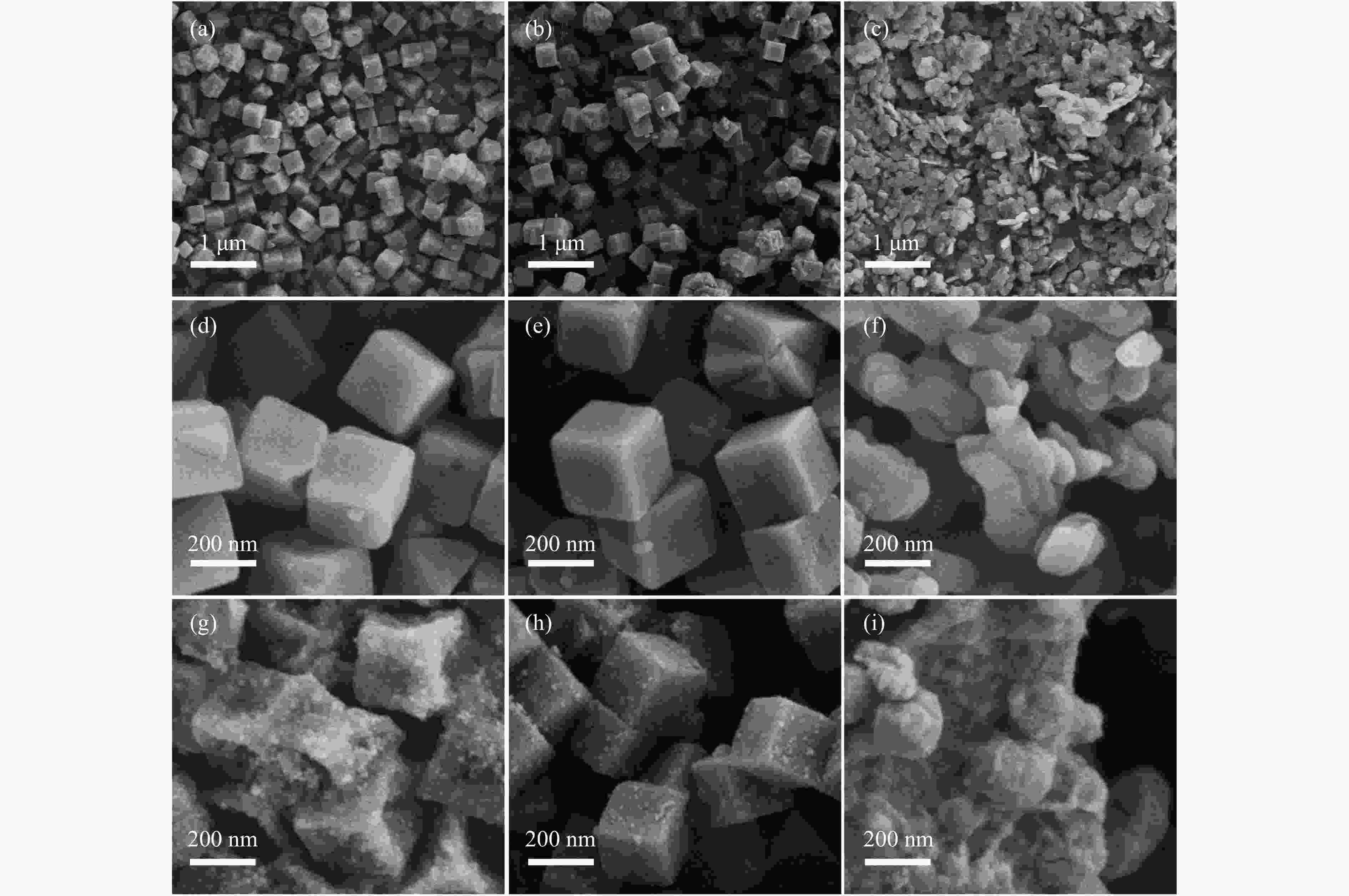
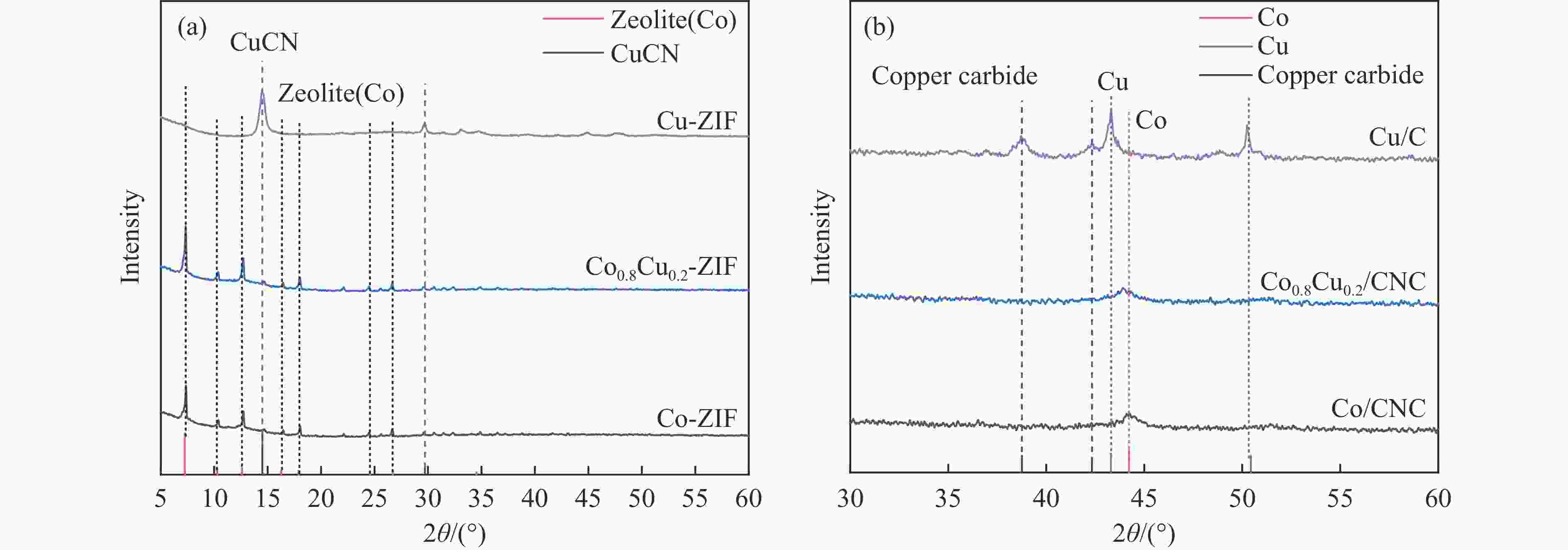
The effects of calcination temperature and dispersants on the catalytic performance of Co0.8Cu0.2/CNC for the hydrolysis of ammonia borane to hydrogen were investigated
-
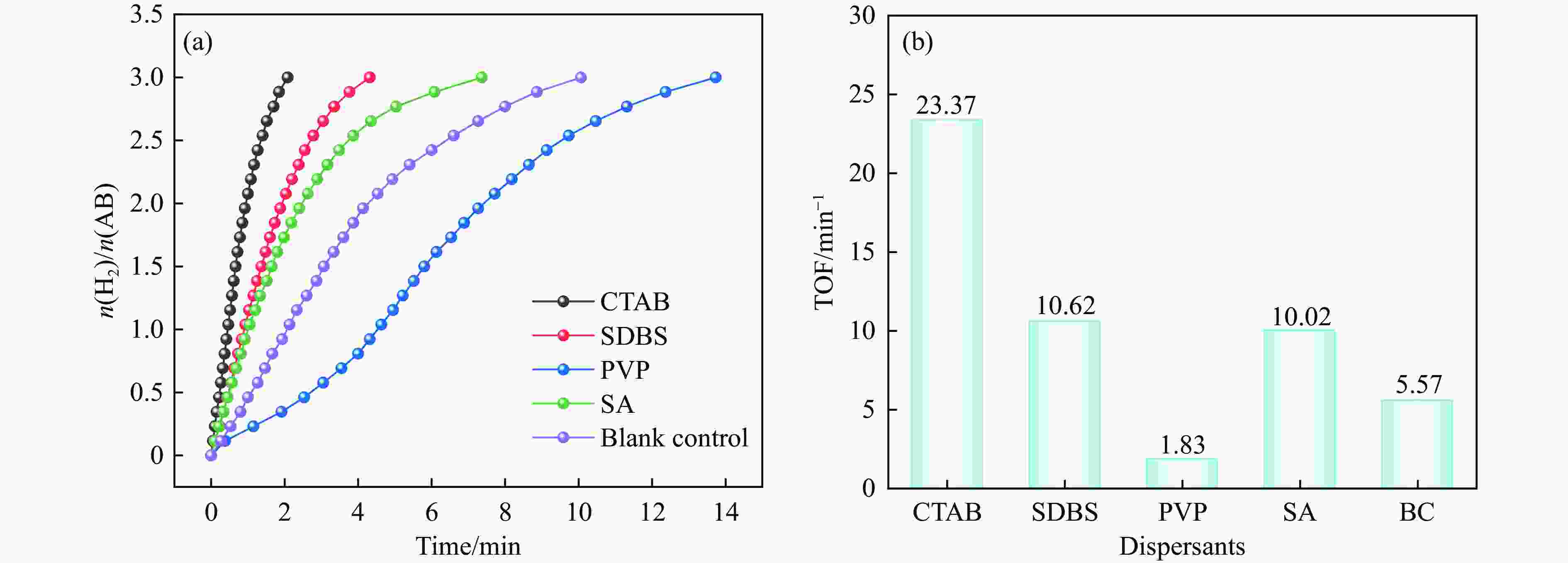
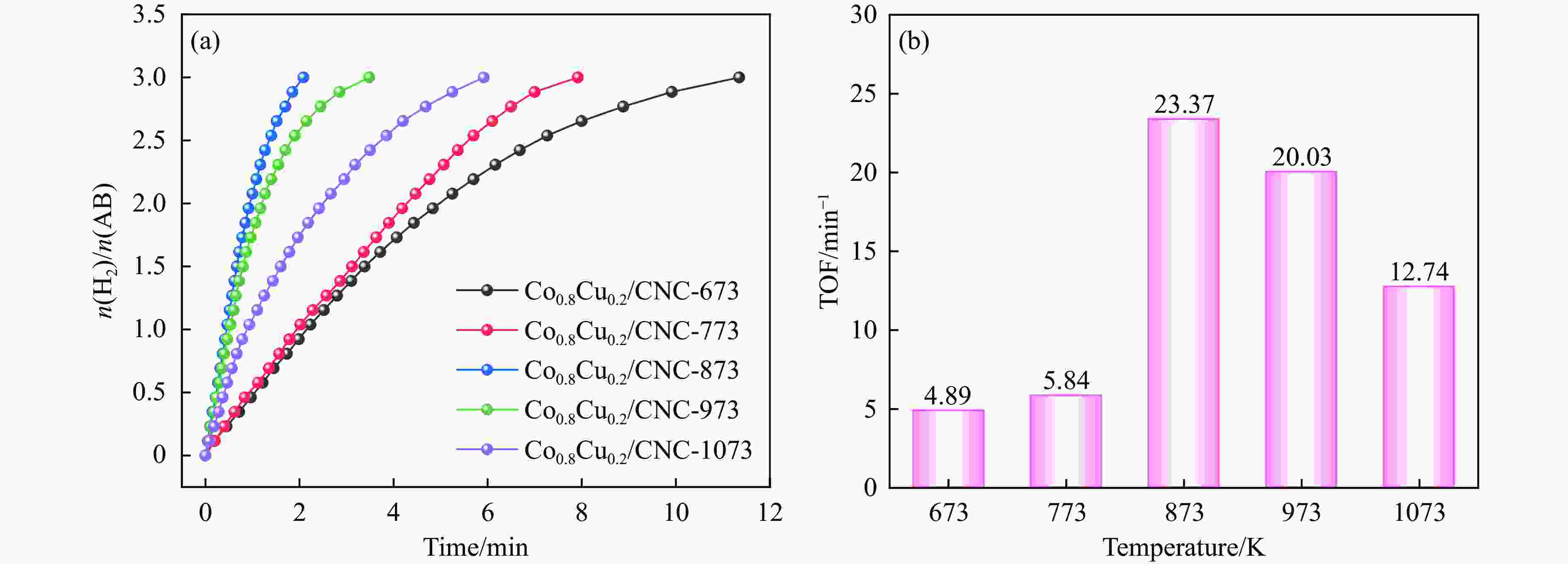
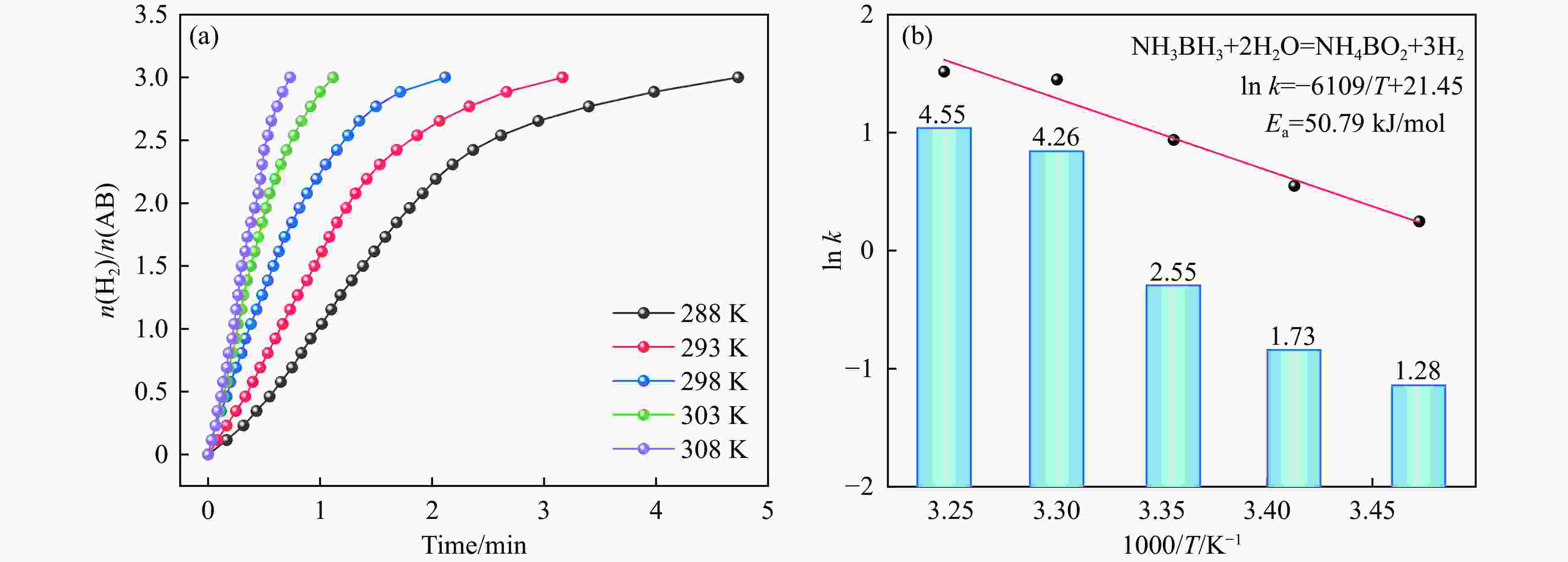
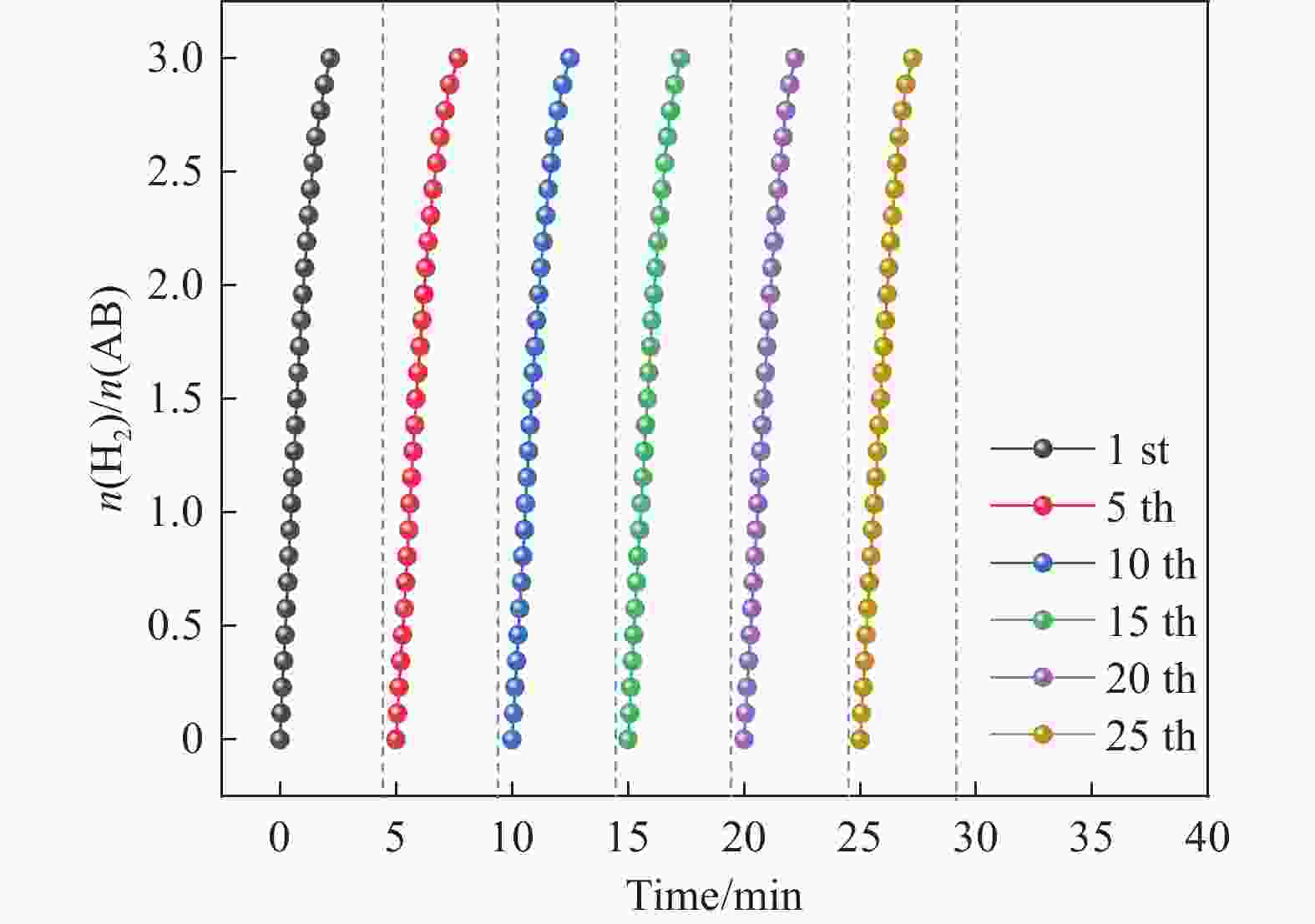
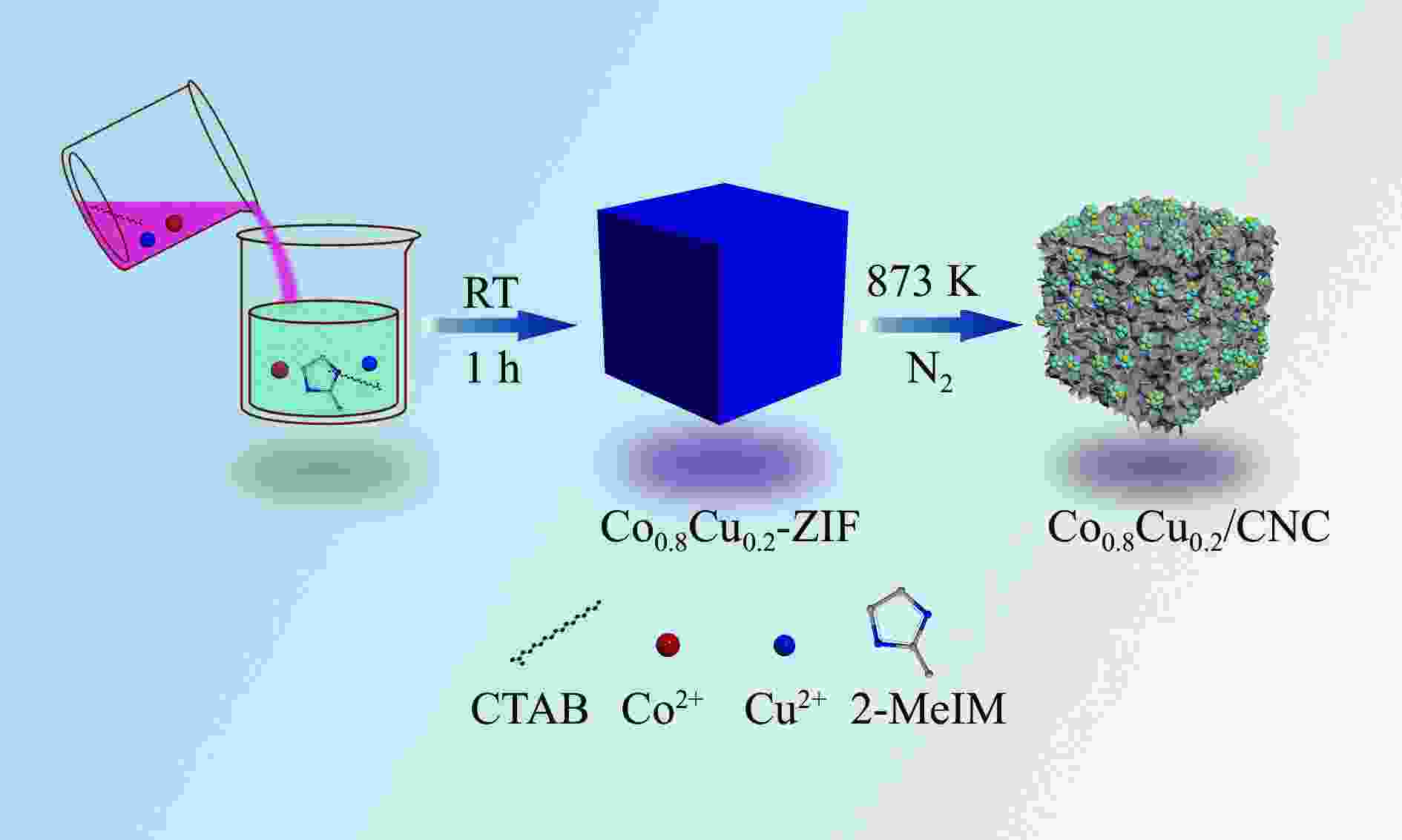
摘要: 氨硼烷(NH3BH3,AB)被认为是一种便携式制氢的理想储氢材料。本工作在常温下搅拌反应物得到Co0.8Cu0.2-ZIF前驱体,并高温焙烧此前驱体制备了一种双金属碳立方体(Co0.8Cu0.2/CNC)催化剂。此外,采用多种表征方法对催化剂的微观结构以及组成成分进行了研究。通过单一变量法探究了催化剂的变化规律。结果表明,少量Cu的加入会对Co0.8Cu0.2/CNC催化剂的立方体形貌有一定的稳固作用。当分散剂为CTAB且焙烧温度为873 K时,其催化AB水解制氢的活化能(Ea)为50.79 kJ/mol,转化频率(TOF)值高达23.37 min−1。此外,该催化剂经过25次循环后仍然可以催化AB完全水解制氢,表明该催化剂的稳定性能良好。Abstract: Ammonia borane (NH3BH3, AB) is considered as an ideal hydrogen storage material for portable hydrogen production. In this paper, Co0.8Cu0.2-ZIF precursor was obtained by stirring the reactants at room temperature, and a bimetallic carbon cube (Co0.8Cu0.2/CNC) catalyst was prepared by roasting this precursor at high temperature. In addition, the microstructure as well as the composition of the catalyst was investigated using various characterization methods. The change rule of the catalyst was explored by the single-variable method. The results showed that the addition of a small amount of Cu had a stabilizing effect on the cubic morphology of the Co0.8Cu0.2/CNC catalyst. When the dispersant was CTAB and the roasting temperature was 873 K, the activation energy (Ea) for catalyzing the hydrolysis of AB to hydrogen was 50.79 kJ/mol, and the transition frequency (TOF) value was as high as 23.37 min−1. In addition, the catalyst could still catalyze the complete hydrolysis of AB to hydrogen after 25 cycles, which indicated that the catalyst had good stability performance.
-
Key words:
- Bimetallic carbon cube /
- ammonia borane /
- hydrogen hydrolysis
-
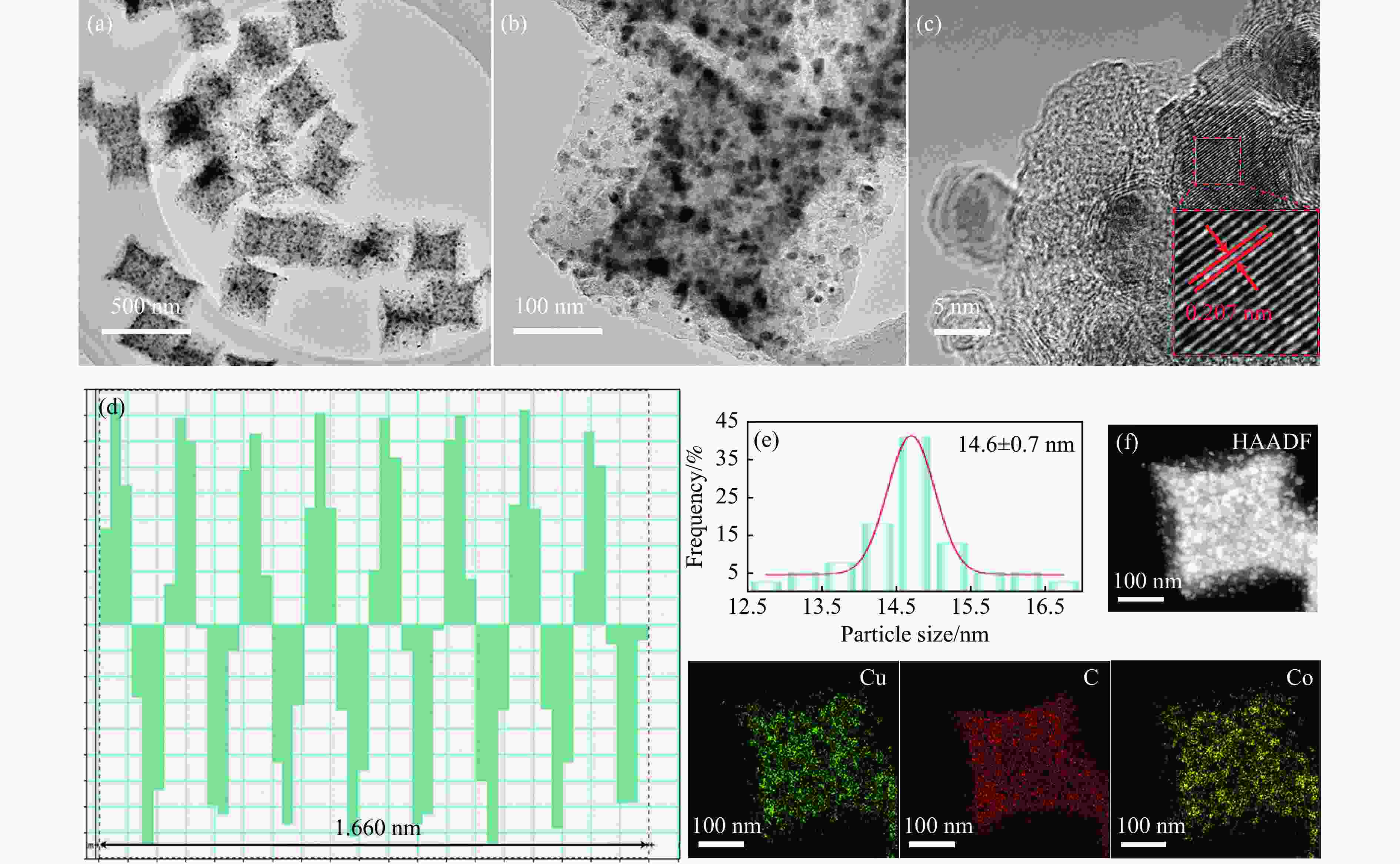
图 3 (a)−(c)Co0.8Cu0.2/CNC不同放大倍率的TEM图像;(d)晶格条纹计算;(e)粒径分布;(f)Co0.8Cu0.2/CNC中Cu、C和Co的相应元素映射
Figure 3 (a)−(c) TEM image of Co0.8Cu0.2/CNC with different magnification; (d) calculation image of lattice fringe; (e) particle size distribution of Co0.8Cu0.2/CNC; (f) the corresponding elemental mapping of Cu, C and Co
表 1 Co/CNC、Co0.8Cu0.2/CNC和Cu/C催化剂的ICP-AES测试
Table 1 ICP-AES results of Co/CNC、Co0.8Cu0.2/CNC and Cu/C catalysts
Catalyst Co w/% Cu w/% Co-Cu initaial composition
(molor ratio)Cu-Co final composition
(molor ratio)Final metals/catalyst
(mmol·100 mg−1)Co/CNC 20.90 — — — 0.354 Co0.8Cu0.2/CNC 18.50 4.36 80∶20 82∶18 0.382 Cu/C 49.73 — — — 0.782 -
[1] XU X, ZHOU Q, YU D. The future of hydrogen energy: Bio-hydrogen production technology[J]. Int J Hydrogen Energy,2022,47(79):33677−33698. doi: 10.1016/j.ijhydene.2022.07.261 [2] 张唯怡, 张议洁, 王进伟, 等. 电解水制氢技术及大电流析氧反应研究与展望[J]. 工程科学学报,2023,45(7):1057−1070.ZHANG Weiyi, ZHANG Yijie, WANG Jinwei, et al. Research and perspectives on electrocatalytic water splitting and large current density oxygen evolution reaction[J]. Chin J Eng,2023,45(7):1057−1070. [3] SCHEFFE J R, HAUSSENER S, PATZKE G R. Solar hydrogen production[J]. Energy Technol,2022,10:2101021. doi: 10.1002/ente.202101021 [4] 吴慧, 郑君宁, 左佑华, 等. NiPd/TiO2催化剂的制备及催化甲酸分解制氢[J]. 精细化工, 2023.WU Hui, ZHENG Junning, ZUO Youhua, et al. Preparation of NiPd/TiO2 catalysts and catalytic hydrogen production from formic acid decomposition[J]. Fine Chem, 2023.) [5] PENG Z, WANG Y, YIN C, et al. Uniform dispersion of ultrafine ruthenium nanoparticles on nano-cube ceria as efficient catalysts for hydrogen production from ammonia-borane hydrolysis[J]. Sustain Energy Fuels,2023,7(3):821−831. doi: 10.1039/D2SE01542K [6] 韦秋红, 褚海亮, 夏永鹏, 等. 氨硼烷水解制氢催化剂的研究进展[J]. 材料导报,2023,37(17):20−31.WEI Qiuhong, CHU Hailiang, XIA Yongpeng, et al. Progress in catalysts for hydrolytic dehydrogenation of ammonia borane[J]. Mater Rep,2023,37(17):20−31. [7] WAN C, LI G, WANG J, et al. Modulating electronic metal-support interactions to boost visible-light-driven hydrolysis of ammonia borane: Nickel-Platinum nanoparticles supported on phosphorus-doped titania[J]. Angew Chem Int Ed,2023,62(40):e202305371. doi: 10.1002/anie.202305371 [8] WEI Q, LIU J, QIU S, et al. Boosting hydrogen evolution from ammonia-borane hydrolysis catalyzed by poly(N-Vinyl-2-Pyrrolidone)-stabilized ruthenium-based nanoclusters catalysts[J]. Adv Sust Syst,2023,7(4):2200464. doi: 10.1002/adsu.202200464 [9] AKBAYRAK S, ÖZKAR S. Ammonia borane as hydrogen storage materials[J]. Int J Hydrogen Energy,2018,43(40):18592−18606. doi: 10.1016/j.ijhydene.2018.02.190 [10] 王小燕, 张若凡, 司航, 等. 椰壳炭负载钌催化剂的制备及其催化氨硼烷水解制氢性能[J]. 石油炼制与化工,2023,54(7):64−70.WANG Xiaoyan, ZHANG Ruofan, SI Hang, et al. Preparation of coconut shell charcoal-loaded ruthenium catalysts and their catalytic performance for hydrogen production from hydrolysis of ammonia borane[J]. Pet Process Petrochem,2023,54(7):64−70. [11] WEI Q, QIU S, YIN C, et al. Nitrogen-doped carbon encapsulated Ru-decorated Co2P supported on graphene oxide as efficient catalysts for hydrogen generation from ammonia borane[J]. J Alloys Compd,2022,921:166207. doi: 10.1016/j.jallcom.2022.166207 [12] AYDIN D S, FILIZ B C, FIGEN A K. Confined ammonia borane nanocarriers: Tubular and fibrous structures based solid-state hydrogen storage composites[J]. Int J Hydrogen Energy, 2024. [13] 曹云钟, 郑君宁, 吴慧, 等. Pt基催化剂催化氨硼烷水解产氢的研究进展[J]. 稀有金属,2023,47(8):1122−1131.CAO Yunzhong, ZHENG Junning, WU Hui, et al. Advances in hydrogen production by ammonia borane hydrolysis over Pt-based catalysts[J]. Rare Met,2023,47(8):1122−1131. [14] CUI B, WU G, QIU S, et al. Ruthenium supported on cobalt-embedded porous carbon with hollow structure as efficient catalysts toward ammonia-borane hydrolysis for hydrogen production[J]. Adv Sust Syst,2021,5(10):2100209. doi: 10.1002/adsu.202100209 [15] 邱小魁, 张若凡, 王小燕, 等. 竹茹丝炭负载钌催化剂光催化氨硼烷水解产氢研究[J]. 无机盐工业,2023,55(10):153−158.QIU Xiaokui, ZHANG Ruofan, WANG Xiaoyan, et al. Hydrogen production by photocatalytic hydrolysis of ammonia borane over bamboo rhizoma pinelliae silk charcoal loaded ruthenium catalysts[J]. Inorg Chem Ind,2023,55(10):153−158. [16] 李贵, 梁雨, 郑君宁, 等. Rh/N-GMCs纳米催化剂的制备及其催化氨硼烷水解产氢性能研究[J]. 燃料化学学报,2023,51(4):528−537.LI Gui, LIANG Yu, ZHENG Junning, et al. Preparation of Rh/N-GMCs nanocatalyst and its catalytic performance for the hydrolytic dehydrogenation of ammonia borane[J]. J Fuel Chem Technol,2023,51(4):528−537. [17] KHALILY M A, EREN H, AKBAYRAK S, et al. Facile Synthesis of three-dimensional Pt-TiO2 nano-networks: a highly active catalyst for the hydrolytic dehydrogenation of ammonia borane[J]. Angew Chem,2016,128(40):12445−12449. doi: 10.1002/ange.201605577 [18] FU F, WANG C, WANG Q, et al. Highly selective and sharp volcano-type synergistic Ni2Pt@ZIF-8-catalyzed hydrogen evolution from ammonia borane hydrolysis[J]. J Am Chem Soc,2018,140(31):10034−10042. doi: 10.1021/jacs.8b06511 [19] ZHANG J, CHEN W, GE H, et al. Synergistic effects in atomic-layer-deposited PtCox/CNTs catalysts enhancing hydrolytic dehydrogenation of ammonia borane[J]. Appl Catal B Environ,2018,235:256−263. doi: 10.1016/j.apcatb.2018.04.070 [20] REJ S, MASCARETTI L, SANTIAGO E Y, et al. Determining plasmonic hot electrons and photothermal effects during H2 evolution with TiN-Pt nanohybrids[J]. ACS Catal,2020,10(9):5261−5271. doi: 10.1021/acscatal.0c00343 [21] ZHANG J, ZHENG X, YU W, et al. Unravelling the synergy in platinum-nickel bimetal catalysts designed by atomic layer deposition for efficient hydrolytic dehydrogenation of ammonia borane[J]. Appl Catal B: Environ,2022,306:121116. doi: 10.1016/j.apcatb.2022.121116 [22] TONG F, LIANG X, WANG Z, et al. Probing the mechanism of plasmon-enhanced ammonia borane methanolysis on a CuAg alloy at a single-particle level[J]. ACS Catal,2021,11(17):10814−10823. doi: 10.1021/acscatal.1c02857 [23] KAHRI H, SEVIM M, METIN Ö. Enhanced catalytic activity of monodispersed AgPd alloy nanoparticles assembled on mesoporous graphitic carbon nitride for the hydrolytic dehydrogenation of ammonia borane under sunlight[J]. Nano Res,2017,10:1627−1640. doi: 10.1007/s12274-016-1345-x [24] REJ S, HSIA C F, CHEN T Y, et al. Facet-dependent and light-assisted efficient hydrogen evolution from ammonia borane using gold-palladium core-shell nanocatalysts[J]. Angew Chem Int Ed,2016,55(25):7222−7226. doi: 10.1002/anie.201603021 [25] GONG Y, ZHONG H, LIU W, et al. General synthetic route toward highly dispersed ultrafine Pd-Au alloy nanoparticles enabled by imidazolium-based organic polymers[J]. ACS Appl Mater Interfaces,2018,10(1):776−786. doi: 10.1021/acsami.7b16794 [26] HAN C, MENG P, WACLAWIK E R, et al. Palladium/Graphitic carbon nitride (g-C3N4) stabilized emulsion microreactor as a store for hydrogen from ammonia borane for use in alkene hydrogenation[J]. Angew Chem Int Ed,2018,57(45):14857−14861. doi: 10.1002/anie.201809882 [27] MANNA J, AKBAYRAK S, ÖZKÂR S. Palladium (0) nanoparticles supported on polydopamine coated CoFe2O4 as highly active, magnetically isolable and reusable catalyst for hydrogen generation from the hydrolysis of ammonia borane[J]. Appl Catal B: Environ,2017,208:104−115. doi: 10.1016/j.apcatb.2017.02.037 [28] WANG L, LI H, ZHANG W, et al. Supported rhodium catalysts for ammonia borane hydrolysis: dependence of the catalytic activity on the highest occupied state of the single rhodium atoms[J]. Angew Chem Int Ed,2017,56(17):4712−4718. doi: 10.1002/anie.201701089 [29] ZOU H, JIN B, WANG R, et al. Iodide-mediated templating synthesis of highly porous rhodium nanospheres for enhanced dehydrogenation of ammonia borane[J]. J Mater Chem A,2018,6(47):24166−24174. doi: 10.1039/C8TA09077G [30] LI W, ZHAO Y, LIU Y, et al. Exploiting Ru-induced lattice strain in CoRu nanoalloys for robust bifunctional hydrogen production[J]. Angew Chem Int Ed,2021,60(6):3290−3298. doi: 10.1002/anie.202013985 [31] WANG Y, LI J, SHI W, et al. Unveiling single atom nucleation for isolating ultrafine fcc Ru nanoclusters with outstanding dehydrogenation activity[J]. Adv Energy Mater,2020,10(43):2002138. doi: 10.1002/aenm.202002138 [32] FANG Y, LI J, TOGO T, et al. Ultra-small face-centered-cubic Ru nanoparticles confined within a porous coordination cage for dehydrogenation[J]. Chem,2018,4(3):555−563. doi: 10.1016/j.chempr.2018.01.004 [33] CAI J, DING J, WEI D, et al. Coupling of Ru and O-vacancy on 2D Mo-based electrocatalyst via a solid-phase interface reaction strategy for hydrogen evolution reaction[J]. Adv Energy Mater,2021,11(26):2100141. doi: 10.1002/aenm.202100141 [34] SHEN R, LIU Y, WEN H, et al. Engineering VO-Ti ensemble to boost the activity of Ru towards water dissociation for catalytic hydrogen generation[J]. Appl Catal B Environ,2022,306:121100. doi: 10.1016/j.apcatb.2022.121100 [35] ZHU R, CAI M, FU T, et al. Fe-Based metal organic frameworks (Fe-MOFs) for bio-related applications[J]. Pharmaceutics,2023,15(6):1599. doi: 10.3390/pharmaceutics15061599 [36] HAN Y, WANG F, ZHANG J. Design and syntheses of hybrid zeolitic imidazolate frameworks[J]. Coord Chem Rev,2022,471:214759. doi: 10.1016/j.ccr.2022.214759 [37] ZHANG J, TAN Y, SONG W-J. Zeolitic imidazolate frameworks for use in electrochemical and optical chemical sensing and biosensing: a review[J]. Microchim Acta,2020,187:234. doi: 10.1007/s00604-020-4173-3 [38] LI X, LIANG H, LIU X, et al. Zeolite imidazolate frameworks (ZIFs) derived nanomaterials and their hybrids for advanced secondary batteries and electrocatalysis[J]. Chem Rec,2022,22:e202200105. doi: 10.1002/tcr.202200105 [39] OH S, LEE S, OH M. Zeolitic Imidazolate framework-based composite incorporated with well-dispersed CoNi nanoparticles for efficient catalytic reduction reaction[J]. ACS Appl Mater Interfaces,2020,12:18625−18633. doi: 10.1021/acsami.0c03756 [40] CHEN J, LONG B, HU H, et al. Synthesis of a novel Co-B/CuNWs/CTAB catalyst via chemical reaction at room temperature for hydrolysis of ammonia borane[J]. Int J Hydrogen Energy,2022,47(5):2976−2991. doi: 10.1016/j.ijhydene.2021.10.255 [41] WANG J, LIU C, FENG J, et al. MOFs derived Co/Cu bimetallic nanoparticles embedded in graphitized carbon nanocubes as efficient Fenton catalysts[J]. J Hazard Mater,2020,394:122567. doi: 10.1016/j.jhazmat.2020.122567 [42] GAO C, WANG H, LI S, et al. Enhanced cobalt-based catalysts through alloying ruthenium to cobalt lattice matrix as an efficient catalyst for overall water splitting[J]. Electrochim Acta,2019,327:134958. doi: 10.1016/j.electacta.2019.134958 [43] WU Y, HUANG Y, DAI X, et al. Alcohol amination catalyzed by copper powder as a self-supported catalyst[J]. ChemSusChem,2019,12(13):3185−3191. doi: 10.1002/cssc.201801877 [44] WAN C, LIANG Y, ZHOU L, et al. Integration of morphology and electronic structure modulation on cobalt phosphide nanosheets to boost photocatalytic hydrogen evolution from ammonia borane hydrolysis[J]. Green Energy Environ, 2022. [45] 吴慧, 郑君宁, 李蓉, 等. NiPt/CeO2催化剂的制备及其催化水合肼分解制氢性能研究[J]. 中国稀土学报, 2023.WU Hui, ZHENG Junning, LI Rong, et al. Preparation of NiPt/CeO2 catalyst and its catalytic performance for hydrogen production from hydrazine hydrate decomposition[J]. J Chin Soc Rare Earths, 2023.) -




 下载:
下载: