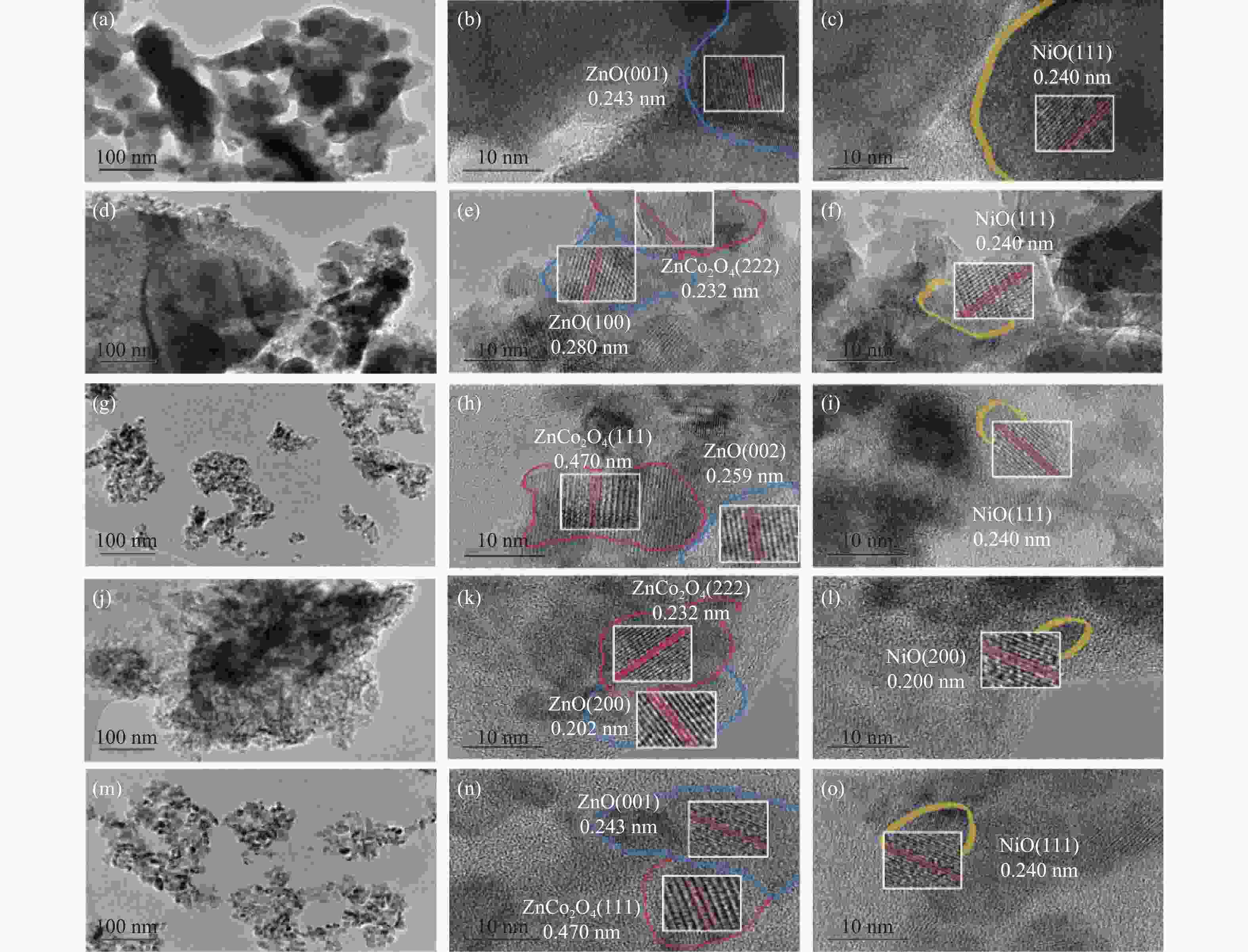
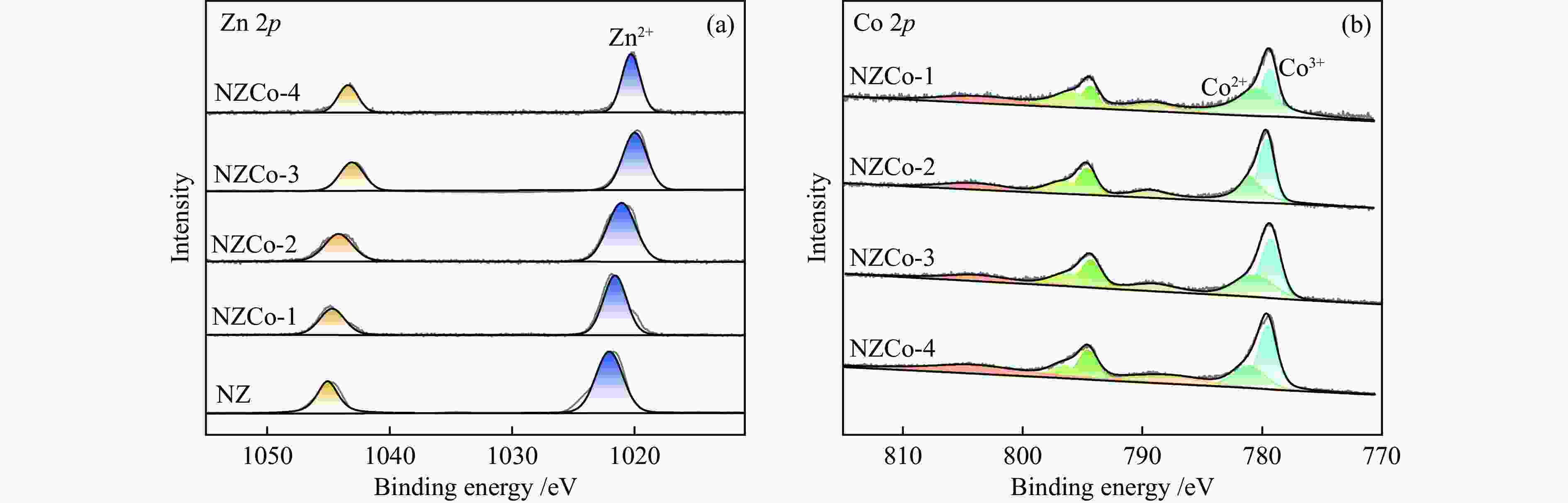
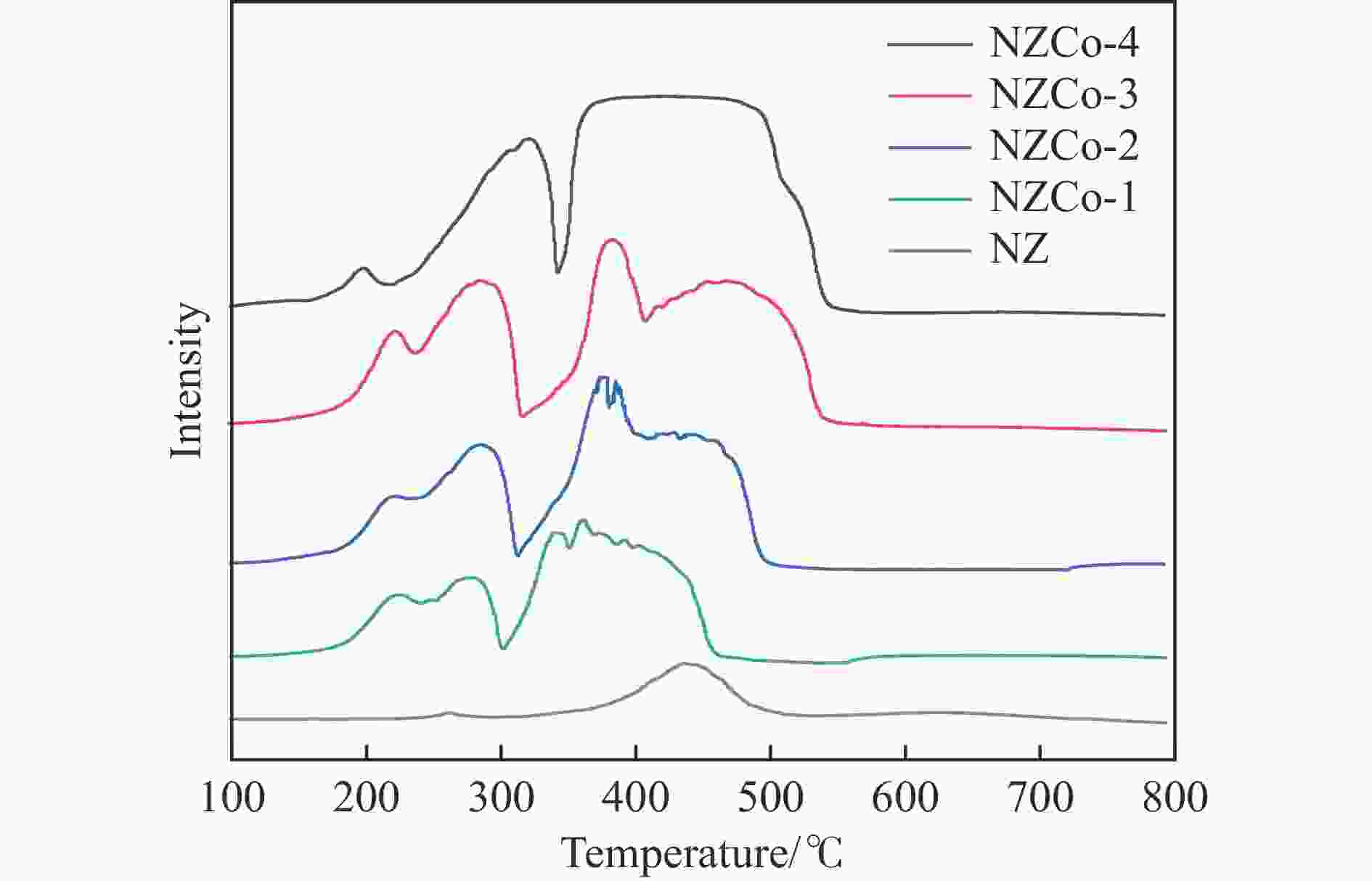
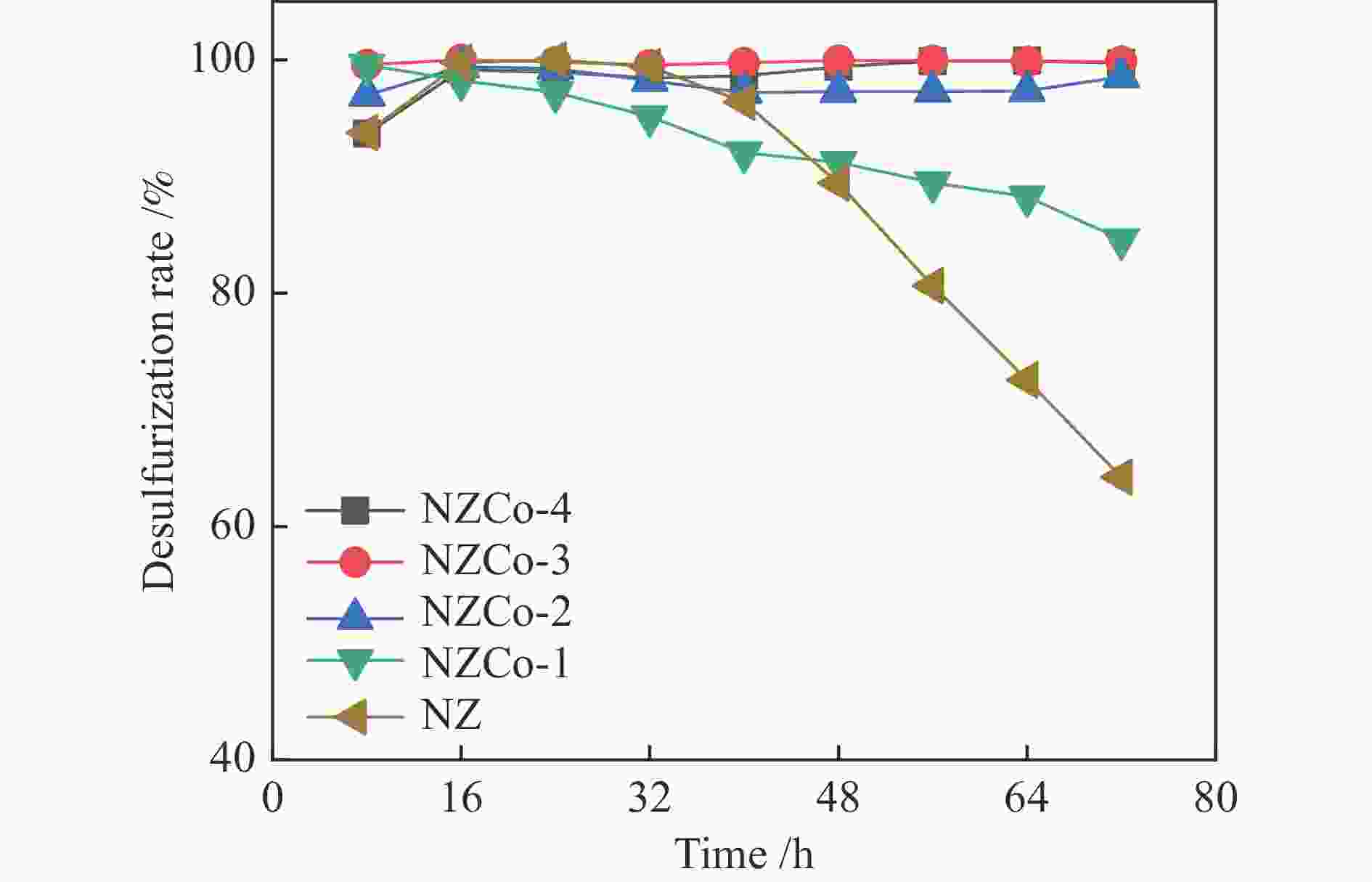
Construction of Ni/ZnCo2O4@ZnO composite metal oxide desulfurization agent and its reactive adsorption desulfurization-regeneration properties
-
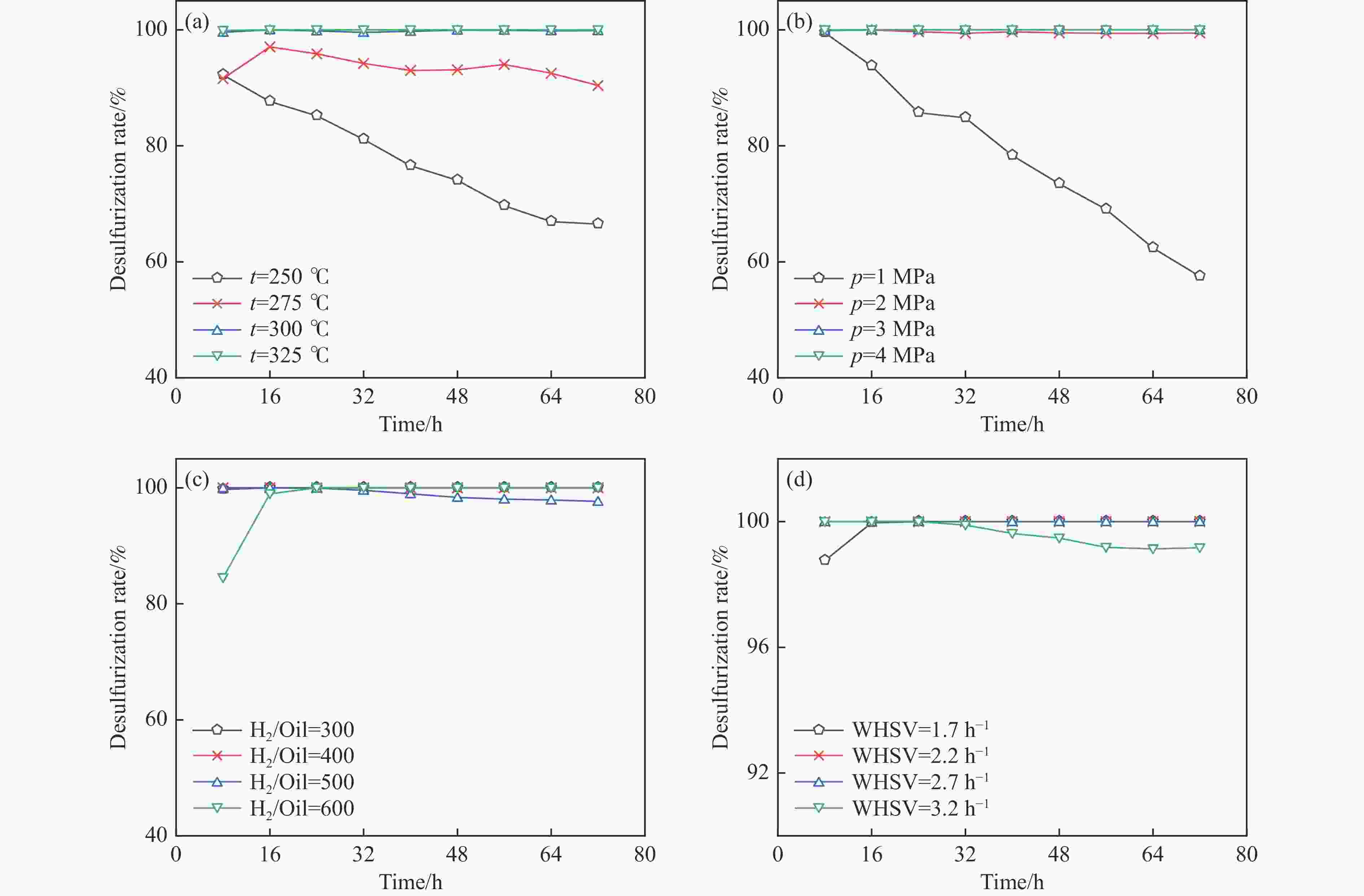
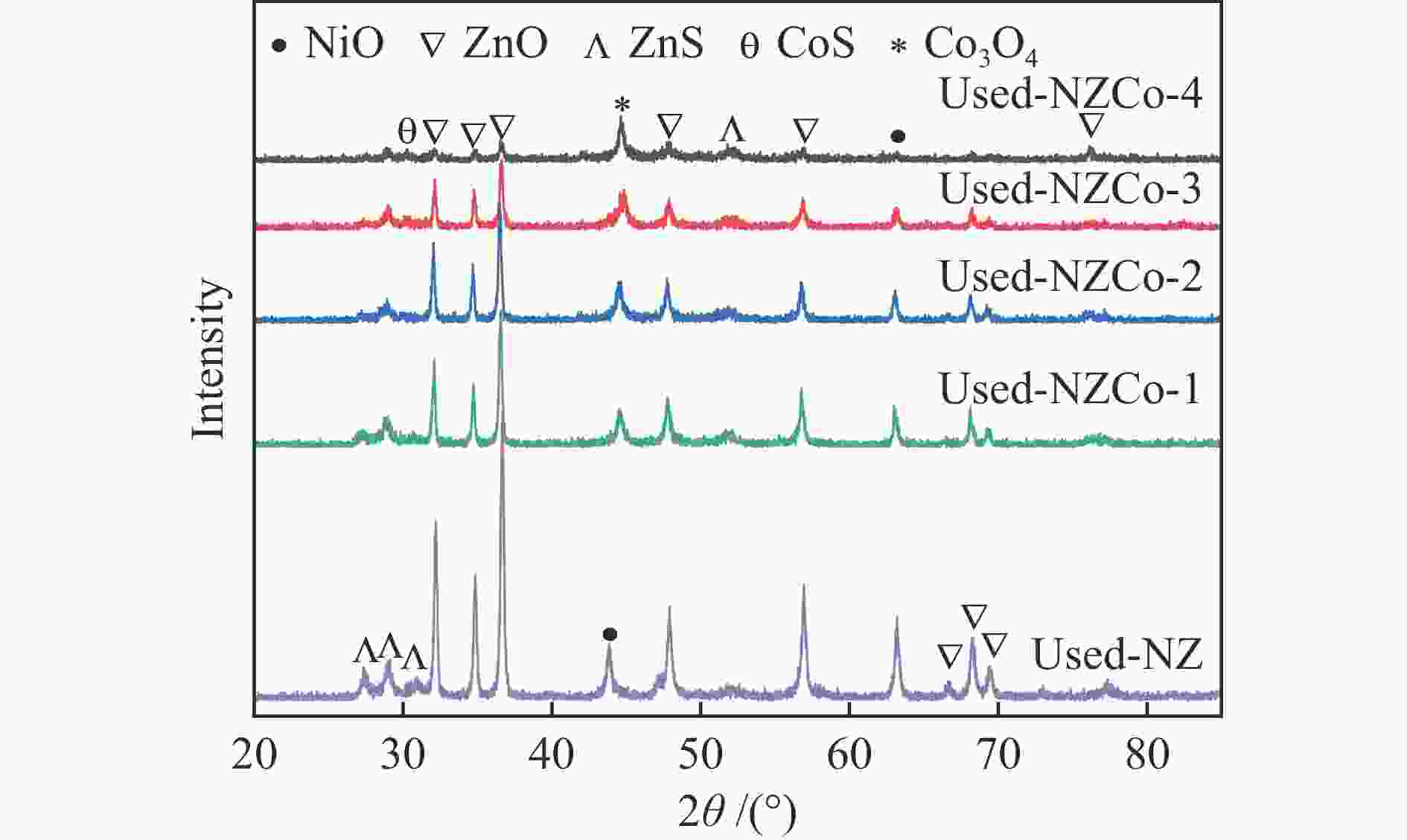
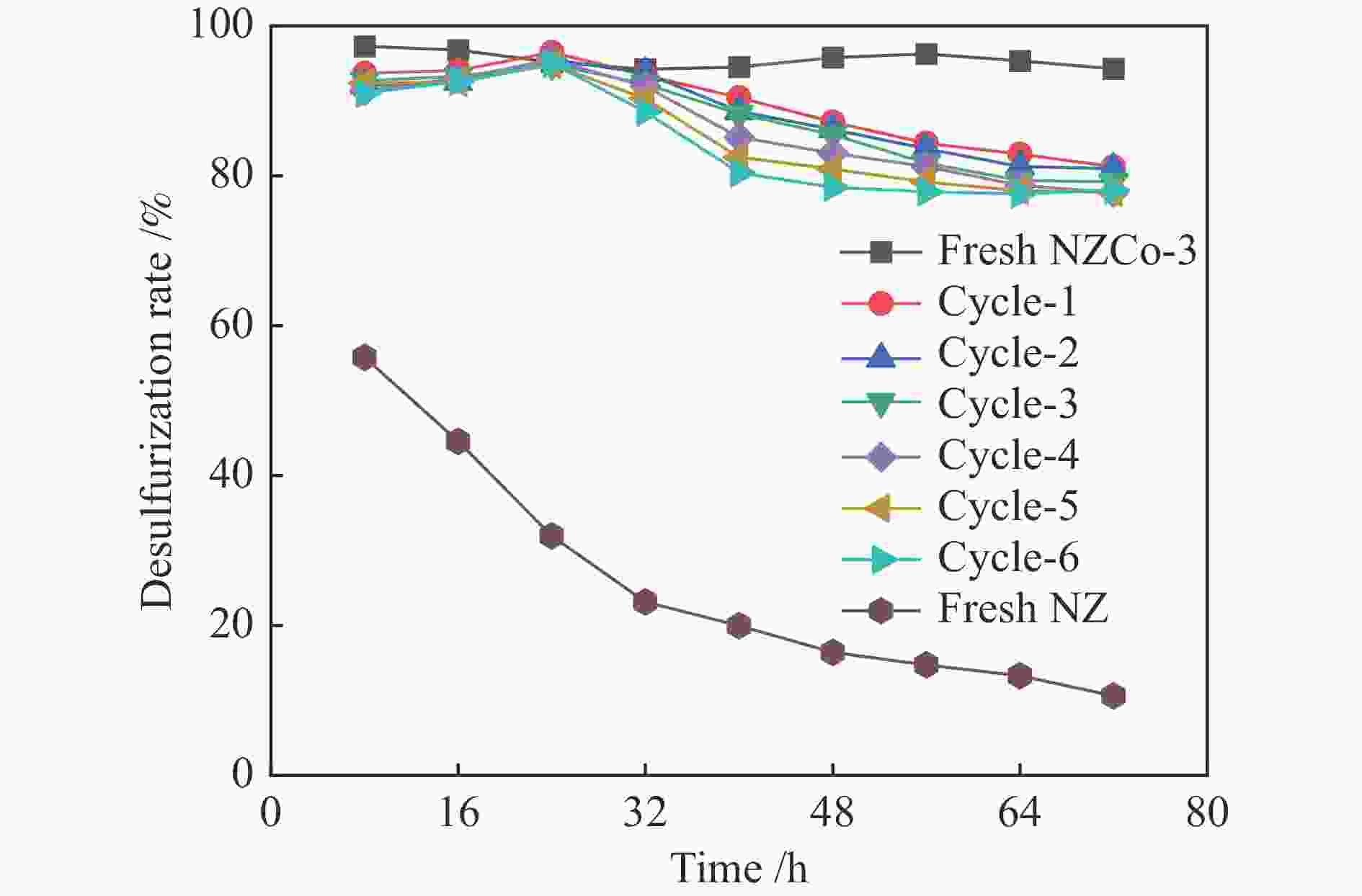
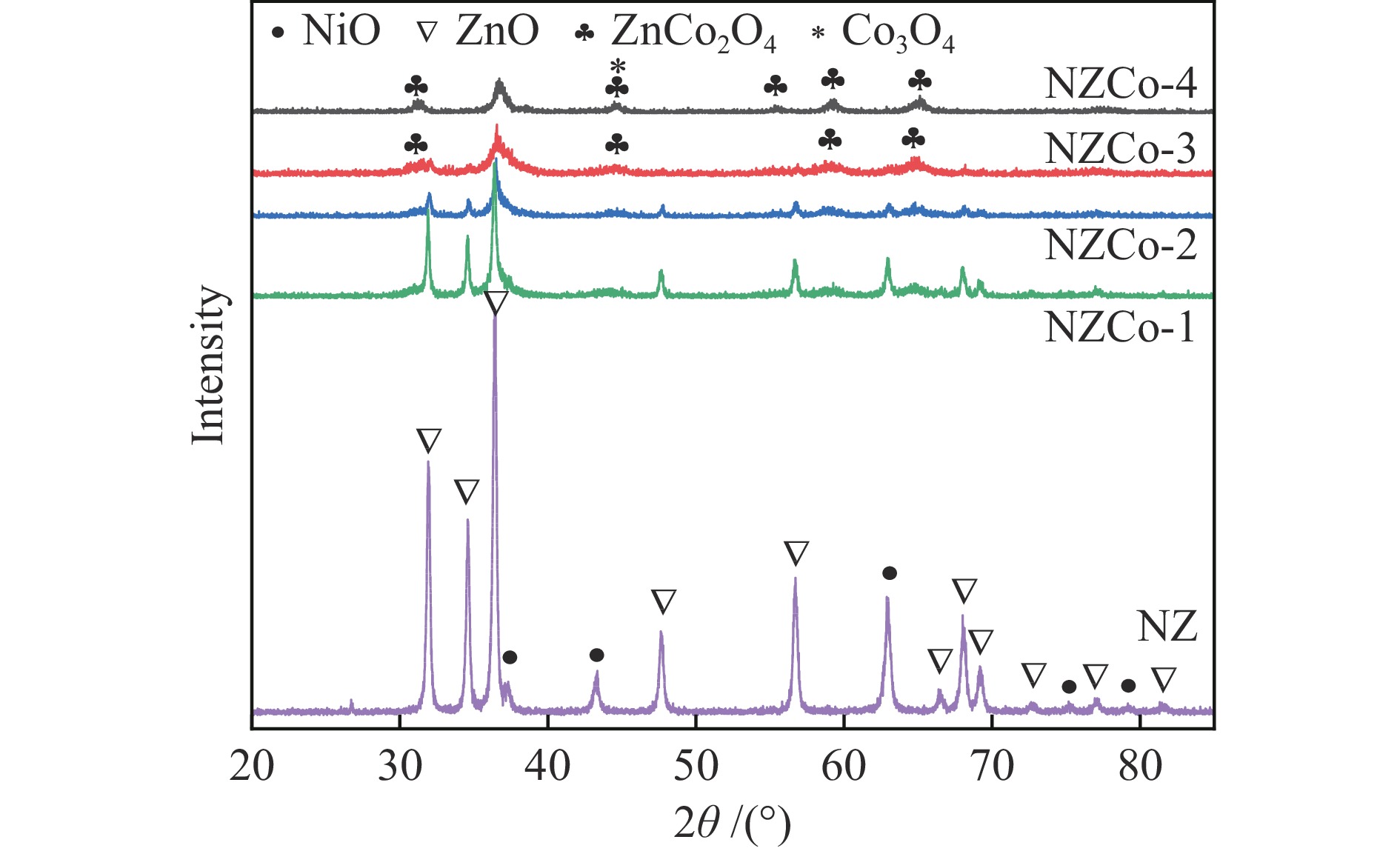
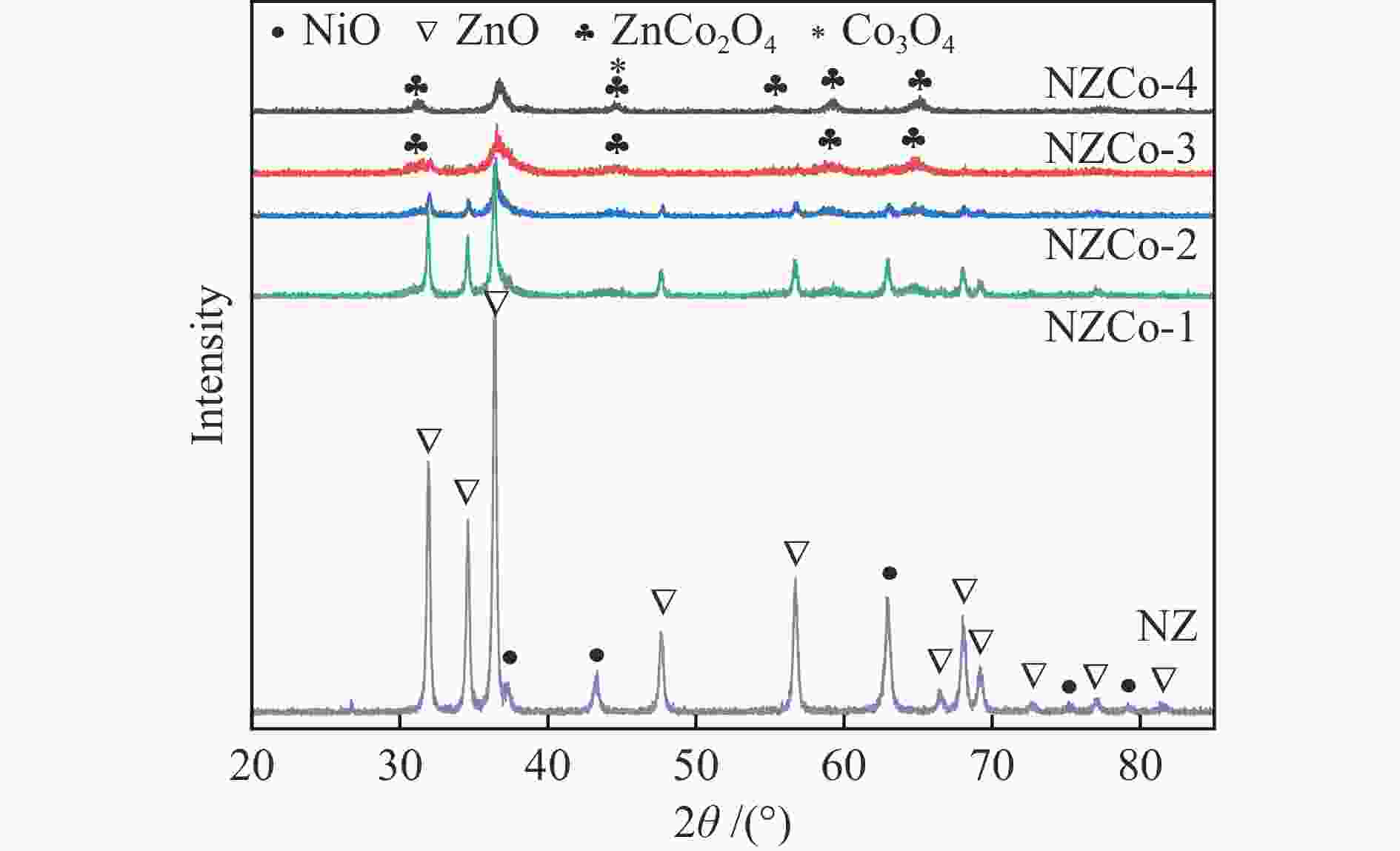
摘要: 采用共沉淀法在ZnO中引入金属Co并使其形成复合金属氧化物,通过共沉淀-浸渍法构筑了不同Co含量的复合金属氧化物脱硫剂,考察其脱硫活性和再生性能。采用XRD、TEM、N2低温吸附-脱附、XPS和H2-TPR等对脱硫剂的结构和性质进行系统表征,证实得到了Ni/ZnCo2O4@ZnO结构的复合金属氧化物脱硫剂。复合金属氧化物脱硫剂中ZnCo2O4的形成有利于脱硫剂的颗粒尺寸减小、分散度提升、比表面积增加。反应后XRD显示,ZnCo2O4也可作为H2S的吸附剂,从而提高了脱硫剂的硫吸附容量。所有的复合金属氧化物脱硫剂的脱硫性能显著优于Ni/ZnO,其中,Zn∶Co物质的量比为1∶1的脱硫剂NZCo-3具有最优的脱硫性能,该脱硫剂在反应温度300 ℃,氢压3 MPa,质量空速2.2 h−1,氢油体积比300的条件下脱硫率为100%,且经过六次循环后仍能够保持优异的脱硫性能。该研究结果为合理设计Ni/ZnO脱硫剂以提高其脱硫性能和再生性能提供新的思路。
-
关键词:
- 反应吸附脱硫 /
- ZnCo2O4复合金属氧化物 /
- Ni/ZnO /
- 脱硫剂再生
Abstract: SOx released from the combustion of sulfur compounds in fuel oil has long been a serious environmental hazard, and there is an urgent need to limit the suifur content in gasoline to about 10×10−6 by using desuifurization technology to protect the environment. Reactive adsorption desuifurization (RADS) combines the advantages of hydrodesuifurization (HDS) and adsorption desuifurization (ADS), in which Ni/ZnO desulfurization agent has excellent RADS performance. Although Ni/ZnO desulfurization agent has been applied in large scale in industry, it still has the problems of insufficient desulfurization depth and poor regeneration performance. In this paper, metal Co was introduced into ZnO by co-precipitation-impregnation method to form composite metal oxides, and the composite metal oxide desulfurization agent with different Co contents was constructed, and its desulfurization activity and regeneration performance were investigated. The results of the desulfurization experiments show that the desulfurization performance of NZCo-x desulfurization agent after the introduction of metal Co is much better than that of NZ desulfurization agent, and its desulfurization performance shows a tendency of increasing and then decreasing with the increase of the Co introduction. Among them, NZCo-3 desuifurization agent has the most excellent desuifurization performance, and its desuifurization rate can reach 100%. The optimum operating conditions for NZCo-3 desuifurization agent were reaction temperature 300 ℃, total pressure 3 MPa, WHSV 2.2 h−1, and H2/Oil (v/v) 300, under which 100% desulfurization rate could be maintained. Systematic characterisation of the structure and properties of the desuifuriser using XRD, TEM, N2 adsorption and desorption, XPS and H2-TPR confirmed that a composite metal oxide desuifuriser with Ni/ZnCo2O4@ZnO structure was obtained. The formation of ZnCo2O4 in the composite metal oxide desuifuriser promotes the reduction of particle size, enhancement of dispersion and increase of specific surface area of the desuifuriser. The smaller NiO grains facilitated the reduction of NiO to Ni, generating more active sites for desuifurization. The smaller ZnO grains were favourable for adsorption of more H2S. The XRD after the reaction showed that the formed ZnCo2O4 structure was able to adsorb the generated H2S, which acted as a suifur adsorbent and improved the suifur adsorption capacity of the desuifurization agent, thus improving the desuifurization activity of the NZCo-x desuifurization agent. Finally, the evaluation results of the cyclic desulfurization experiment with NZCo-3 desulfurization agent showed that, under the reaction conditions of reaction temperature 275 ℃, reaction pressure 3 MPa, H2/Oil (v/v) 300, and mass-air velocity of 2.2 h−1, the desulfurization rate of NZCo-3 desulfurization agent could still be maintained at more than 77% after six reaction and regeneration cycles of the agent, which is only 16.51% lower than that of the fresh NZCo-3 desulfurization agent. The desulfurization rate of fresh NZ desulfurization agent was only 10.6%, which was much lower than that of NZCo-3 desulfurization agent after 6 regeneration cycles. In conclusion, the method of improving the desulfurization and regeneration performance of Ni/ZnO desulfurization agent by constructing a composite metal oxide structure in this paper provides a new idea for further designing high-performance Ni/ZnO desulfurisation adsorbents to meet the requirements of deep desulfurisation of catalytic cracking gasoline. -
表 1 NZ和NZCo-x脱硫剂的元素含量
Table 1 Elemental contents of NZ and NZCo-x desulfurization agent
Sample Ni w/% Zn w/% Co w/% n(Zn∶Co) measured value n(Zn∶Co) theoretical value NZ 9.550 90.45 / / / NZCo-1 10.66 70.95 18.39 1∶0.29 1∶0.3 NZCo-2 10.96 58.16 30.88 1∶0.59 1∶0.6 NZCo-3 11.15 48.14 40.71 1∶0.94 1∶1 NZCo-4 11.18 25.16 63.66 1∶2.81 1∶3 表 2 NZ和NZCo-x脱硫剂的织构参数
Table 2 Weaving parameters of NZ and NZCo-x desulfurization agent
Sample BET surface area/(m2·g−1) Pore volume/(cm3·g−1) Average pore diameter/nm NZ 12.32 0.088 28.68 NZCo-1 41.78 0.126 12.08 NZCo-2 60.17 0.187 12.40 NZCo-3 103.6 0.399 15.40 NZCo-4 64.10 0.251 15.66 表 3 NZ和NZCo-x脱硫剂的平均粒径
Table 3 Average particle size of NZ and NZCo-x desulfurization agent
Sample Average size/nm NZ 42.23 NZCo-1 15.23 NZCo-2 11.76 NZCo-3 7.60 NZCo-4 14.67 -
[1] ZAIDI Z, GUPTA Y, GAYATRI S L, et al. A comprehensive discussion on fuel combustion and desulfurization technologies[J]. Inorg Chem Commun, 2023, 110964. [2] TANG M M, WANG W X, ZHOU L G, et al. Reactive adsorption desulfurization of thiophene over NiMo/ZnO, a new adsorbent with high desulfurization performance and sulfur capacity at moderate temperature[J]. Catal Sci Technol,2019,9(22):6318−6326. doi: 10.1039/C9CY01070J [3] NIU H, LI C, LUO J, et al. Modulation of Pt states on Pt/ZnO for atmospheric ultra-deep desulfurization of dibenzothiophene and sustainable utilization[J]. Fuel,2024,357:129883. doi: 10.1016/j.fuel.2023.129883 [4] 瞿国华. 我国清洁汽油生产的热点和难点[J]. 石油化工技术与经济,2019,35(2):1−6. doi: 10.3969/j.issn.1674-1099.2019.02.001ZHAI Guohua. Hot spots and difficulties in China's clean petrol production[J]. Technol. Econ. Petrochem,2019,35(2):1−6. doi: 10.3969/j.issn.1674-1099.2019.02.001 [5] BABICH I V, MOULIJN J A. Science and technology of novel processes for deep desulfurization of oil refinery streams: a review[J]. Fuel,2003,82(6):607−631. doi: 10.1016/S0016-2361(02)00324-1 [6] TANG M X, SI J K, XIA L C, et al. Thermodynamic evaluation and experimental validation of candidate sulfur acceptors for reactive adsorption desulfurization adsorbent[J]. Fuel,2019,257:115968. doi: 10.1016/j.fuel.2019.115968 [7] CAMPBELL K C, HOLLIMAN P J, HOYLE R W, et al. Cobalt-zinc oxide absorbents for low temperature gas desulfurization[J]. J Mater Chem,1999,9(2):599−605. doi: 10.1039/a806909c [8] PETERÁWILLIAMS B. Mixed Co-Zn-Al oxides as absorbents for low-temperature gas desulfurisation[J]. J Chem Soc,1995,91(18):3219−3230. [9] YANG C, YANG S, FAN H L, et al. A sustainable design of ZnO-based adsorbent for robust H2S uptake and secondary utilization as hydrogenation catalyst[J]. Chem Eng J,2020,382:122892. doi: 10.1016/j.cej.2019.122892 [10] ZHANG Y, YANG Y, HAN H, et al. Ultra-deep desulfurization via reactive adsorption on Ni/ZnO: The effect of ZnO particle size on the adsorption performance[J]. Appl Catal B-Environ,2012,119:13−19. [11] SINGH S B, DE M. Room temperature adsorptive removal of thiophene over zinc oxide-based adsorbents[J]. J Mater Eng Perform,2018,27:2661−2667. doi: 10.1007/s11665-018-3192-2 [12] DHAGE P, SAMOKHVALOV A. Regenerable Fe-Mn-ZnO/SiO2 sorbents for room temperature removal of H2S from fuel reformates: performance, active sites, Operando studies[J]. Phys Chem Chem Phys.,2011,13:2179−2187. doi: 10.1039/C0CP01355B [13] LIU M M, MA S Y, CAI Y H, et al. ZnO/ZnCo2O4 composite prepared by one-step hydrothermal method for high-performance ethylene glycol sensor[J]. Ceram Int,2022,48(15):22346−22353. [14] 唐明兴, 李学宽, 吕占军, 等. 苯中硫在Ni/ZnO催化剂上加氢吸附脱除的研究[J]. 燃料化学学报,2009,37(6):707−712.TANG Mingxing, LI Xuekaun, LV Zhanjun, et al. Ultra-deep hydrodesulfurization of benzene over Ni/ZnO catalyst[J]. J Fuel Chem Technol,2009,37(6):707−712. [15] INGER M, WILK M, SARAMOK M, et al. Cobalt Spinel Catalyst for N2O Abatement in the Pilot Plant Operation-Long-Term Activity and Stability in Tail Gases[J]. Ind Eng Chem Res,2014,53:10335−10342. doi: 10.1021/ie5014579 [16] LIU Y, TIAN J, ZHENG Y, et al. Tuning of surface structure of porous glass-supported titania with fibrous silica for efficient coupling adsorption-catalysis-resorption desulfurization scheme[J]. Surf Interfaces,2022,33:102264. doi: 10.1016/j.surfin.2022.102264 [17] HUANG L, WANG G, QIN Z, et al. In situ XAS study on the mechanism of reactive adsorption desulfurization of oil product over Ni/ZnO[J]. Appl Catal B-Environ,2011,106(1):26−38. [18] WANG H, TANG M, SHI F, et al. Amorphous Cr2WO6-modified WO3 nanowires with a large specific surface area and rich Lewis acid sites: a highly efficient catalyst for oxidative desulfurization[J]. Acs Appl Mater Inter,2020,12(34):38140−38152. doi: 10.1021/acsami.0c10118 [19] 魏延雨. Ni/ZnO反应吸附脱除汽油中硫化物的研究[D]. 天津大学, 2013.WEI Yanyu. Adsorptive removal of sulfides from gasoline by Ni/ZnO reaction [D]. Tianjin University, 2013.) [20] BAIRD T, CAMPBELL K C, HOLLIMAN P J, et al. Cobalt-zinc oxide absorbents for low temperature gas desulfurisation[J]. J Mater Chem,1999,9(2):599−605. doi: 10.1039/a806909c [21] WANG G, WEN Y, FAN J, et al. Reactive characteristics and adsorption heat of Ni/ZnO-SiO2-Al2O3 adsorbent by reactive adsorption desulfurization[J]. Ind Eng Chem Res,2011,50(22):12449−12459. doi: 10.1021/ie201144u -




 下载:
下载: