Influence of Ni-Ag/SiO2 catalyst preparation method on its performance in hydrogenation of dimethyl oxalate to methyl glycolate
-
摘要: 本研究采用均匀沉淀-浸渍和浸渍-浸渍两种分步法分别制备了Ni-Ag/SiO2-p和Ni-Ag/SiO2-i两种Ni-Ag双金属催化剂,用于催化草酸二甲酯(DMO)选择性加氢制乙醇酸甲酯(MG)。利用X射线衍射、红外光谱、透射电镜、N2物理吸附、程序升温脱附实验和X射线光电子能谱表征技术对两种催化剂进行了系统的表征和结构解析,发现催化剂的制备方法对双金属催化剂的结构和性能有着重要影响,Ni-Ag/SiO2-p催化剂中由于层状硅酸镍结构的存在,Ag、Ni活性物种的金属分散度相比于Ni-Ag/SiO2-i催化剂更高,这促进了反应物H2和DMO的吸附与活化;催化剂性能评价结果表明,均匀沉淀-浸渍法制备的Ag负载量仅为0.48%的Ni-Ag/SiO2-p催化剂显著提升了DMO加氢活性,在220 ℃、2.0 MPa、液时空速0.5 h-1、氢酯比50的条件下,DMO转化率和MG选择性达到了99.1%和87.6%。研究结果可为设计和优化DMO加氢制MG催化剂提供一定借鉴。Abstract: Methyl glycolate (MG), as a high value-added organic chemical intermediate, possesses broad prospects for downstream applications. In this study, nickel-silver bimetallic catalysts, denoted as Ni-Ag/SiO2-p and Ni-Ag/SiO2-i, were synthesized via homogenous precipitation-impregnation and impregnation-impregnation methods, respectively, for selective hydrogenation of dimethyl oxalate (DMO) to MG. Various characterization techniques, including X-ray diffraction, infrared spectroscopy, transmission electron microscopy, N2 physisorption, and X-ray photoelectron spectroscopy, were employed to extensively analyze the catalysts' structures. The results unveiled that the Ni-Ag/SiO2-p catalyst, prepared through the precipitation of the nickel precursor followed by impregnation of the silver precursor, exhibited a laminated nickel silicate structure and a higher specific surface area compared to the Ni-Ag/SiO2-i catalyst, synthesized via sequential impregnation of both metal precursors over SiO2. This enhanced surface area facilitated improved metal-support interaction and the reduction of smaller metal particles, thereby Ni-Ag/SiO2-p demonstrated superior metal dispersion compared to Ni-Ag/SiO2-i, providing more active sites for adsorption and activation of reactant molecules. Specifically, Ni species with small particle sizes facilitated the adsorption and activation of DMO molecules , while the introduction of Ag not only promoted the adsorption of DMO molecules but also significantly enhanced the adsorption and activation capacity of H2. This resulted in H2 predominating in competitive adsorption with DMO molecules, substantially augmenting the hydrogenation activity of DMO on the catalyst. Remarkably, Ni-Ag/SiO2-p achieved outstanding results with a low Ag loading of 0.48% under operating conditions of 220 ℃, 2.0 MPa, a liquid hourly space velocity of 0.5 h-1, and a hydrogen-to-ester ratio of 50. Specifically, Ni-Ag/SiO2-p catalyst demonstrated DMO conversion and MG selectivity of 99.1% and 87.6%, respectively. These findings underscore the substantial impact of catalyst preparation method on the structure and catalytic performance of bimetallic catalysts, offering valuable insights for the design and optimization of catalysts for DMO hydrogenation to MG.
-
Key words:
- dimethyl oxalate /
- hydrogenation /
- catalyst preparation /
- methyl glycolate
-
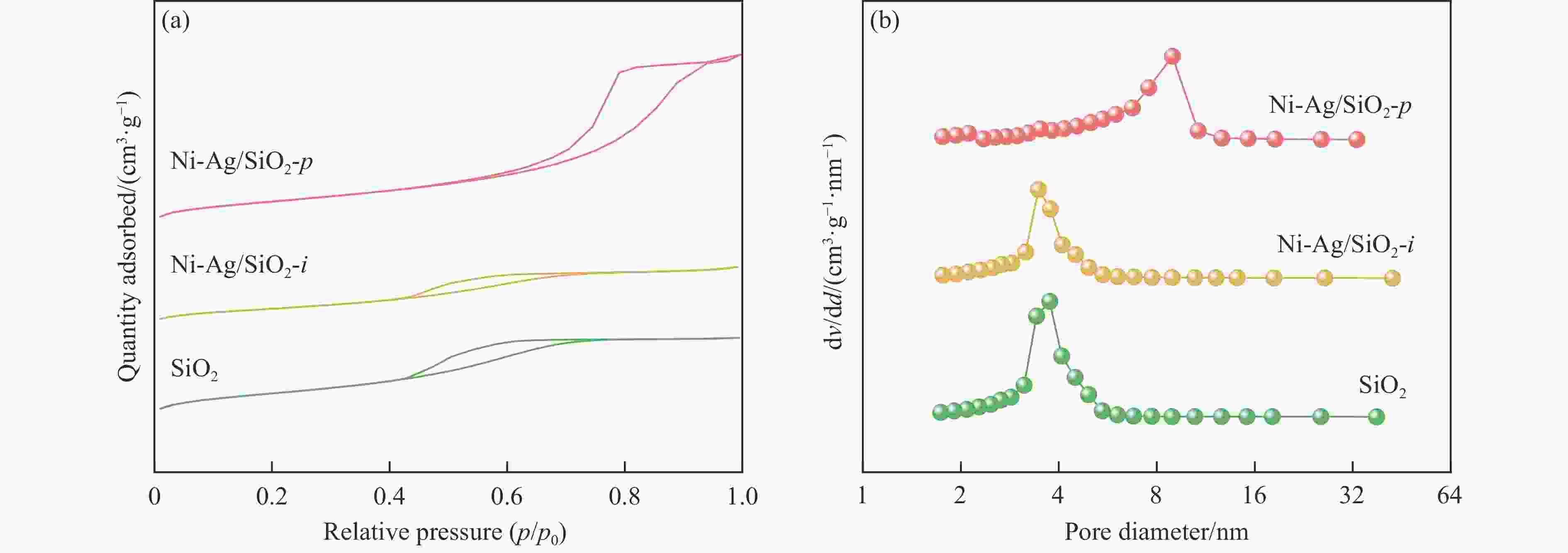
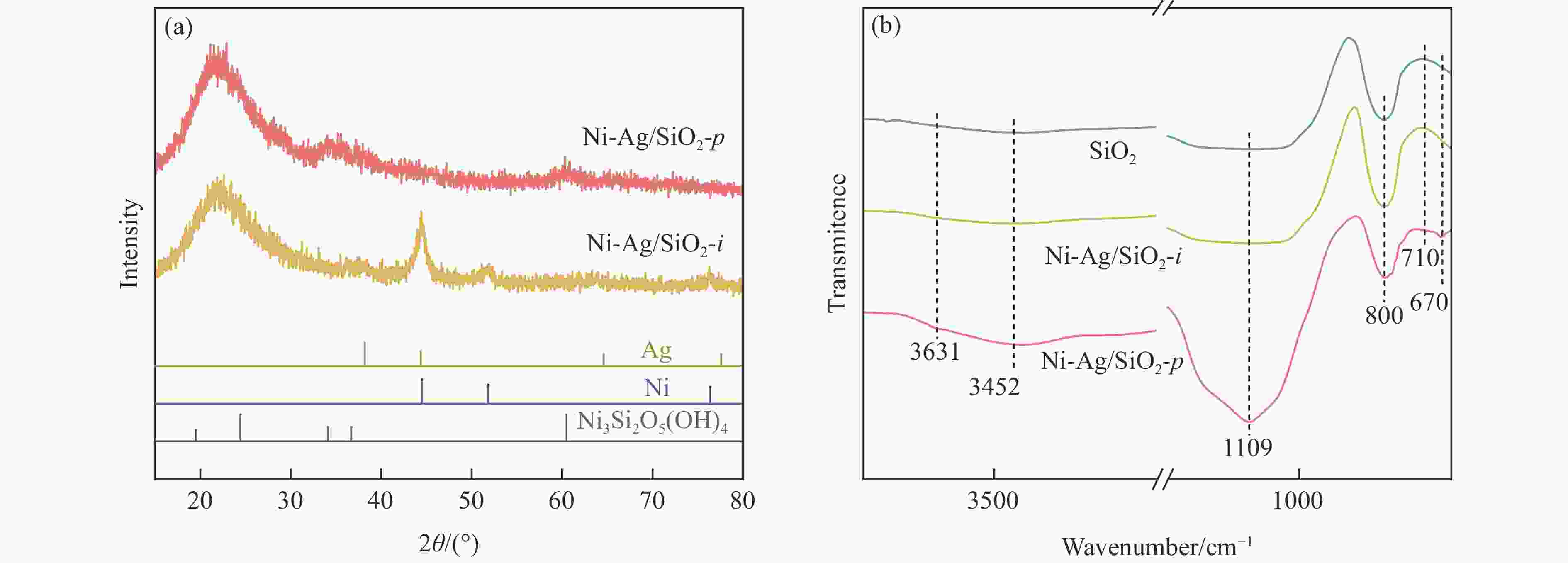
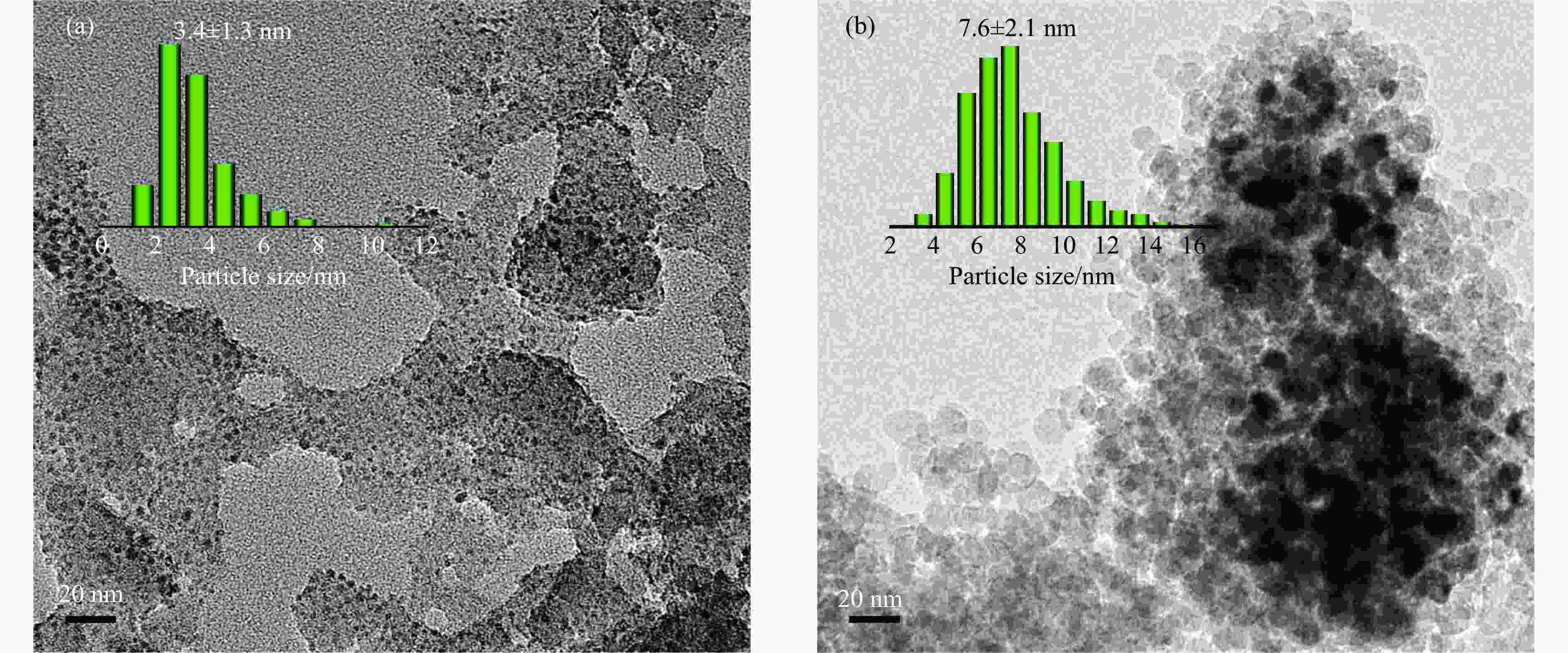
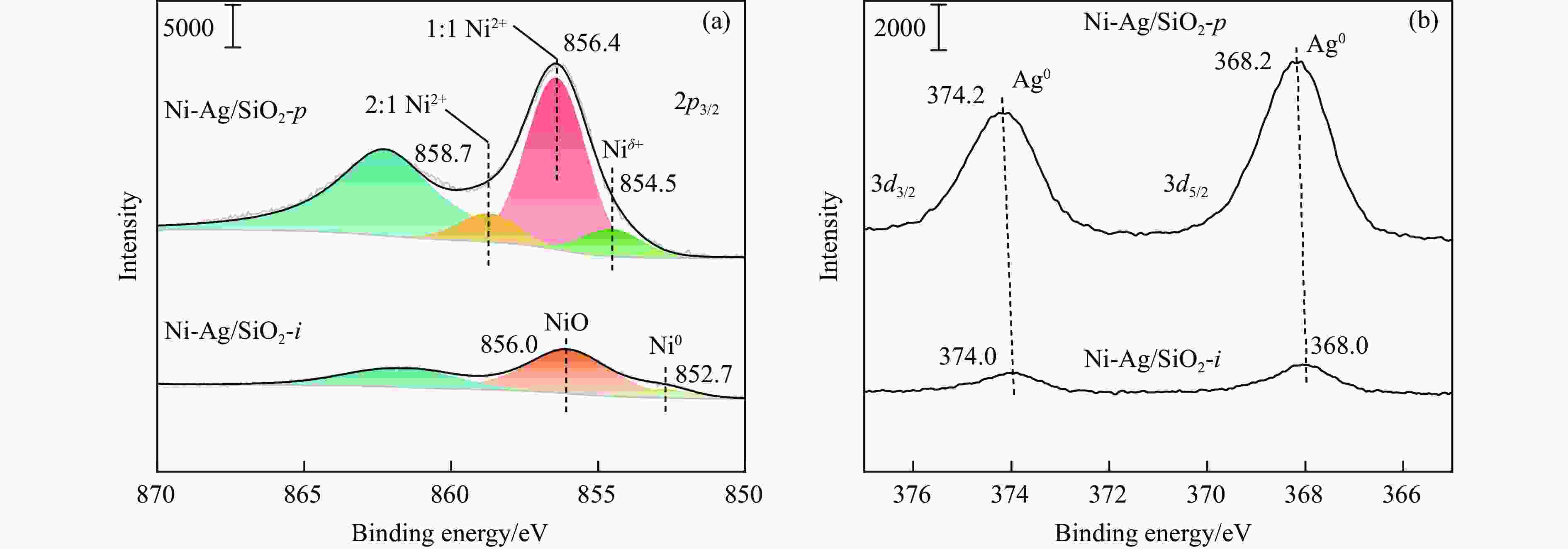
表 1 SiO2和Ni-Ag/SiO2催化剂的理化特征
Table 1 Physicochemical properties of SiO2 and Ni-Ag/SiO2 catalysts
Sample Loadinga w/% SBETb/
(m2·g−1)vporec/
(cm3·g−1)dBJHc/
nmdmetald/
nmQCOe/
(mmol·g−1)$Q_{\Delta {\mathrm{H}}_2} $f/
(mmol·g−1)Ni Ag SiO2 − − 248 0.27 3.72 − − − Ni-Ag/SiO2-p 8.9 0.48 267 0.58 7.86 3.4 0.0041 0.077 Ni-Ag/SiO2-i 9.3 0.51 173 0.20 3.96 7.6 0.0017 0.026 a: Ni content and Ag content determined by ICP; b: Specific surface area calculated by BET method; c: Pore volume and pore size calculated by BJH method; d: Average size of metal particles determined by TEM; e: CO adsorption amount was determined by CO-chem; f: H2 consumption of Ag was determined by the difference between the H2 consumption for H2-TPR test after the oxidation by N2O and the CO adsorption amount for CO-Chem. -
[1] 张大洲, 卢文新, 商宽祥, 等. 草酸二甲酯加氢制乙醇酸甲酯反应网络分析及其多相加氢催化剂研究进展[J]. 化工进展,2023,42(1):204−214.ZHANG Dazhou, LU Wenxin, SHANG Kuanxiang, et al. Reaction network analysis of dimethyl oxalate hydrogenation to methyl glycolate and recent progress in the heterogeneous catalysts[J]. Chem Ind Eng Prog,2023,42(1):204−214. [2] QU R, JUNGE K, BELLER M. Hydrogenation of carboxylic acids, esters, and related compounds over heterogeneous catalysts: A step toward sustainable and carbon−neutral processes[J]. Chem Rev,2023,123(3):1103−1165. doi: 10.1021/acs.chemrev.2c00550 [3] OUYANG M, WANG J, PENG B, et al. Effect of Ti on Ag catalyst supported on spherical fibrous silica for partial hydrogenation of dimethyl oxalate[J]. Appl Surf Sci,2019,466(2):592−600. [4] CHENG S, MENG T, MAO D, et al. Ni-modified Ag/SiO2 catalysts for selective hydrogenation of dimethyl oxalate to methyl glycolate[J]. Nanomaterials,2022,12(3):407. doi: 10.3390/nano12030407 [5] DONG G, CAO Y, ZHENG S, et al. Catalyst consisting of Ag nanoparticles anchored on amine-derivatized mesoporous silica nanospheres for the selective hydrogenation of dimethyl oxalate to methyl glycolate[J]. J Catal,2020,391:155−162. doi: 10.1016/j.jcat.2020.08.018 [6] LUO Z, XU X, DONG G, et al. Regulating mesopore structures of support toward enhanced selective hydrogenation of dimethyl oxalate to methyl glycolate on Ag catalysts[J]. Chem Eng J,2022,450:138397 doi: 10.1016/j.cej.2022.138397 [7] WEN C, CUI Y, CHEN X, et al. Reaction temperature controlled selective hydrogenation of dimethyl oxalate to methyl glycolate and ethylene glycol over copper-hydroxyapatite catalysts[J]. Appl Catal, B,2015,162:483−493. doi: 10.1016/j.apcatb.2014.07.023 [8] CHEN H M, TAN J J, ZHU Y L, et al. An effective and stable Ni2P/TiO2 catalyst for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. Catal Commun,2016,73(73):46−49. [9] ZHU J, CAO L, LI C, et al. Nanoporous Ni3P evolutionarily structured onto a Ni foam for highly selective hydrogenation of dimethyl oxalate to methyl glycolate[J]. ACS Appl Mater Interfaces,2019,11(41):37635−37643. doi: 10.1021/acsami.9b11703 [10] 周静红, 罗祖伟, 曹约强, 等. 一种镍基双金属催化剂及其制备方法和应用, CN116273055A [P]. 2023-06-23.ZHOU Jinghong, LUO Zuwei, CAO Yueqiang, et al. A nickel-based bimetallic catalyst and its preparation method and application, CN116273055A [P]. 2023-06-23.) [11] 申志兵, 柯明, 张君涛, 等. 活性金属不同浸渍顺序对Mo−Ni/Al2O3催化剂硫醚化反应性能的影响[J]. 燃料化学学报,2017,45(5):616−623.SHEN Zhibing, KE Ming, ZHANG Juntao, et al. Effect of impregnation sequence of Mo and Ni on the performance of Mo−Ni/Al2O3 catalyst in thioetherification[J]. J Fuel Chem Technol,2017,45(5):616−623. [12] NA H, ZHU T, LIU Z. Effect of preparation method on the performance of Pt−Au/TiO2 catalysts for the catalytic co-oxidation of HCHO and CO[J]. Catal Sci Technol,2014,4(7):2051−2057. doi: 10.1039/c4cy00020j [13] WANG B, XU Q, SONG H, et al. Synthesis of methyl glycolate by hydrogenation of dimethyl oxalate over Cu-Ag/SiO2 catalyst[J]. J Nat Gas Chem,2007,16(1):78−80. doi: 10.1016/S1003-9953(07)60030-9 [14] YIN A Y, WEN C, DAI W L, et al. Nanocasting of CuAu alloy nanoparticles for methyl glycolate synthesis[J]. J Mater Chem,2011,21(25):8997−8999. doi: 10.1039/c1jm10646e [15] Hu M, Yan Y, Duan X, et al. Effective anchoring of silver nanoparticles onto N-doped carbon with enhanced catalytic performance for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. Catal Commun,2017,100:148−152. doi: 10.1016/j.catcom.2017.06.025 [16] OUYANG M, WANG Y, ZHANG J, et al. Three dimensional Ag/KCC-1 catalyst with a hierarchical fibrous framework for the hydrogenation of dimethyl oxalate[J]. RSC Adv,2016,6(16):12788−12791. doi: 10.1039/C5RA26602E [17] DUAN X P, CHEN T, CHEN T, et al. Intercalating lithium into the lattice of silver nanoparticles boosts catalytic hydrogenation of carbon−oxygen bonds[J]. Chem Sci,2021,12(25):8791−8802. doi: 10.1039/D1SC01700D [18] 杨春雁, 杨卫亚, 凌凤香, 等. 负载型金属催化剂表面金属分散度的测定[J]. 化工进展,2010,29(8):1468−1473,1501.YANG Chunyan, YANG Weiya, LING Fengxiang, et al. Determination of metal dispersion on supported metal catalyst surface[J]. Chem Ind Eng Prog,2010,29(8):1468−1473,1501. [19] 董桂霖, 罗祖伟, 曹约强, 等. 液相还原温度对草酸酯加氢制乙醇酸甲酯银硅催化剂性能的影响[J]. 化工学报,2022,73(1):232−240.DONG Guilin, LUO Zuwei, CAO Yueqiang, et al. Effect of liquid−phase reduction temperature on performance of silver−silica catalysts for hydrogenation of dimethyl oxalate to methyl glycolate[J]. CIESC J,2022,73(1):232−240. [20] WU C, WANG L, WILLIAMS P T, et al. Hydrogen production from biomass gasification with Ni/MCM-41 catalysts: Influence of Ni content[J]. Appl Catal, B,2011,108(1-2):6−13. [21] ZHOU J, DUAN X, YE L, et al. Enhanced chemoselective hydrogenation of dimethyl oxalate to methyl glycolate over bimetallic Ag−Ni/SBA-15 catalysts[J]. Appl Catal, A,2015,505:344−353. doi: 10.1016/j.apcata.2015.08.022 [22] KONG X, ZHU Y, ZHENG H, et al. Ni nanoparticles inlaid nickel phyllosilicate as a metal−acid bifunctional catalyst for low-temperature hydrogenolysis reactions[J]. ACS Catal,2015,5(10):5914−5920. doi: 10.1021/acscatal.5b01080 [23] ZHU S, GAO X, ZHU Y, et al. A highly efficient and robust Cu/SiO2 catalyst prepared by the ammonia evaporation hydrothermal method for glycerol hydrogenolysis to 1, 2-propanediol[J]. Catal Sci Technol,2015,5(2):1169−1180. doi: 10.1039/C4CY01148A [24] DU H, MA X, JIANG M, et al. Efficient Ni/SiO2 catalyst derived from nickel phyllosilicate for xylose hydrogenation to xylitol[J]. Catal Today,2021,365:265−273. doi: 10.1016/j.cattod.2020.04.009 [25] YAN L, LIU X, DENG J, et al. Molybdenum modified nickel phyllosilicates as a high performance bifunctional catalyst for deoxygenation of methyl palmitate to alkanes under mild conditions[J]. Green Chem,2017,19(19):4600−4609. doi: 10.1039/C7GC01720K [26] HE Q, LI P, FANG W, et al. High-efficiency hydrogenation of methyl furoate to valuable methyl tetrahydrofuran-2-carboxylate over Ni−SiO2 catalysts with high Ni content and dispersion[J]. Catal Lett,2023,153(2):398−407. doi: 10.1007/s10562-022-03983-8 [27] KORYTKOVA E N, MASLOV A V, PIVOVAROVA L N, et al. Synthesis of nanotubular Mg3Si2O5(OH)4–Ni3Si2O5(OH)4 silicates at elevated temperatures and pressures[J]. Inorg Mater,2005,41(7):743−749. doi: 10.1007/s10789-005-0202-1 [28] SIVAIAH M V, PETIT S, BARRAULT J, et al. CO2 reforming of CH4 over Ni-containing phyllosilicates as catalyst precursors[J]. Catal Today,2010,157(1):397−403. [29] GHIAT I, BOUDJEMAA A, SAADI A, et al. Efficient hydrogen generation over a novel Ni phyllosilicate photocatalyst[J]. J Photochem Photobiol, A,2019,382:111952. doi: 10.1016/j.jphotochem.2019.111952 [30] WANG J, FU Y, KONG W, et al. Design of a carbon-resistant Ni@S-2 reforming catalyst: Controllable Ni nanoparticles sandwiched in a peasecod-like structure[J]. Appl Catal, B,2021,282:119546. doi: 10.1016/j.apcatb.2020.119546 [31] LI Z, MO L, KATHIRASER Y, et al. Yolk–satellite–shell structured Ni–yolk@Ni@SiO2 nanocomposite: superb catalyst toward methane CO2 reforming reaction[J]. ACS Catal,2014,4(5):1526−1536. doi: 10.1021/cs401027p [32] MA L, YAN L, LU A-H, et al. Effects of Ni particle size on amination of monoethanolamine over Ni-Re/SiO2 catalysts[J]. Chin J Catal,2019,40(4):567−579. doi: 10.1016/S1872-2067(19)63302-4 [33] LI J, LI P, LI J, et al. Highly-dispersed Ni-NiO nanoparticles anchored on an SiO2 support for an enhanced CO methanation performance[J]. Catalysts,2019,9(6):506. doi: 10.3390/catal9060506 [34] DONG G, LUO Z, CAO Y, et al. Understanding size-dependent hydrogenation of dimethyl oxalate to methyl glycolate over Ag catalysts[J]. J Catal,2021,401:252−261. doi: 10.1016/j.jcat.2021.07.028 [35] Venezia A M. X-ray photoelectron spectroscopy (XPS) for catalysts characterization[J]. Catal Today,2003,77(4):359−370. doi: 10.1016/S0920-5861(02)00380-2 [36] Yan S, Sun D, Tan Y, et al. Synthesis and formation mechanism of Ag–Ni alloy nanoparticles at room temperature[J]. J Phys Chem Solids,2016,98:107−114. doi: 10.1016/j.jpcs.2016.06.013 [37] ZHOU R-J, YAN W-Q, CAO Y-Q, et al. Probing the structure sensitivity of dimethyl oxalate partial hydrogenation over Ag nanoparticles: A combined experimental and microkinetic study[J]. Chem Eng Sci,2022,259:117830. doi: 10.1016/j.ces.2022.117830 [38] LI P, ZHANG M, WANG S, et al. Pd−Ni−Fe nanoparticles supported on UiO-66 for selective hydrogenation of fatty acid methyl esters to alcohols[J]. ACS Appl Nano Mater,2023,6(20):18892−18904. doi: 10.1021/acsanm.3c03297 [39] CHEN X, CHEN X, WANG J, et al. Steerable engineering of nickel molybdenum carbonitride nanostructures for catalytic regioselectivity cleavage of C−O and C−C bonds in deoxygenation of methyl palmitate[J]. ACS Appl Nano Mater,2022,5(10):14987−14998. doi: 10.1021/acsanm.2c03210 [40] LI M M-J, YE L, ZHENG J, et al. Surfactant-free nickel−silver core@shell nanoparticles in mesoporous SBA-15 for chemoselective hydrogenation of dimethyl oxalate[J]. Chem Commun,2016,52(12):2569−2572. doi: 10.1039/C5CC09827K [41] VELU S, GANGWAL S K. Synthesis of alumina supported nickel nanoparticle catalysts and evaluation of nickel metal dispersions by temperature programmed desorption[J]. Solid State Ionics,2006,177(7):803−811. [42] CHEN S, PAN X, MIAO C, et al. Study of catalytic hydrodeoxygenation performance for the Ni/KIT-6 catalysts[J]. J Saudi Chem Soc,2018,22(5):614−627. doi: 10.1016/j.jscs.2017.11.002 [43] Liu K, Yan P, Jiang H, et al. Silver initiated hydrogen spillover on anatase TiO2 creates active sites for selective hydrodeoxygenation of guaiacol[J]. J Catal,2019,369:396−404. doi: 10.1016/j.jcat.2018.11.033 [44] Chen Y, Fan S, Chen J, et al. Catalytic membrane nanoreactor with Cu–Agx bimetallic nanoparticles immobilized in membrane pores for enhanced catalytic performance[J]. ACS Appl Mater Interfaces,2022,14(7):9106−9115. doi: 10.1021/acsami.1c22753 [45] Luo Z, Shen Y, Fang D, et al. Insights into support effects of Ag/SiO2 catalysts for dimethyl glycolate semi-hydrogenation to methyl glycolate[J]. Mol Catal,2024,559:114109. doi: 10.1016/j.mcat.2024.114109 [46] ZAHIDI E, CASTONGUAY M, MCBREEN P. RAIRS and TPD study of methyl formate, ethyl formate, and methyl acetate on Ni(111)[J]. J Am Chem Soc,1994,116(13):5847−5856. doi: 10.1021/ja00092a040 -




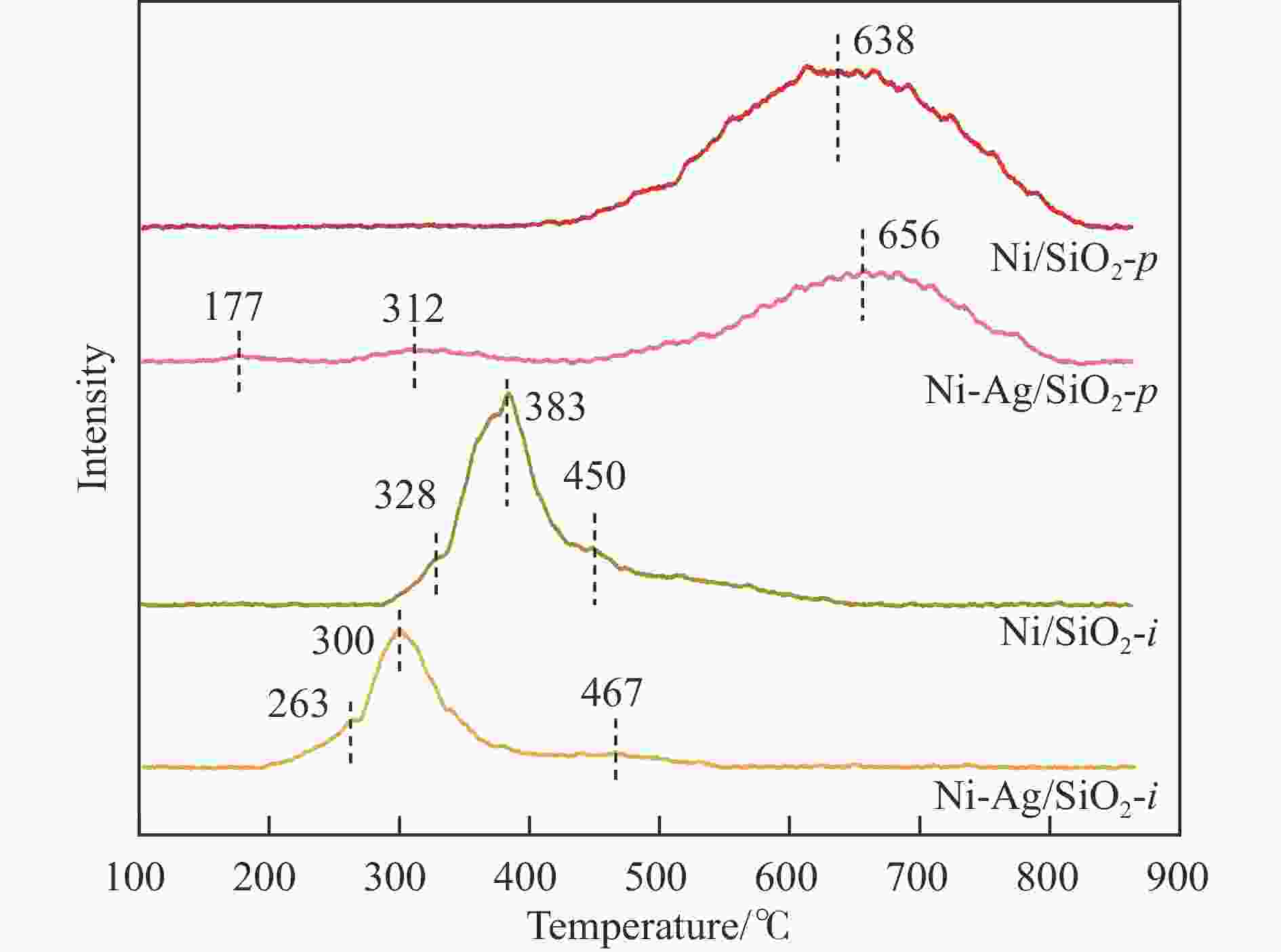
 下载:
下载: