DBD plasma-assisted dry reforming of methane over Ni/SiO2
-
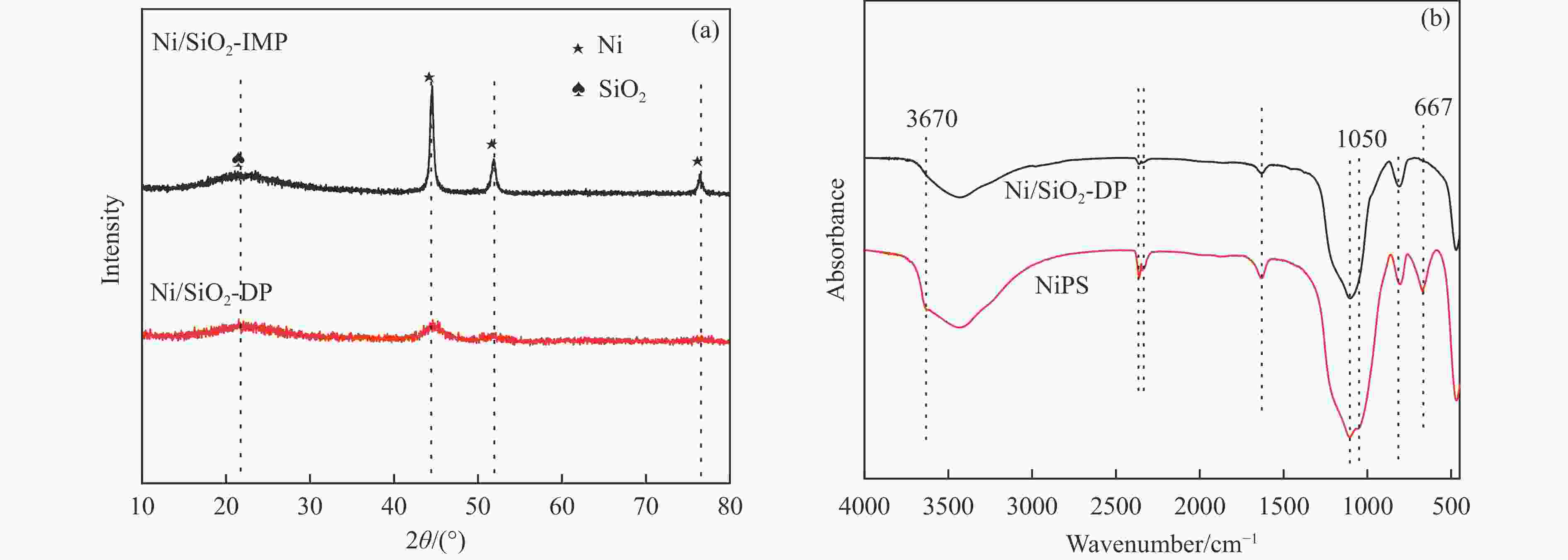
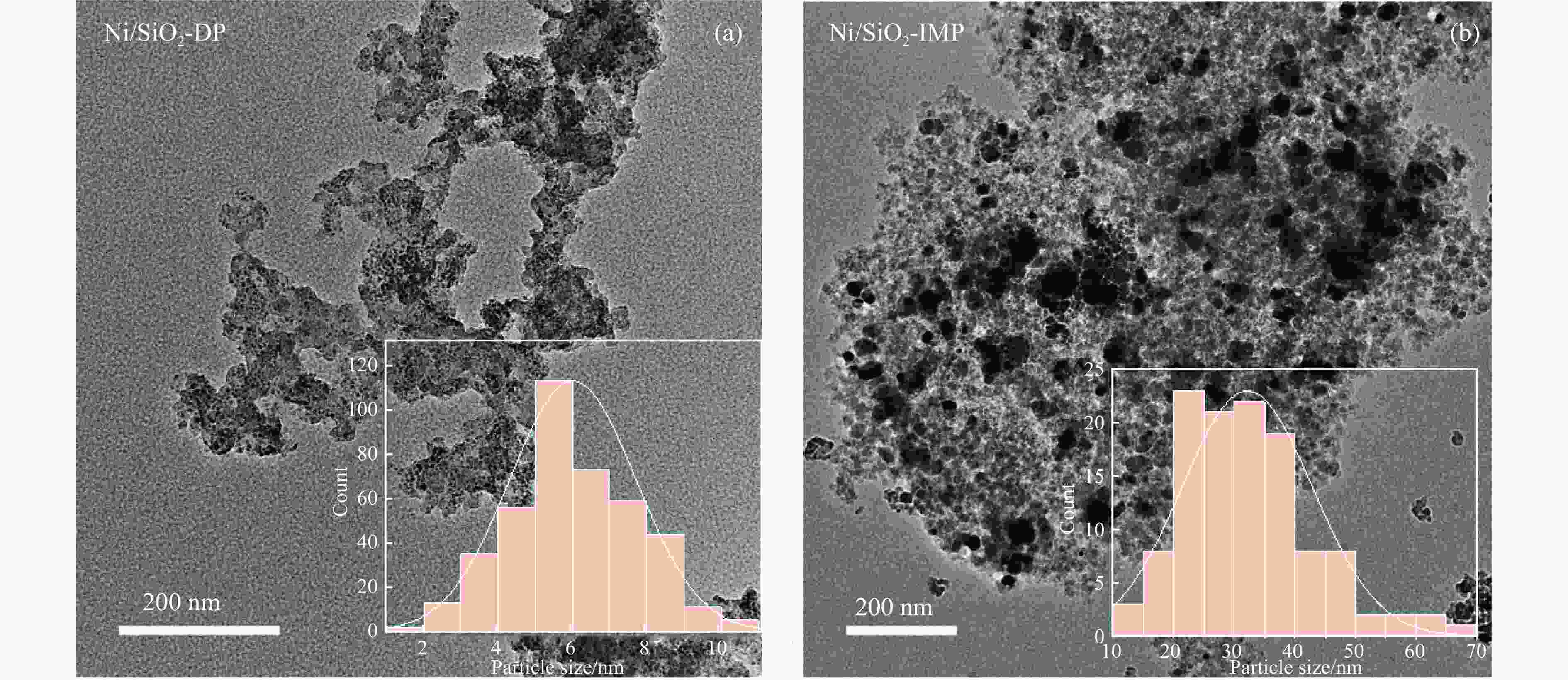
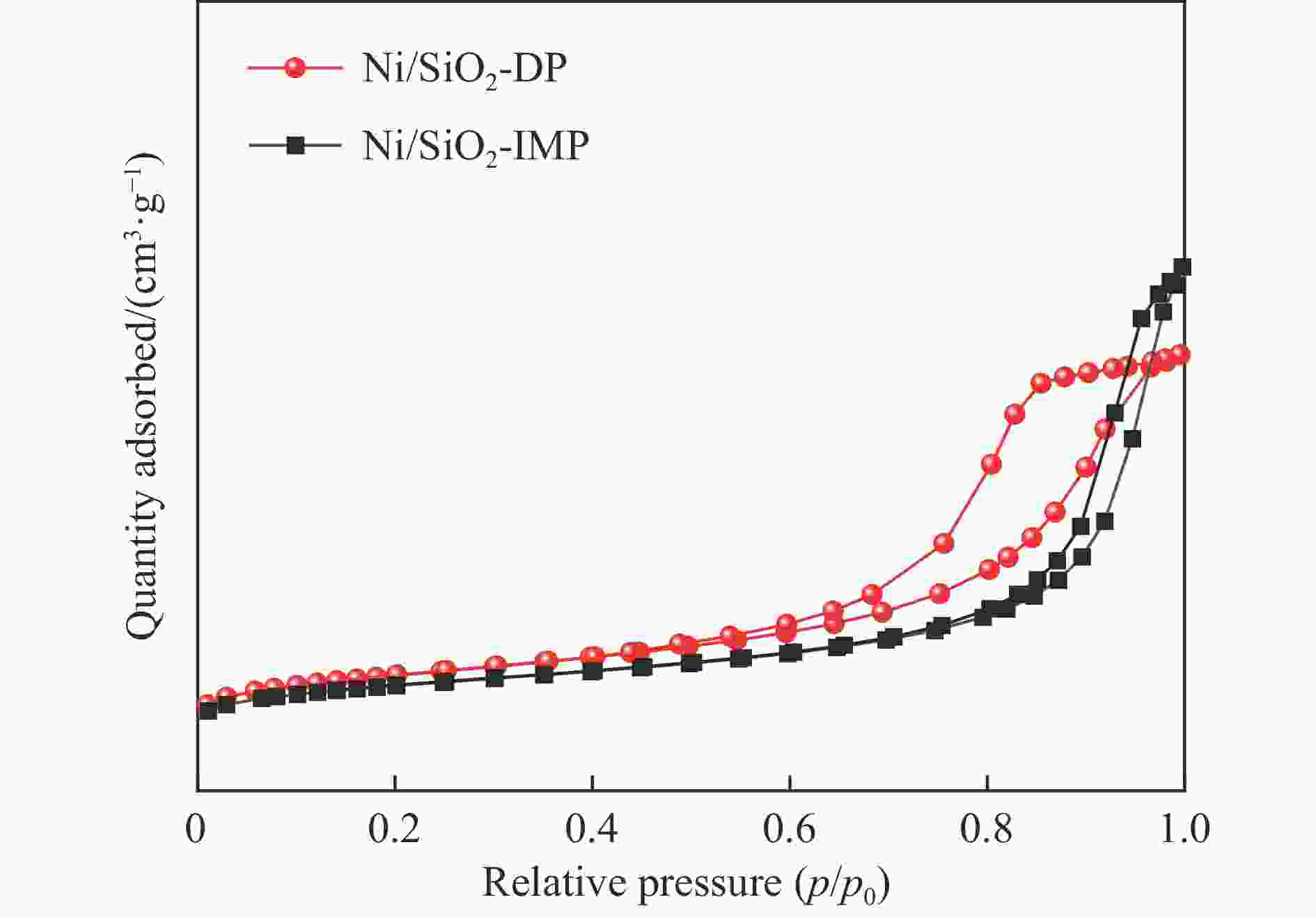
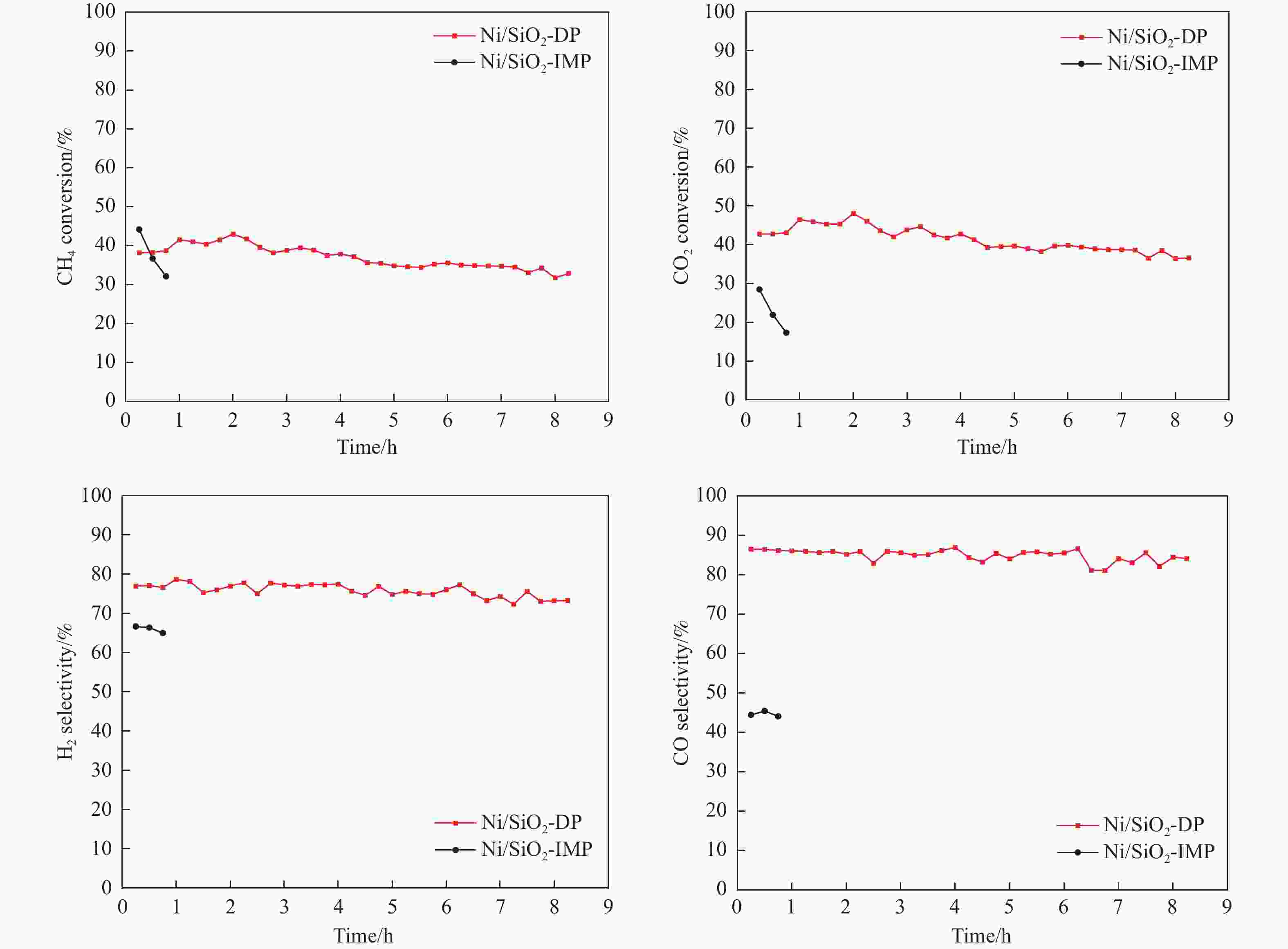
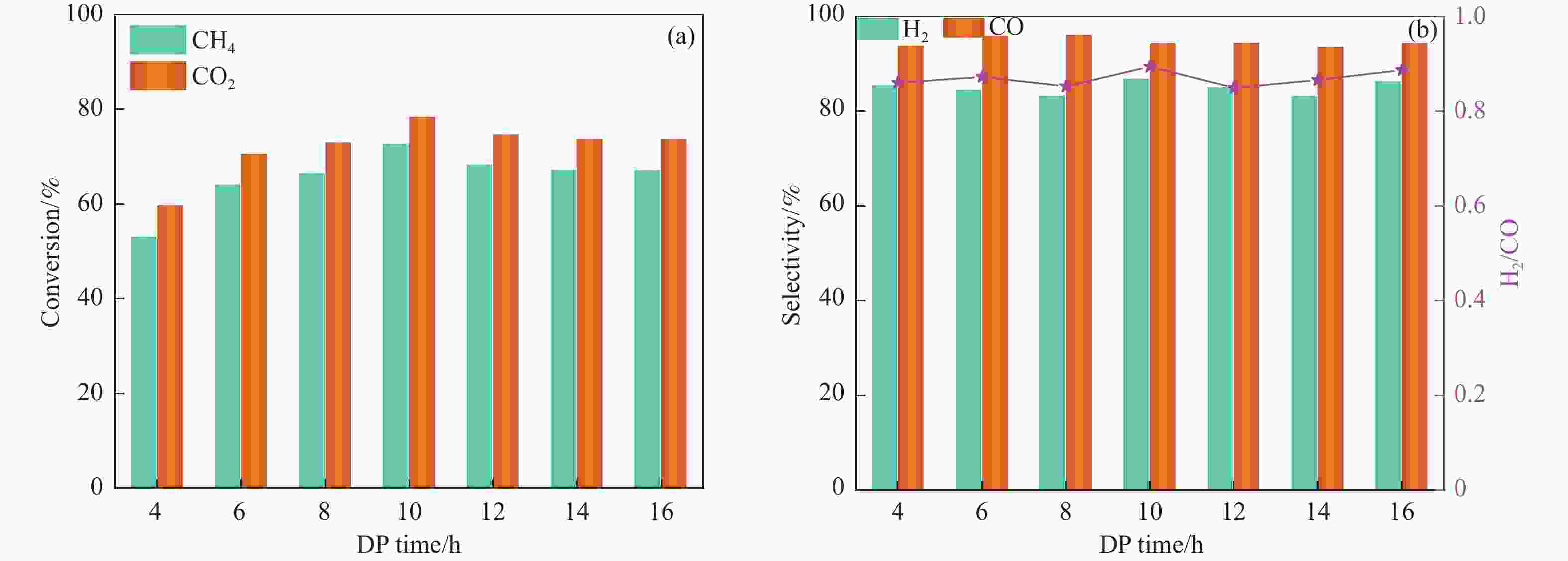
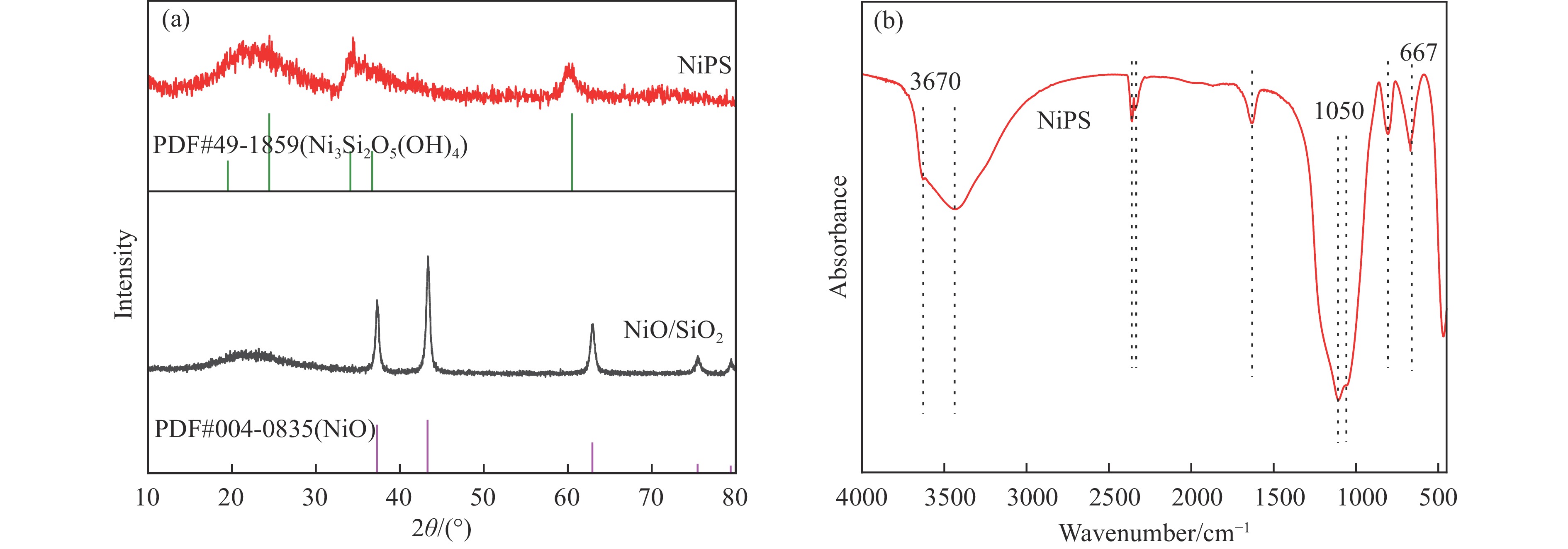
摘要: 分别采用沉积沉淀法(DP)和浸渍法(IMP)制备Ni/SiO2催化剂前体,前体经H2还原得到Ni/SiO2-DP和Ni/SiO2-IMP。对所制备的催化剂进行X射线衍射、X射线光电子能谱、N2吸附-脱附、化学吸附、傅里叶变换红外、透射电镜和拉曼光谱表征,并考察其与介质阻挡放电等离子体(DBD)协同催化甲烷干重整(DRM)制合成气反应性能。研究结果表明,相较于Ni/SiO2-IMP,Ni/SiO2-DP因其较小的Ni颗粒尺寸、Ni与载体的强相互作用以及对反应物分子较强的吸附活化能力,具有更高的催化活性和稳定性。对Ni/SiO2-DP制备条件考察结果表明,H2等离子体还原(PR)的Ni/SiO2-DP-PR比程序升温还原(TPR)的Ni/SiO2-DP-TPR具有更高的催化活性。沉积沉淀时间为10 h,H2等离子体还原时间为30 min时,CH4和CO2转化率分别为72.5%和78.2%,H2和CO选择性分别为86.7%和94.2%,能量利用率为4.36 mmol/kJ。
-
关键词:
- CH4/CO2重整 /
- Ni/SiO2 /
- 介质阻挡放电等离子体
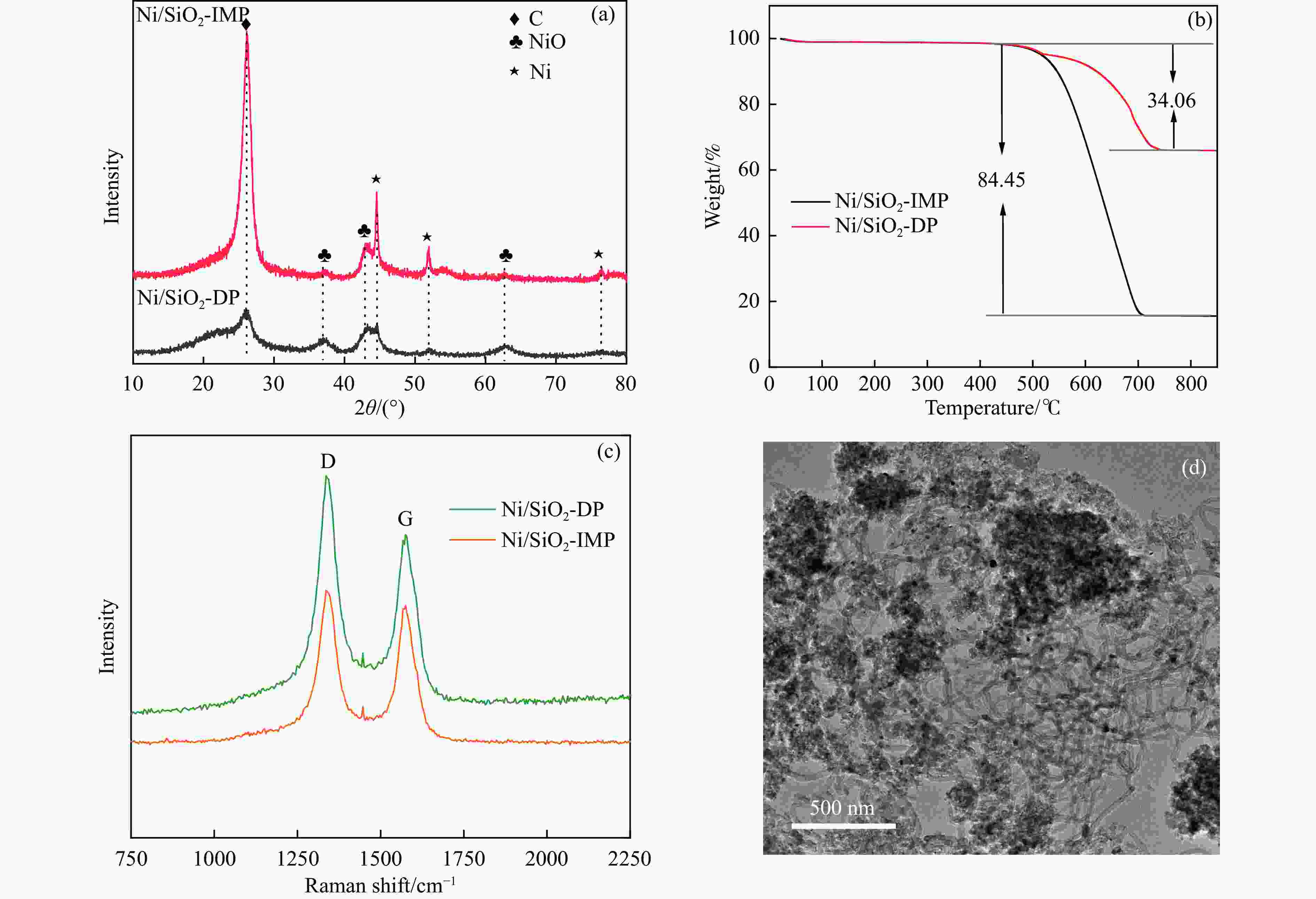
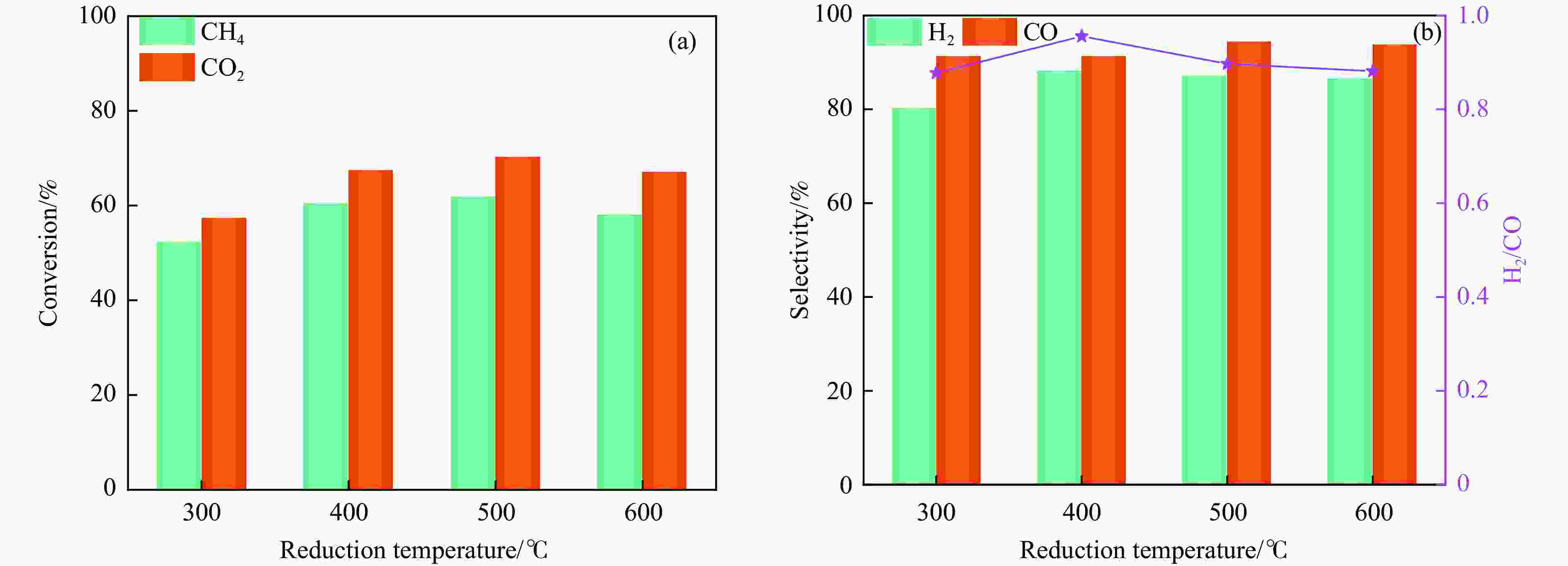
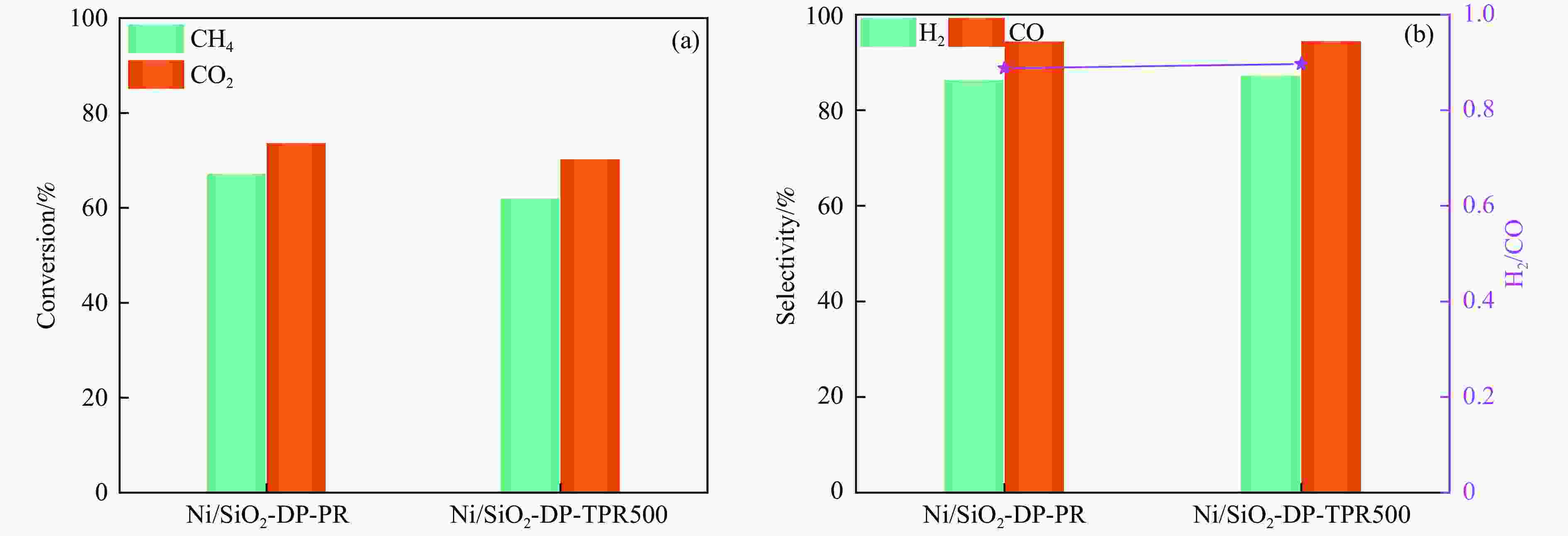
Abstract: Dry reforming of methane reaction(DRM) utilizes both CH4 and CO2 greenhouse gases to convert them into synthesis gas (H2 and CO), which can be further synthesized into value-added chemicals such as hydrocarbons or liquid oxygen-containing compounds. The traditional thermal method for catalyzing DRM reaction often uses Ni-based catalysts. And it requires high reaction temperature (>700 ℃). The high temperature leads to sintering and carbon deposition of Ni-based catalysts, as well as low energy efficiency, which limits the application of this reaction. Dielectric barrier discharge plasma (DBD) can synergistically drive the reaction with Ni-based catalysts at low temperature, thereby addressing the drawbacks of thermal catalysis. The key of this technology is to develop catalysts that have synergistic effects with plasma and strong resistance to carbon deposition. Therefore, this paper uses nickel phyllosilicate as precursor and H2 plasma reduction to prepare highly dispersed Ni-based catalyst, which synergistically catalyze the DRM reaction with DBD plasma. Nickel phyllosilicate was prepared by deposition-precipitation method, and Ni/SiO2-DP was obtained after calcination and reduction. Ni/SiO2-IMP was prepared by impregnation method. The prepared catalysts were characterized by X-ray diffraction, X-ray photoelectron spectroscopy, N2-adsorption-desorption, chemisorption, Fourier transform infrared, transmission electron microscope, thermogravimetric analysis and Raman spectra. The catalytic performance for dry reforming of methane (DRM) to synthesis gas was investigated in the dielectric barrier discharge(DBD) reactor. The research results indicate that Ni/SiO2 -DP has higher catalytic activity and stability, which benefits from its smaller Ni particles size, stronger interaction between Ni and support, and stronger adsorption ability for the reactants, compared with Ni/SiO2 -IMP. The CH4 and CO2 conversions of Ni/SiO2-DP are 61.7% and 70.0%. The selectivities of H2 and CO are 86.9% and 94.3%, and H2/CO is 0.92. The CH4 and CO2 conversions of Ni/SiO2-IMP are 44.2% and 28.4%. The selectivities of H2 and CO are 62.7% and 42.4%, and H2/CO is 1.48. After stability testing, the catalysts were characterized by TG. The weight loss of Ni/SiO2- IMP is 84.45%, while the weight loss of Ni/SiO2-DP is only 34.06%. The preparation conditions of Ni/SiO2 -DP were investigated. The results show that the Ni/SiO2-DP-PR from H2 plasma reduction(PR) exhibits higher catalytic activity than the Ni/SiO2-DP-TPR from temperature programmed reduction(TPR). DBD plasma reactor contains a large number of high energy particles, including H atoms, excited state H atoms, and ionic hydrogen (H+, H2+, H3+). The reduction ability of H2 plasma is much higher than that of temperature programmed reduction. H2 plasma can fully reduce the precursor at low temperature, avoiding the aggregation of Ni particles caused by temperature programmed reduction, resulting in smaller Ni particles size in the obtained catalyst. When the deposition-precipitation time is 10 hours, the catalytic activity of the catalyst is optimal. When the deposition-precipitation time is less than 10 hours, the content of Ni in the catalyst is relatively low, resulting in low activity. When the deposition-precipitation time exceeds 10 hours, long deposition-precipitation time may lead to an increase in the crystallinity and nickel phyllosilicate particles size. As the deposition-precipitation time increases, the deposition components gradually block the pores and reduce the specific surface area of the catalyst, resulting in a decrease in the catalytic activity of the catalyst. Under optimal preparation conditions, the conversions of CH4 and CO2 are 72.5% and 78.2%. The selectivities of H2 and CO are 86.7% and 94.2%, and H2/CO is 0.89. The energy efficiency is 4.36 mmol/kJ.-
Key words:
- CH4/CO2 reforming /
- Ni/SiO2 /
- dielectric barrier discharge plasma
-
图 10 (a)反应后Ni/SiO2-DP和Ni/SiO2-IMP的XRD谱图(b)反应后Ni/SiO2-DP和Ni/SiO2-IMP的TG曲线(c)反应后Ni/SiO2-DP和Ni/SiO2-IMP的拉曼光谱(d)反应后Ni/SiO2-DP的TEM图像
Figure 10 (a) XRD patterns of the Ni/SiO2-DP and Ni/SiO2-IMP after reaction (b) TG curves of the Ni/SiO2-DP and Ni/SiO2-IMP after reaction (c) Raman spectra of the Ni/SiO2-DP and Ni/SiO2-IMP after reaction (d) TEM image of Ni/SiO2-DP after reaction
表 1 不同沉积沉淀时间的Ni/SiO2-DP的物理结构特征参数
Table 1 Physical structural charateristics of Ni/SiO2-DP with different DP time
Catalyst SBET/(m2·g−1) v/(cm3·g−1) d/nm Ni/SiO2-DP-4 h 247.7 0.64 8.86 Ni/SiO2-DP-10 h 242.0 0.66 9.11 Ni/SiO2-DP-16 h 223.5 0.68 9.78 表 2 Ni/SiO2-DP协同DBD等离子体催化干重整性能
Table 2 The catalytic performance of Ni/SiO2-DP for DRM with DBD plasma
Input power/W Conversion/% Selectivity/% CH4 CO2 H2 CO 19 25.3 23.2 60.1 68.3 21 34.6 37.8 71.6 85.1 23 45.3 51.4 77.5 89.6 25 54.1 62.6 80.1 92.1 27 72.5 78.2 86.7 94.2 表 3 Ni/SiO2-DP热法催化干重整性能
Table 3 The catalytic performance of Ni/SiO2-DP for DRM with thermal catalysis
Reaction temp./℃ Conversion/% Selectivity/% CH4 CO2 H2 CO 600 50.6 54.8 76.9 93.9 650 67.3 72.8 81.2 96.1 700 81.1 84.5 83.6 97.6 750 89.9 91.0 84.7 98.7 800 95.0 94.2 86.1 99.1 -
[1] ZACHOS J C, DICKENS G R, ZEEBE R E. An early cenozoic perspective on greenhouse warming and carbon-cycle dynamics[J]. Nature,2008,451(7176):279−283. doi: 10.1038/nature06588 [2] WANG W, WANG S P, MA X B, et al. Recent advances in catalytic hydrogenation of carbon dioxide[J]. Chem. Soc. Rev.,2011,40(7):3703−3727. doi: 10.1039/c1cs15008a [3] BAHMANPOUR A M, SIGNORILE M, KRöCHER O. Recent progress in syngas production via catalytic CO2 hydrogenation reaction[J]. Appl. Catal. , B,2021,295:120319. doi: 10.1016/j.apcatb.2021.120319 [4] SAQIB N, RADULESCU M, USMAN M, et al. Environmental technology, economic complexity, renewable electricity, environmental taxes and CO2 emissions: Implications for low-carbon future in G-10 bloc[J]. Heliyon,2023,9(6):e16457. doi: 10.1016/j.heliyon.2023.e16457 [5] DIAO Y A, WANG H Y, CHEN B B, et al. Ordered mesoporous Ni-La2O3/Al2O3 catalysts towards efficient plasma-assisted dry reforming of methane[J]. Fuel Process. Technol.,2023,243:107676. doi: 10.1016/j.fuproc.2023.107676 [6] CHUNG W C, CHANG M B. Review of catalysis and plasma performance on dry reforming of CH4 and possible synergistic effects[J]. Renewable Sustainable Energy Rev.,2016,62:13−31. doi: 10.1016/j.rser.2016.04.007 [7] LI K, LIU J L, LI X S, et al. Novel power-to-syngas concept for plasma catalytic reforming coupled with water electrolysis[J]. Chem. Eng. J.,2018,353:297−304. doi: 10.1016/j.cej.2018.07.111 [8] TU X, GALLON H J, TWIGG M V, et al. Dry reforming of methane over a Ni/Al2O3 catalyst in a coaxial dielectric barrier discharge reactor[J]. J. Phys. D: Appl. Phys.,2011,44(27):274007. doi: 10.1088/0022-3727/44/27/274007 [9] TU X, WHITEHEAD J C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Understanding the synergistic effect at low temperature[J]. Appl. Catal. , B,2012,125:439−448. doi: 10.1016/j.apcatb.2012.06.006 [10] KHOJA A H, TAHIR M, AMIN N A S. Recent developments in non-thermal catalytic DBD plasma reactor for dry reforming of methane[J]. Energy Convers. Manage.,2019,183:529−560. doi: 10.1016/j.enconman.2018.12.112 [11] UYTDENHOUWEN Y, BAL K M, NEYTS E C, et al. On the kinetics and equilibria of plasma-based dry reforming of methane[J]. Chem. Eng. J.,2021,405:126630. doi: 10.1016/j.cej.2020.126630 [12] KUAI P Y, LIU C J, HUO P P. Characterization of CuO-ZnO catalyst prepared by decomposition of carbonates using dielectric-barrier discharge plasma[J]. Catal. Lett.,2009,129(3-4):493−498. doi: 10.1007/s10562-008-9829-2 [13] ZENG Y X, ZHU X B, MEI D H, et al. Plasma-catalytic dry reforming of methane over γ-Al2O3 supported metal catalysts[J]. Catal. Today,2015,256:80−87. doi: 10.1016/j.cattod.2015.02.007 [14] LI J W, DOU L G, GAO Y, et al. Revealing the active sites of the structured Ni-based catalysts for one-step CO2/CH4 conversion into oxygenates by plasma-catalysis[J]. J. CO2 Util.,2021,52:101675. doi: 10.1016/j.jcou.2021.101675 [15] SCARDUELLI G, GUELLA G, ASCENZI D, et al. Synthesis of liquid organic compounds from CH4 and CO2 in a dielectric barrier discharge operating at atmospheric pressure[J]. Plasma Processes Polym.,2011,8(1):25−31. doi: 10.1002/ppap.201000044 [16] SONG H K, CHOI J W, YUE S H, et al. Synthesis gas production via dielectric barrier discharge over Ni/γ-Al2O3 catalyst[J]. Catal. Today,2004,89(1-2):27−33. doi: 10.1016/j.cattod.2003.11.009 [17] TAO X M, YANG C, HUANG L, et al. DBD plasma combined with catalysts derived from NiMgAlCe hydrotalcite for CO2 reforming of CH4[J]. Mater. Chem. Phys.,2020,250:123118. doi: 10.1016/j.matchemphys.2020.123118 [18] ZHANG Q L, WANG M Z, ZHANG T F, et al. A stable Ni/SBA-15 catalyst prepared by the ammonia evaporation method for dry reforming of methane[J]. Rsc Adv.,2015,5(114):94016−94024. doi: 10.1039/C5RA18845H [19] BIAN Z F, KAWI S. Highly carbon-resistant Ni-Co/SiO2 catalysts derived from phyllosilicates for dry reforming of methane[J]. J. CO2 Util.,2017,18:345−352. doi: 10.1016/j.jcou.2016.12.014 [20] 李振宇. 高耐磨环氧树脂纳米复合材料的制备及性能 [D]. 安徽理工大学, 2020LI Zhenyu. Preparation and characterization of epoxy nanocomposites with excellent anti-wear performance [D]. AnHui University of Science and Technology, 2020.) [21] HONGMANOROM P, ASHOK J, ZHANG G H, et al. Enhanced performance and selectivity of CO2 methanation over phyllosilicate structure derived NiMg/SBA-15 catalysts[J]. Appl. Catal. , B,2021,282:119564. doi: 10.1016/j.apcatb.2020.119564 [22] YAN L, LIU X X, DENG J, et al. Molybdenum modified nickel phyllosilicates as a high performance bifunctional catalyst for deoxygenation of methyl palmitate to alkanes under mild conditions[J]. Green Chem.,2017,19(19):4600−4609. doi: 10.1039/C7GC01720K [23] TAN J J, XIA X L, CUI J L, et al. Efficient tuning of surface nickel species of the Ni-phyllosilicate catalyst for the hydrogenation of maleic anhydride[J]. J. Phys. Chem. C,2019,123(15):9779−9787. doi: 10.1021/acs.jpcc.8b11972 [24] WANG X, ZHU S H, WANG S, et al. Ni nanoparticles entrapped in nickel phyllosilicate for selective hydrogenation of guaiacol to 2-methoxycyclohexanol[J]. Appl. Catal. , A,2018,568:231−241. doi: 10.1016/j.apcata.2018.10.009 [25] BARTON R R, CARRIER M, SEGURA C, et al. Ni/HZSM-5 catalyst preparation by deposition-precipitation. part 1. effect of nickel loading and preparation conditions on catalyst properties[J]. Appl. Catal. , A,2017,540:7−20. doi: 10.1016/j.apcata.2017.03.040 [26] BIAN Z F, KAWI S. Preparation, characterization and catalytic application of phyllosilicate: A review[J]. Catal. Today,2020,339:3−23. doi: 10.1016/j.cattod.2018.12.030 [27] SIVAIAH M V, PETIT S, BEAUFORT M F, et al. Nickel based catalysts derived from hydrothermally synthesized 1: 1 and 2: 1 phyllosilicates as precursors for carbon dioxide reforming of methane[J]. Microporous Mesoporous Mater.,2011,140(1-3):69−80. doi: 10.1016/j.micromeso.2010.09.015 [28] LIU Z C, ZHOU J, CAO K, et al. Highly dispersed nickel loaded on mesoporous silica: One-spot synthesis strategy and high performance as catalysts for methane reforming with carbon dioxide[J]. Appl. Catal. , B,2012,125:324−330. doi: 10.1016/j.apcatb.2012.06.003 [29] YE R P, LIAO L, REINA T R, et al. Engineering Ni/SiO2 catalysts for enhanced CO2 methanation[J]. Fuel,2021,285:119151. doi: 10.1016/j.fuel.2020.119151 [30] BANG S, HONG E, BAEK S W, et al. Effect of acidity on Ni catalysts supported on P-modified Al2O3 for dry reforming of methane[J]. Catal. Today,2018,303:100−105. doi: 10.1016/j.cattod.2017.08.013 [31] ZHANG T F, LIU Q. Lanthanum-modified MCF-derived nickel phyllosilicate catalyst for enhanced CO2 methanation: A comprehensive study[J]. ACS Appl. Mater. Interfaces,2020,12(17):19587−19600. doi: 10.1021/acsami.0c03243 [32] SOGHRATI E, ONG T K C, POH C K, et al. Zeolite-supported nickel phyllosilicate catalyst for C-O hydrogenolysis of cyclic ethers and polyols[J]. Appl. Catal. , B,2018,235:130−142. doi: 10.1016/j.apcatb.2018.04.053 [33] XIONG K, GAO Y, CHEN J, et al. Ordered porous Ni decorated by thin-layer amorphous nickel-phosphorus mild electrochemical phosphorization for enhancing the hydrogen evolution performance[J]. Chem. Commun.,2020,56(4):611−614. doi: 10.1039/C9CC08698F [34] KAWI S, KATHIRASER Y, NI J, et al. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane[J]. Chemsuschem,2015,8(21):3556−3575. doi: 10.1002/cssc.201500390 [35] WANG J J, WANG X X, ALQAHTANI M S, et al. Synergetic effect of non-thermal plasma and supported cobalt catalyst in plasma-enhanced CO2 hydrogenation[J]. Chem. Eng. J.,2023,451:138661. doi: 10.1016/j.cej.2022.138661 [36] PENG W, LI Z B, LIU B, et al. Enhanced activity and stability of Ce-doped PrCrO3-supported nickel catalyst for dry reforming of methane[J]. Sep. Purif. Technol.,2022,303:122245. doi: 10.1016/j.seppur.2022.122245 [37] QU H, YANG H, HAN L B, et al. Sandwich-structured nickel/kaolinite catalyst with boosted stability for dry reforming of methane with carbon dioxide[J]. Chem. Eng. J.,2023,453:139694. doi: 10.1016/j.cej.2022.139694 [38] WANG F G, HAN K H, YU W S, et al. Low temperature CO2 reforming with methane reaction over CeO2-modified Ni@SiO2 catalysts[J]. ACS Appl. Mater. Interfaces,2020,12(31):35022−35034. doi: 10.1021/acsami.0c09371 [39] 赵俊. 金属磷化物的制备及其甲烷化性能研究 [D]; 天津大学, 2019.ZHAO Jun. Study on preparation of metal phosphides and its methanation performance [D]. Tianjin University, 2019.) [40] 王伟. 氢等离子还原法制备磷化镍及加氢精制催化性能研究[D]. 大连理工大学, 2018WANG Wei. Preparation of nickel phosphides by hydrogen plasma reduction and their catalytic performance in hydrotreatment [D]. Dalian University of Technology, 2018.) [41] GUAN J, WANG Y, QIN M L, et al. Synthesis of transition-metal phosphides from oxidic precursors by reduction in hydrogen plasma[J]. J. Solid State Chem.,2009,182(6):1550−1555. doi: 10.1016/j.jssc.2009.03.026 [42] XIA L H, FANG X Z, XU X L, et al. The promotional effects of plasma treating on Ni/Y2Ti2O7 for steam reforming of methane (SRM): Elucidating the NiO-support interaction and the states of the surface oxygen anions[J]. Int. J. Hydrogen Energy,2020,45(7):4556−4569. doi: 10.1016/j.ijhydene.2019.12.119 [43] LIU C J, ZOU J J, YU K L, et al. Plasma application for more environmentally friendly catalyst preparation[J]. Pure Appl. Chem.,2006,78(6):1227−1238. doi: 10.1351/pac200678061227 [44] 关杰. 氢等离子体法制备金属磷化物及其加氢脱硫性能[D]. 大连理工大学, 2009(GUAN Jie. Synthesis of transition-metal phosphides by reduction in hydrogen plasma and its hydrodesulfurization performance [D]. Dalian University of Technology, 2009.) [45] 遇治权. Ni3P基催化剂的制备及苯酚加氢脱氧性能[D]. 大连理工大学, 2019YU Zhiquan. Preparation of Ni3P-based catalysts and their catalytic performances in phenol hydrodeoxygenation [D]. Dalian University of Technology, 2019.) [46] YAN X L, ZHAO B R, LIU Y, et al. Dielectric barrier discharge plasma for preparation of Ni-based catalysts with enhanced coke resistance: Current status and perspective[J]. Catal. Today,2015,256:29−40. doi: 10.1016/j.cattod.2015.04.045 [47] RAY D, REDDY P M K, SUBRAHMANYAM C. Ni-Mn/γ-Al2O3 assisted plasma dry reforming of methane[J]. Catal. Today,2018,309:212−218. doi: 10.1016/j.cattod.2017.07.003 -




 下载:
下载: