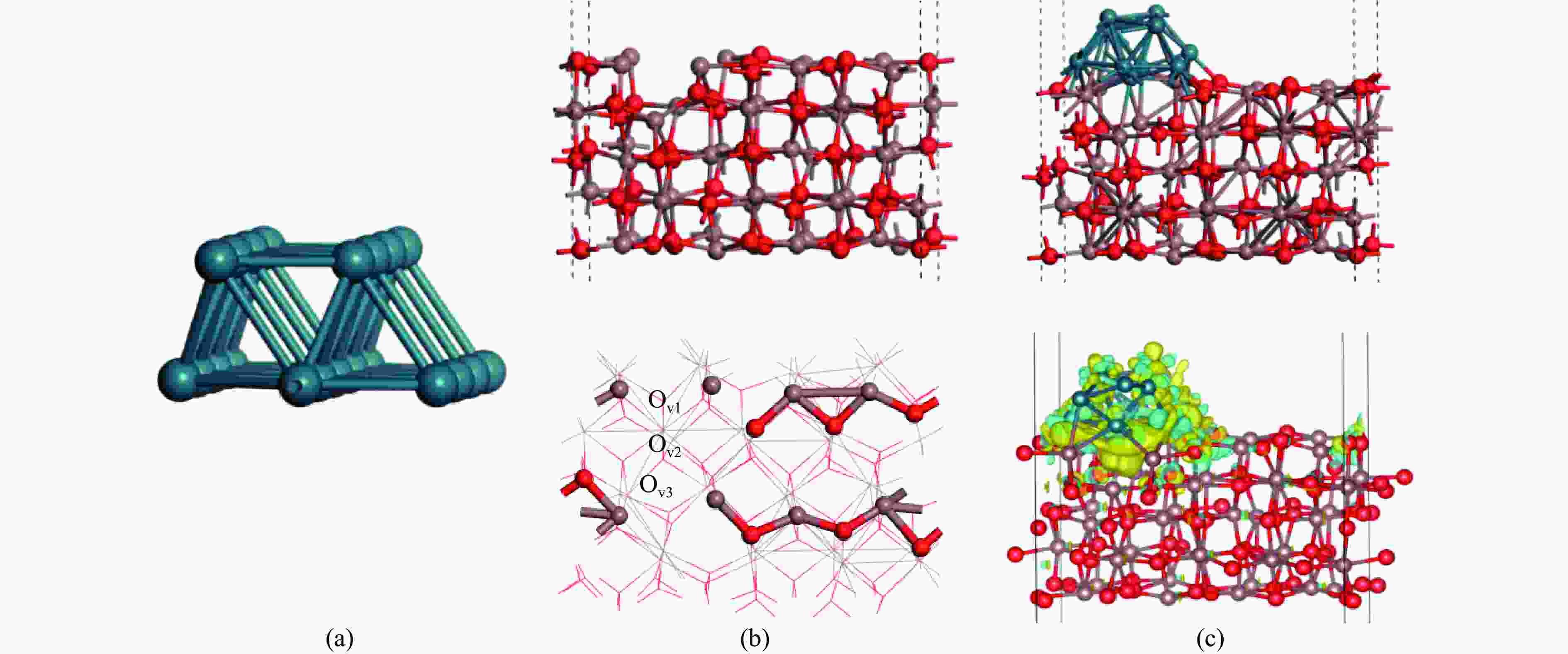
图 2 (a)由20个Rh原子组成的Rh棒状模型,将Rh模型加载到有缺陷的In2O3表面上,In2O3表面上的四个 O 原子与四个 Rh 原子相互作用,导致 Rh 以 Rh16 的形式结合;(b)Rh16/In2O3基底:侧视图(上)、俯视图(下);(c)Rh16/In2O3(110) 模型及Rh16 /In2O3模型中的差分电荷分布图,模型(上),差分电荷分布图(下)
Figure 2 2(a) Rh rod-shaped model composed of 20 Rh atoms, the Rh model is loaded onto the defective In2O3 surface, the four O atoms on the In2O3 surface interact with the four Rh atoms, resulting in Rh in the form of Rh16 combine; combine (b) Rh16/In2O3 substrate: side view (top), top view (bottom); (c) Differential charge distribution diagram in Rh16/In2O3(110) model and Rh16/In2O3 model, model (top), differential charge distribution diagram (bottom)
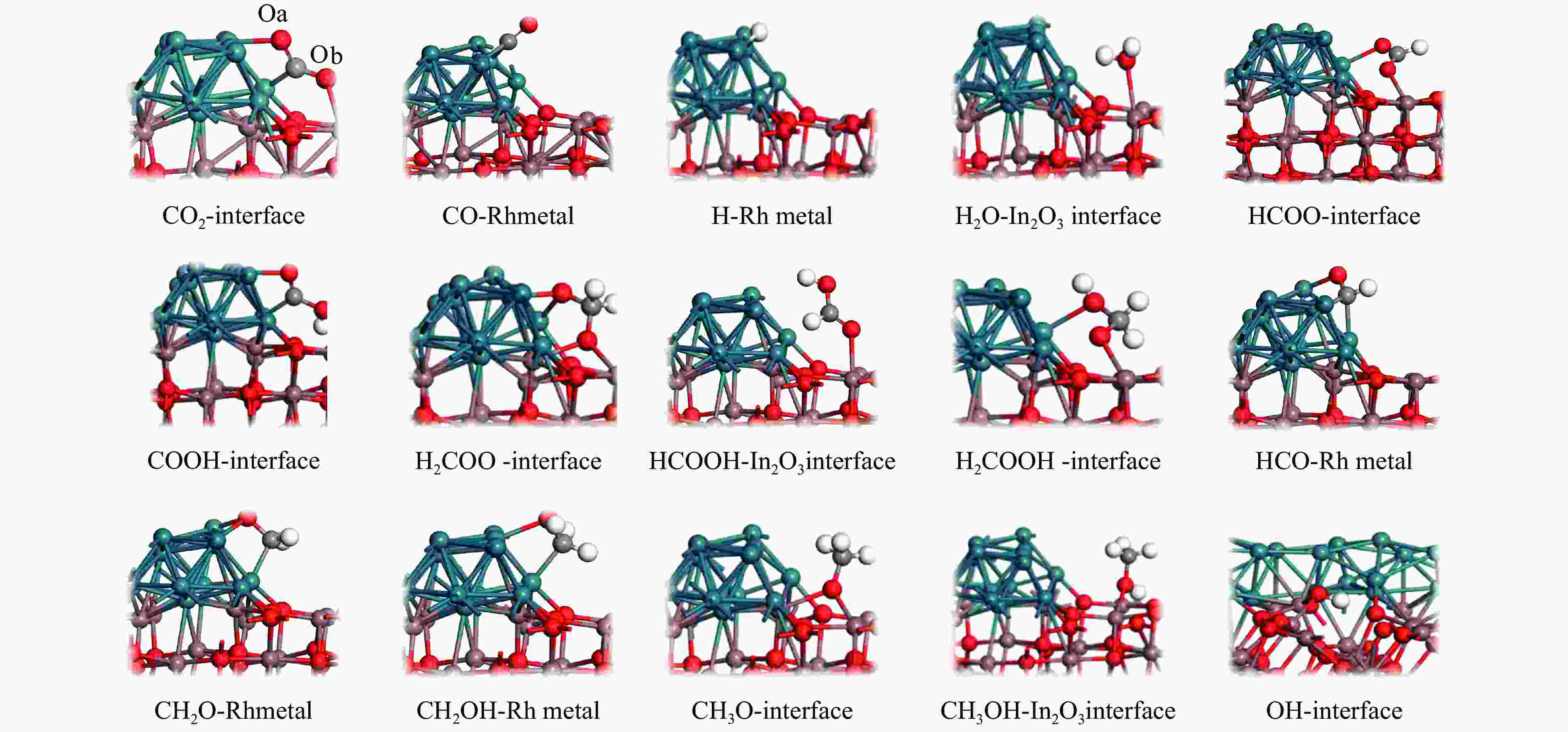
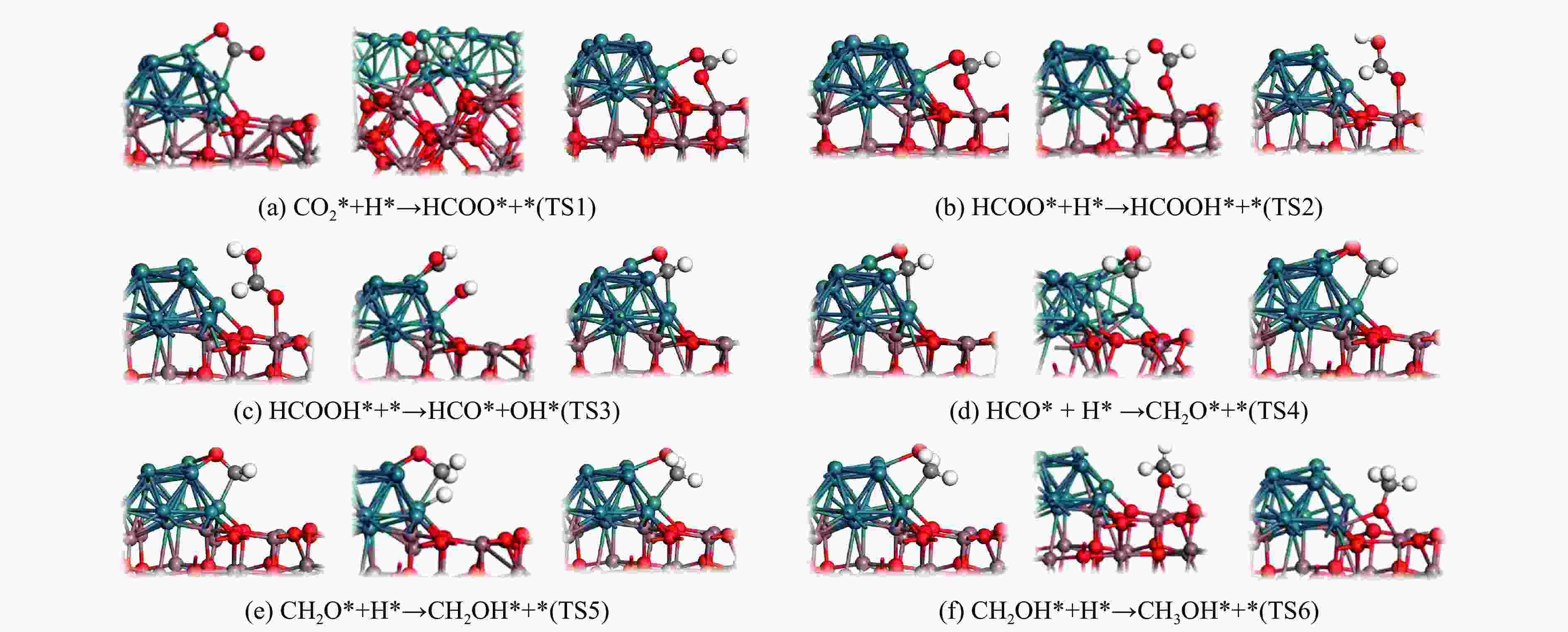
图 9 Rh16/In2O3上CO2加氢合成甲醇,对 COOH 通道各个自由基反应的初始、过渡和最终状态进行了优化结构,其余分支反应如图7所示
Figure 9 Methanol is synthesized from CO2 hydrogenation on Rh16/In2O3. The initial, transition and final states of each free radical reaction in the COOH channel are optimized. The remaining branch reactions are shown in Figure 7
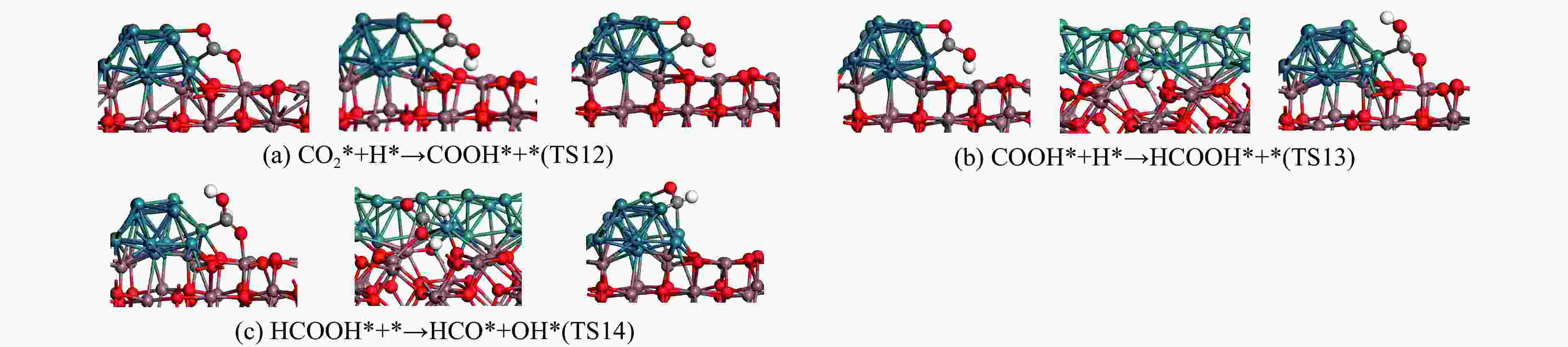
图 11 对RWGS + CO-HydrO通道的二氧化碳加氢反应合成甲醇的单个原始反应的初始、过渡和最终状态的优化结构,其余分支反应见图7
Figure 11 Optimized structures of the initial, transition and final states of a single original reaction for the synthesis of methanol from carbon dioxide hydrogenation of the RWGS + CO-HydrO channel. The remaining branch reactions are shown in Figure 7
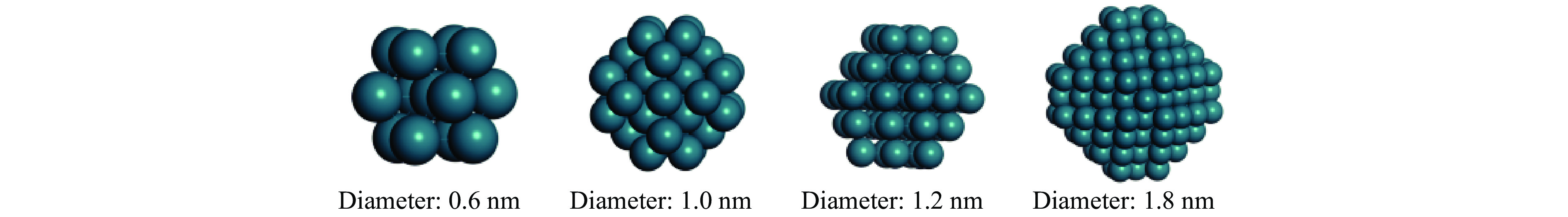
表 1 五种Rh金属团簇的功函数
Table 1 Work functions of seVeral Rh metal clusters
Model Diameter/nm Number of atoms Work function Rh13 0.6 13 4.039 Rh43 1.0 43 4.130 Rh55 1.2 55 4.177 Rh165 1.8 165 4.317 Rh(rods) − 16 4.418 表 2 Rh16/In2O3上的吸附能、吸附位和反应种类的结构参数
Table 2 Structural parameters of adsorption energies, adsorption sites and reaction types on Rh16/In2O3
Specie Eads/eV Site Bond length (Å) and bond angle (°) CO2 −1.16 interface d(Rh−O)=2.126;d(In−O)= 2.247;
d(Rh−C)=1.973;∠Oa−C−Ob=121.9°H(1/2 H2) −0.68 Rh metal d(Rh−H)=1.709/1.772 H2O −0.83 In2O3 interface d(In−O)= 2.352 HCOOH −0.94 In2O3 interface d(In−O)=2.314 CH2O −1.51 Rh metal d(Rh−O)=2.016;d(Rh−C)=2.149/2.124 CH3OH −1.43 In2O3 interface d(In−O)= 2.264;d(O−H)= 1.577 CO −2.57 Rh metal d(Rh−C)=1.964/1.982 表 3 HCOO 途径中甲醇合成所涉及的基本步骤的反应能ΔE 和势垒Eb
Table 3 Reaction energy ΔE and potential barrier Eb of the basic steps involved methanol synthesis in the HCOO pathway
Elementary reaction step Eb/eV △E/eV CO2* + H* → HCOO* + * 0.69 −0.86 HCOO* + H* → H2COO* + * 1.72 0.17 H2COO* + H* → H2COOH* + * 0.97 0.41 H2COOH* + *→CH2O*+ OH* 0.79 −0.06 HCOO* + H* → HCOOH* + * 1.15 0.14 HCOOH* + * → HCO* + OH* 0.54 −1.02 HCO* + H* → CH2O* + * 1.10 −0.06 CH2O* + H* → CH3O* + * 1.07 0.57 CH3O* + H* → CH3OH* + * 0.30 0.10 CH2O* + H* → CH2OH* + * 1.02 0.20 CH2OH* + H* → CH3OH* + * 0.37 0.10 表 4 COOH途径中甲醇合成所涉及的基本步骤的反应能 ΔE 和势垒Eb
Table 4 Reaction energies ΔE and potential barriers Eb for the basic steps involved in methanol synthesis in the COOH pathway
Elementary reaction step Eb/eV △E/eV CO2* + H* → COOH* + * 0.99 −0.70 COOH* + H* → HCOOH* + * 0.88 0.14 HCOOH* + *→HCO* + OH* 0.68 −1.01 COOH* + * → CO* + OH* 0.51 −1.88 HCO* + H* → CH2O* + * 1.10 −0.06 表 5 RWGS + CO-Hydro 通道中甲醇合成所涉及的基本步骤的反应能 ΔE和能垒 Eb
Table 5 Reaction energy ΔE and energy barrier Eb for the basic steps involved in methanol synthesis in the RWGS +CO-Hydro channel
Elementary reaction step Eb/eV △E/eV CO2* + H* → COOH* + * 0.99 −0.70 COOH* + * → CO* + OH* 0.51 −1.88 CO* + H* → HCO* + * 1.37 −1.01 HCO* + H* → CH2O* + * 1.10 −0.06 CO2* + * → CO* + O* 2.18 −1.18 CO* +O* → C* + O* 2.94 0.72 -
[1] ABAS N, KALAIR A, KHAN N. ReView of fossil fuels and future energy technologies[J]. Futures,2015,69:31−49. doi: 10.1016/j.futures.2015.03.003 [2] OLAH G A, PRAKASH G K S, GOEPPERT A. Anthropogenic chemical carbon cycle for a sustainable future[J]. J. Am. Chem. Soc,2011,133(33):12881−12898. doi: 10.1021/ja202642y [3] Li Y, CHAN S H, SUN Q. Heterogeneous catalytic conversion of CO2: A comprehensive theoretical review[J]. Nanoscale,2015,7(19):8663−8683. doi: 10.1039/C5NR00092K [4] HE M, SUN Y, HAN B. Green carbon science: Efficient carbon resource processing, utilization, and recycling towards carbon neutrality[J]. Angewandte Chemie,2022,134(15):e202112835. doi: 10.1002/ange.202112835 [5] LI Y N, MA R, HE L N, et al. Homogeneous hydrogenation of carbon dioxide to methanol[J]. Catal Sci Technol,2014,4(6):1498−1512. doi: 10.1039/C3CY00564J [6] WANG L, WANG D, LI Y. Single-atom catalysis for carbon neutrality[J]. Carbon Energy,2022,4(6):1021−1079. doi: 10.1002/cey2.194 [7] MARTINEZ-SUAREZ L, SIEMER N, FRENZEL J, MARX D. Reaction network of methanol synthesis over Cu/ZnO nanocatalysts[J]. Acs Catal,2015,5(7):4201−4218. doi: 10.1021/acscatal.5b00442 [8] DUBOIS J L, SAYAMA K, ARAKAWA H. CO2 hydrogenation over carbide catalysts[J]. Chem Lett,1992,21(1):5−8. doi: 10.1246/cl.1992.5 [9] SOLYMOSI F, OSZKO A, BANSAGI T, et al. Adsorption and reaction of CO2 on Mo2C catalyst[J]. J Phys Chem B,2002,106(37):9613−9618 doi: 10.1021/jp0203696 [10] BONURA G, CORDORA M, CANNILLA C, et al. The changing nature of the active site of Cu-Zn-Zr catalysts for the CO2 hydrogenation reaction to methanol[J]. Appl Catal B-Environ Energy,2014,152:152−161. [11] BAHRUJI H, BOWKER M, HUTCHINGS G, et al. Pd/ZnO catalysts for direct CO2 hydrogenation to methanol[J]. J Catal,2016,343:133−146. doi: 10.1016/j.jcat.2016.03.017 [12] SHEN C, SUN K, ZHANG Z, et al. Highly active Ir/In2O3 catalysts for selective hydrogenation of CO2 to methanol: experimental and theoretical studies[J]. Acs Catal,2021,11(7):4036−4046. doi: 10.1021/acscatal.0c05628 [13] FANG H, YANG J, WEN M, et al. Nanoalloy materials for chemical catalysis[J]. Adv Mater,2018,30(17):1705698. doi: 10.1002/adma.201705698 [14] STANGELAND K, Li H, Yu Z. CO2 hydrogenation to methanol: the structure–activity relationships of different catalyst systems[J]. Energy Ecol Environ,2020,5:272−285. doi: 10.1007/s40974-020-00156-4 [15] SHA F, HAN Z, TANG S, et al. Hydrogenation of Carbon Dioxide to Methanol over Non− Cu‐based Heterogeneous Catalysts[J]. Chemsuschem,2020,13(23):6160−6181. doi: 10.1002/cssc.202002054 [16] YE J Y, LIU C J, GE Q F. DFT study of CO2 adsorption and hydrogenation on the In2O3 surface[J]. J Phys Chem C,2012,116(14):7817−7825. doi: 10.1021/jp3004773 [17] YE J Y, LIU C J, MEI D H, et al. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3 (110): A DFT Study[J]. Acs Catal,2013,3(6):1296−1306. doi: 10.1021/cs400132a [18] YE J Y, LIU C J, GE Q F. A DFT study of methanol dehydrogenation on the PdIn(110) surface[J]. Phys Chem Chem Phys,2012,14(48):16660−16667. doi: 10.1039/c2cp42183f [19] WANG J, SUN K, JIA X, et al. CO2 hydrogenation to methanol over Rh/In2O3 catalyst[J]. Catal Today,2021,365:341−347. doi: 10.1016/j.cattod.2020.05.020 [20] MARTIN O, MARTIN A J, Mondelli C, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angewandte Chemie,2016,128(21):6369−6373. doi: 10.1002/ange.201600943 [21] RUI N, ZHANG F, SUN K, et al. Hydrogenation of CO2 to Methanol on a Auδ+–In2O3 –x Catalyst[J]. Acs Catal,2020,10(19):11307−11317. doi: 10.1021/acscatal.0c02120 [22] VA DEN BERG T J. First-principles microkinetic modelling of Rh doped In2O3 (111) for CO2 hydrogenation to methanol[J]. 2020. [23] WANG Y, LI S. Computational screening of single-atom doped In2O3 catalysts for the reverse water gas shift reaction[J]. Phys Chem Chem Phys,2024,26(1):381−389. doi: 10.1039/D3CP04352E [24] KRESSE, G, Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6 , 15–50. [25] KRESSE, G; FURTHMULLER, J. Efficient Iterative Schemes for AbInitio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. ReV. B 1996, 54 , 11169−11186. [26] PENG H, PERDREW J P. Rehabilitation of the Perdew-Burke-Ernzerhof generalized gradient approximation for layered materials[J]. Phys Rev B,2017,95(8):081105. doi: 10.1103/PhysRevB.95.081105 [27] SUN K H, FAN Z G, YE J Y, YAN J M, GE Q F, LI Y N, HE W, YANG W J, LIU C J. Hydrogenation of CO2 to methanol over In2O3 catalyst[J]. J CO2 Util,2015,12:1−6. doi: 10.1016/j.jcou.2015.09.002 [28] CHADI D J, COHEN M L. Special points in the Brillouin zone[J]. Phys Rev B,1973,8(12):5747. doi: 10.1103/PhysRevB.8.5747 [29] LAX M, HOPFIELD J J. Selection rules connecting different points in the Brillouin zone[J]. Physcial Review,1961,124(1):115. doi: 10.1103/PhysRev.124.115 [30] 祁萌. Pt掺杂In2O3表面二氧化碳加氢合成甲醇的理论研究[D]. 武汉: 武汉轻工大学, 2023.QI Meng. Theoretical study on the synthesis of methanol by surface suspension hydrogenation of Pt-doped In2O3[D]. Wuhan: Wuhan Polytechnic University, 2023.) [31] 郑晋楠, 安康, 王嘉明, 等. Co/La-Ga-O 复合氧化物用于催化二氧化碳加氢制乙醇[J]. 燃料化学学报,2019,47(6):698−707ZHENG Jinnan, AN kang, Wang Jiaming, et al. Co/La-Ga-O composite oxide is used to catalyze the hydrogenation of carbon dioxide to ethanol[J]. J Fuel Chem Technol,2019,47(6):698−707 [32] DOSTAGIR N H M D, Thompson C, Kobayashi H, et al. Rh promoted In2O3 as a highly active catalyst for CO2 hydrogenation to methanol[J]. Catal Sci Technol,2020,10(24):8196−8202. doi: 10.1039/D0CY01789B [33] 彭必先, 赵翔. 大小选择的金属支撑团簇研究进展[J]. 化学进展,2000,12(3):245. doi: 10.3321/j.issn:1005-281X.2000.03.002PENG Bixian, ZHAO Xiang. Research progress on size-selected metal-supported clusters[J]. Prog Chem,2000,12(3):245. doi: 10.3321/j.issn:1005-281X.2000.03.002 [34] YANG X F, WANG A, QIAO B, et al. Single-atom catalysts: a new frontier in heterogeneous catalysis[J]. Acs. Chem. Res,2013,46(8):1740−1748. doi: 10.1021/ar300361m [35] LANG N D, KOHN W. Theory of metal surfaces: work function[J]. Phys Rev B,1971,3(4):1215. doi: 10.1103/PhysRevB.3.1215 [36] GARG R, DUTTA N K, Roy Choudhury N. Work function engineering of graphene[J]. Nanomaterials,2014,4(2):267−300. doi: 10.3390/nano4020267 [37] CAO A, WANG Z, Li H, et al. Relations between surface oxygen vacancies and activity of methanol formation from CO2 hydrogenation over In2O3 surfaces[J]. Acs Catal,2021,11(3):1780−1786. doi: 10.1021/acscatal.0c05046 [38] 薛继龙, 方镭, 罗伟, 等. Cu-Pt-Au三元合金催化水煤气变换反应的密度泛函研究[J]. 燃料化学学报,2019,47(6):688−696. doi: 10.1016/S1872-5813(19)30030-1XUE Jilong, FANG Lei, LUO Wei, et al. Density functional study of Cu-Pt-Au ternary alloy catalyzed water gas shift reaction[J]. J Fuel Chem Technol,2019,47(6):688−696. doi: 10.1016/S1872-5813(19)30030-1 [39] 赵炳坤, 陈镇, 吴玉龙, 等. 甲氧基在Rh(111)表面吸附的密度泛函研究[J]. 燃料化学学报,2010,38(3):365−369.ZHAO Bingkun, CHEN Zhen, WU Yulong, et al. Density functional study of methoxy group adsorption on Rh(111) surface[J]. J Fuel Chem Technol,2010,38(3):365−369. [40] 厉志鹏, 牛胜利, 赵改菊, 等. Sr掺杂对CaO(100)表面吸附甲醇影响的分子模拟[J]. 燃料化学学报,2020,48(2):172−178. doi: 10.1016/S1872-5813(20)30008-6LI Zhipeng, NIU Shengli, ZHAO Gaiju, et al. Molecular simulation study of strontium doping on the adsorption of methanol on CaO(100) surface[J]. J Fuel Chem Technol,2020,48(2):172−178. doi: 10.1016/S1872-5813(20)30008-6 [41] LIN D, LI S, WEN J, et al. Patterns and driving forces of dimensionality-dependent charge density waves in 2 H-type transition metal dichalcogenides[J]. Nat Commun,2020,11(1):2406. doi: 10.1038/s41467-020-15715-w [42] KASTNER J, SHERWOOD P. Superlinearly converging dimer method for transition state search[J]. J Chem Phys, 2008, 128 (1). [43] HEYDEN A, BELL A T, Keil F J. Efficient methods for finding transition states in chemical reactions: Comparison of improved dimer method and partitioned rational function optimization method[J]. J Chem Phys, 2005, 123 (22). [44] BISHOP C. Exact calculation of the Hessian matrix for the multilayer perceptron[J]. 1992. [45] CAMPBELL C T. The degree of rate control: a powerful tool for catalysis research[J]. Acs Catal,2017,7(4):2770−2779. doi: 10.1021/acscatal.7b00115 [46] JIANG X, WANG X, NIE X, et al. CO2 hydrogenation to methanol on Pd-Cu bimetallic catalysts: H2/CO2 ratio dependence and surface species[J]. Catal Today,2018,316:62−70. doi: 10.1016/j.cattod.2018.02.055 [47] PECHUKAS P. Transition state theory[J]. Annu Rev Phys Chem,1981,32(1):159−177. doi: 10.1146/annurev.pc.32.100181.001111 [48] WANG X, PAN J, WEI H, et al. Mechanism of Methanol Synthesis from CO2 Hydrogenation over Pt8/In2O3 Catalysts: A Combined Study on Density Functional Theory and Microkinetic Modeling[J]. J Chem Phys,2022,126(4):1761−1769. [49] 窦茂斌. In2O3 表面状态及 CO2 催化加氢制甲醇机理的模拟研究[D]. 天津: 天津大学, 2020.DOU Maobin. Simulation study on the surface state of In2O3 and the mechanism of CO2 catalytic hydrogenation to methanol [D]. Tianjin: Tianjin University, 2020.) [50] FREI M S, CAPDEVILA-CORTADE M, GARCIA-MUELAS R, et al. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide[J]. J Catal,2018,361:313−321. doi: 10.1016/j.jcat.2018.03.014 [51] YE J, LIU C, MEI D, et al. Methanol synthesis from CO2 hydrogenation over a Pd4/In2O3 model catalyst: A combined DFT and kinetic study[J]. J Catal,2014,317:44−53. doi: 10.1016/j.jcat.2014.06.002 [52] KARELOVIC A, RUIZ P. Mechanistic study of low temperature CO2 methanation over Rh/TiO2 catalysts[J]. J Catal,2013,301:141−153 doi: 10.1016/j.jcat.2013.02.009 -





 下载:
下载: